Abstract
Background
We aimed to investigate the prognostic significance of insulin resistance (IR) markers fasting triglyceride-glucose (TyG) index and triglyceride high-density lipoprotein cholesterol (TG/HDL-C) ratio in HER2-positive breast cancer (BC) patients with brain metastasis (BM).
Methods
In this single-center study, 120 patients who met the criteria were included. TyG and TG/HDL-C at the time of diagnosis were computed retrospectively. For TyG and TG/HDL-C, the median values of 9.32 and 2.95 were taken as the cut-off, respectively. TyG values <9.32 and <2.95 were considered low, and TG/HDL-C values ≥9.32 and ≥2.95 were considered high.
Results
The median overall survival (OS) was 47 months (95% CI: 40.54–53.45). Time to BM was 22 months (95% CI: 17.22–26.73). The median time to BM was 35 months (95% CI: 20.90–49.09) in the low TyG group and 15 months (95% CI: 8.92–21.07) in the high TyG group (p < 0.001). The time to BM was 27 months (95% CI: 20.49–33.50) in the low TG/HDL-C group and 20 months (95% CI: 16.76–23.23) in the high TG/HDL-C group (p=0.084). In the multivariate Cox regression analysis, the TyG index (HR: 20.98, 95% CI: 7.14–61.59, p < 0.001) was an independent risk factor for time to BM.
Conclusion
These findings suggest that the TyG index could be used as a predictive biomarker at the time of diagnosis for risk of time BM in patients with HER2-positive BC. The TyG index can be used as a standard potential marker with prospective studies confirming these data.
Introduction
Breast cancer (BC) is the most common cancer in women and the most common cause of cancer death.Citation1 BC is a genetically and pathologically heterogeneous disease. Therefore, the disease’s spread, course, and prognosis are also very different between subgroups. BC subgroups have the potential to metastasize to different sites. BC can metastasize to the bone, lungs, liver, and brain.Citation2 Brain metastasis (BM) is commonly seen in triple-negative (25–75%) and HER2-positive subgroups (11–20%).Citation3,Citation4 BM is common during HER2-positive metastatic BC and leads to poor survival and worse quality of life.Citation5,Citation6
Studies have shown the role of the brain microenvironment and nutrient sources in developing tumor metabolism and growth.Citation7 Glucose metabolism is the main energy source, but different metabolic mechanisms can be used for energy production depending upon the tumor subtype.Citation8 Another important metabolic pathway is lipid metabolism and fatty acid (FA) oxidation.Citation9 Some subtypes of BC have enzymes and molecules in the lipid metabolism steps expressed at different levels as a function of the metastasis site. Acyl-CoA oxidase 1 (ACOX1) and tatty acid synthase (FASN) are expressed at higher levels in HER2-positive BM.Citation9
In recent years, studies have shown that metabolic syndrome and insulin resistance (IR) are associated with BC and its prognosis.Citation10,Citation11 The role of IR in tumor development and carcinogenesis is well-documented.Citation12 Many studies have revealed that the fasting triglyceride-glucose (TyG) index and triglyceride high-density lipoprotein cholesterol (TG/HDL-C) ratio reflected IR and can be used as IR markers.Citation13 These inexpensive and easily accessible markers are mostly used to predict the possibility of carcinogenesis and can predict the risk of various cancers.Citation14,Citation15 However their role in metastasis has not yet been adequately investigated. Despite the detection of various molecules mentioned in the above paragraph, no marker has yet been used in clinical practice for BM of BC. Thus, TyG index and TG/HDL-C ratio may reflect glucose-lipid metabolism, which is thought to play an important role for tumor cells in brain metastases; it may also play a predictive-prognostic role.
To the best of our knowledge, there is no data in the English literature investigating the relationship between HER2-positive BC patients with BM and TyG index and TG/HDL-C ratio. Hence, this study aimed to investigate the predictive significance of the TyG index and TG/HDL-C ratio in HER2-positive BC with BM.
Methods
Patients
This retrospective study reviewed data from 3225 HER2-positive BC patients who were followed in our oncology center (Health Sciences University, Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital, Ankara/Turkey) between 2000 and 2021. Patients (n=120) older than 18 years who had HER2-positive BC with BM were included in the study. We excluded patients with secondary malignancies (had cancer other than BC), those under 18 years of age, and patients with comorbidities and conditions that might impact lipid and glucose levels [eg, diabetes mellitus (DM), dyslipidemia, coronary artery disease, hypertension (HT), liver metastasis, active infection and steroid use], as well as those with missing data. In addition to demographic data, complete baseline blood counts and biochemistry parameters at the time of BC diagnosis were recorded.
Measurements
Fasting TG [mg/dl] × fasting glucose[mg/dl] was used to calculate the ln TyG index (ln: natural logarithm of a number).Citation10 The TG/HDL-C ratio was calculated as TG divided by HDL-C. The median values were the cut-off values for the TyG index and TG/HDL-C ratio. They were divided into two groups with values above the median high and below the median low.
Statistical Analysis
Statistical analyses were conducted with SPSS 25.0 software (SPSS, Chicago, IL, USA). The Mann–Whitney U-test compared nonparametric data while the Student’s t-test compared parametric data. Chi-square or Fisher’s exact test was used to compare categorical data. The Kaplan-Meier method was used for survival analysis, and the Log rank test was used for intergroup comparisons. Predictive factors impacting the overall survival (OS) were determined by multivariate analysis with the Cox proportional hazards model in which p < 0.05 was considered statistically significant. The time to BM was considered the primary endpoint.
Results
All patients were female (n=120). The median age at diagnosis was 48 years old (IQR: 42–55), and 13 patients (10.8%) were smokers. Pathological examination showed that 72 patients (60%) had hormone receptor positivity. Except for BM, the most common metastasis area was the lymph nodes (80%). Patient characteristics are summarized in .
Table 1 Baseline Characteristics of Patients
The median follow-up duration was 44.5 months (IQR: 29–69.75), and 101 patients died (84.2%). The median OS was 47 months (95% CI: 40.54–53.45). The time from the first BC diagnosis to brain metastasis was defined as “time to BM” and was 22 months (95% CI: 17.22–26.73). Survival after BM was 13 months (95% CI: 10.01–15.98).
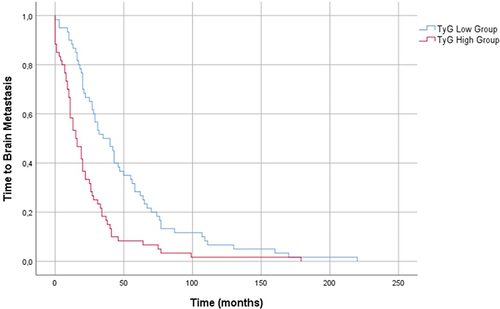
In the correlation analysis, there was a negative correlation between time to BM and TyG index as well as the TG/HDL-C ratio (for TyG r:-0.488, p<0.001 for TG/HDL-C r:-0.269 p=0.003) (). Patients were divided into low and high TyG groups as well as TG/HDL-C groups based on the median TyG (9.32) and TG/HDL-C (2.95). The median time to BM in the low TyG group (< 9.32) was 35 months (95% CI: 20.90–49.09). Time to BM was 15 months in the high TyG group (≥ 9.32) (95% CI: 8.92–21.07) (p < 0.001) ().
Table 2 Correlation Analyzes for Time to Brain Metastasis
In the low TG/HDL-C group (<2.95), the time to BM was 27 months (95% CI: 20.49–33.50) and 20 months in the high TG/HDL-C group (≥2.95), (95% CI: 16.76–23.23). Although there was a numerical difference, there was no statistical significance (p=0.084). presents a summary of survival outcomes. Multivariate Cox regression analysis with TyG index, TG/HDL-C ratio, TG, HDL-C, and glucose showed that TyG (HR: 20.98, 95% CI: 7.14–61.59, p < 0.001) was an independent risk factor for time to BM.
Table 3 Survival Outcomes
Discussion
Our study found that the TyG index at diagnosis is an independent prognostic factor for time to BM in patients with HER2-positive BC. Moreover, our findings showed a negative correlation between time to BM and TG/HDL-C ratio. Changes in glucose and lipid metabolism play key and complex roles in tumor growth, invasion, and metastasis.Citation16 The Warburg effect is one of the main glucose metabolism phenomena. Here, a metabolic shift in energy production occurs from oxidative phosphorylation in normal cells towards aerobic glycolysis in cancer cells.Citation9,Citation17 BC exhibits unique characteristics according to its pathologic subtypes and metastatic sites. Different glucose-lipid metabolism pathways are activated in each subtype.Citation9 One of these pathways is lipid metabolism, which involves lipid synthesis, lipid degradation, catabolism, and FA oxidation.Citation18 FASN and ACOX1 are involved in lipid synthesis and oxidation and play key roles in the metabolism of BC metastasis.Citation19,Citation20 The expression of these enzymes was higher in HER2 type BC and BM in different studies.Citation9,Citation16,Citation18 The brain has different metabolic and anatomical features versus other metastasis sites in BC. It produces energy by glycolysis using glucose and not by oxidative phosphorylation—thus, the brain’s metabolic environment is differentiated.Citation9 Although endothelial cells form a continuous barrier that prevent cancer cells from penetrating easily, brain-tropic HER2-positive BC cells that outcompete the proximate cells for glucose uptake and metabolize lactate can initiate BM.Citation7
Various studies have reported a relationship between hyperglycemia-dyslipidemia and malignancy.Citation21,Citation22 Hyperglycemia and dyslipidemia may play roles in tumor initiation and progression.Citation23 A trial found that high TG levels are a risk factor for lymphatic metastasis of early cancer in the upper gastrointestinal tract.Citation24 Another study determined that hyperglycemia activates intranuclear nuclear factor kappa B (NF-κB), which regulate several genes regarding neoplasia and metastasis.Citation25
Despite all of these data, no markers are yet standard in clinical practice to reflect glucose-lipid metabolism and BC metastases. The biomarkers used in studies on metabolism typically have one component (eg, FASN, ACOX1), and they fail to adequately reflect the BM-BC relationship with different metabolic pathways and mechanisms. The TyG index is used initially in metabolic syndrome consisting of TG and glucose components and is a practical and reliable measurement for IR.Citation26,Citation27 Many studies have demonstrated that the TyG index could predict the incidence of type 2 DM and cardiovascular disease.Citation28,Citation29 Metabolic syndrome and its indicator, IR, play an important role in the development of cancer.Citation30 The adipose tissue and related products are the main factors in metabolic syndrome physiopathology. The changed production of adipokines, cytokines, and hormones generate a tumorigenic micro-environment resulting in increased tumor progression and reduced survival.Citation31,Citation32
Moreover, IR induces hyperinsulinemia and activates the PI3K/AKT/mTOR/S6K signaling pathway in malignancy.Citation33 Fritz et al studied patients with obesity-related cancers. The authors reported that the TyG index, an IR indicator, was associated with an increased risk of cancers of the digestive system such as the liver, pancreas, colon, and rectum.Citation15 Different studies on patients with gynecologic cancers, non-small cell lung cancer, and colorectal cancer showed that the TyG index predicted the incidence of cancer.Citation34–36
The TyG index was used to predict cancer incidence relative to the normal population in BC as in other previously mentioned malignancy studies. A multicenter study conducted by Panigoro et al revealed that a high TyG index was associated with the risk of occurring BC.Citation10 Another study on breast mass patients demonstrated the TyG index’s predictive effect in distinguishing benign and malignant lesions of the breast.Citation14 In addition to all these publications, our study found that the TyG index may also effectively predict the risk of developing BM in HER2-positive BC. The TyG index activates the AKT signaling pathway, which promotes the metastasis process by G protein-coupled receptor.Citation37 Another study supports these findings and demonstrates that FA promotes glioblastoma multiforme proliferation via triglyceride metabolism.Citation38 A meta-analysis of seven studies on brain tumors revealed that hyperglycemia has an obvious prognostic significance in patients with brain tumors and is associated with shorter OS.Citation39
We found a negative correlation between time to BM and TG/HDL-C ratio, but it did not reach statistical significance probably due to the low number of patients when divided low-and high grouped with reference to median cut off value. A study conducted with colorectal cancer patients demonstrated that HDL-C was related to increased proinflammatory cytokines, which promote cancer cell proliferation.Citation40 A Chinese study found that TG/HDL-C ratio was associated with a higher risk of colorectal cancer.Citation13 Some studies have failed to find associations between lipid lipoproteins and the risk of BC, while other large clinical studies have demonstrated an inverse association between HDL-C and BC risk.Citation41 In contrast, a population-based survival study suggested no relationships observed between lipids and prognostic outcomes among patients with luminal A and luminal B subtypes. However, the authors reported that TG and the HDL-C/total-cholesterol ratio could independently provide information regarding prognostic outcomes among triple-negative BC patients. TG levels were inversely associated with OS in HER2 patients.Citation42
This study does have some limitations. The most important of these is the inability to report body mass index data, which is one of the factors that may affect the TyG index. Moreover, it was a retrospective and single-center study; thus, despite impressive results, a prospective multicenter study can be more effective in evaluating the TyG index. There is also a risk of bias in some results due to the low number of patients and missing data.
Conclusion
This study demonstrated that the TyG index could be used as a prognostic biomarker at the time of diagnosis for risk of time BM in patients with HER2-positive BC. In this patient group, a closer follow-up may be required regarding the risk of BM. This was the first study to investigate the predictive value of TyG with BM HER2-positive BC in English literature. Large prospective studies on this subject will provide better information, thus reducing the possibility of bias.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee from Health Sciences University, Ankara Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital (Decision No: 2022-12/2167). All participants were adequately informed of the aims, methods, and risks of the study as well as of voluntary participation and confidentiality of the responses at the introduction of the survey. The responses were anonymous and participants’ confidentiality was maintained.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
Data Sharing Statement
All data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sadeghi M, Kashanian S, Naghib SM, Arkan E. A high performance electrochemical aptasensor based on graphene decorated rhodium nanoparticles to detect HER2-ECD oncomarker in liquid biopsy. Sci Rep. 2022;12:3299. doi:10.1038/s41598-022-07230-3
- Karakaya S, Karadag I, Ates O, Cakmak Oksuzoglu OB, Clinical Outcomes DU. Prognostic Factors in HER-2 Positive Breast Cancer with Brain Metastasis: a Single-centre Experience. J Coll Physicians Surg Pak. 2021;31:166–170.
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi:10.1200/JCO.2009.25.9820
- Martin AM, Cagney DN, Catalano PJ, et al. Brain Metastases in Newly Diagnosed Breast Cancer: a Population-Based Study. JAMA Oncol. 2017;3:1069–1077. doi:10.1001/jamaoncol.2017.0001
- Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22:525–531. doi:10.1016/j.breast.2012.12.006
- Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4:33. doi:10.1186/s40164-015-0028-8
- Parida PK, Marquez-Palencia M, Nair V, et al. Metabolic diversity within breast cancer brain-tropic cells determines metastatic fitness. Cell Metab. 2022;34(90–105.e7). doi:10.1016/j.cmet.2021.12.001
- Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi:10.1111/j.1742-4658.2007.05686.x
- Jung YY, Kim HM, Koo JS. Expression of Lipid Metabolism-Related Proteins in Metastatic Breast Cancer. PLoS One. 2015;10:e0137204. doi:10.1371/journal.pone.0137204
- Panigoro SS, Sutandyo N, Witjaksono F, et al. DK, Pranata R. The Association Between Triglyceride-Glucose Index as a Marker of Insulin Resistance and the Risk of Breast Cancer Front Endocrinol (Lausanne). 2021;12:745236.
- Pan K, Chlebowski RT, Mortimer JE, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126:3638–3647. doi:10.1002/cncr.33002
- Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7:1003. doi:10.7150/ijbs.7.1003
- Liu T, Zhang Q, Wang Y, et al. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. 2022;22:1007. doi:10.1186/s12885-022-10100-w
- Alkurt EG, Özkan MB, Turhan VB. Predictive value of triglyceride/glucose index (TyG) in predicting breast cancer in patients with breast mass. Eur Rev Med Pharmacol Sci. 2022;26:4671–4676. doi:10.26355/eurrev_202207_29191
- Fritz J, Bjørge T, Nagel G, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol. 2020;49:193–204. doi:10.1093/ije/dyz053
- Wang L, Zhang S, Wang X. The Metabolic Mechanisms of Breast Cancer Metastasis. Front Oncol. 2021;10:602416. doi:10.3389/fonc.2020.602416
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi:10.1126/science.123.3191.309
- Kim S, Lee Y, Koo JS. Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS One. 2015;10:e0119473. doi:10.1371/journal.pone.0119473
- Albiges L, Andre F, Balleyguier C, Gomez-Abuin G, Chompret A, Delaloge S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol. 2005;16:1846–1847. doi:10.1093/annonc/mdi351
- Arnold SM, Young AB, Munn RK, Patchell RA, Nanayakkara N, Markesbery WR. Expression of p53, bcl-2, E-cadherin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 in paired primary tumors and brain metastasis. Clin Cancer Res. 1999;5:4028–4033.
- Hiatt RA, Friedman GD, Bawol RD, Ury HK. Breast cancer and serum cholesterol. J Natl Cancer Inst. 1982;68:885–889.
- Howe GR, Aronson KJ, Benito E, et al. The relationship between dietary fat intake and risk of colorectal cancer: evidence from the combined analysis of 13 case-control studies. Cancer Causes Control. 1997;8:215–228. doi:10.1023/A:1018476414781
- O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi:10.1111/obr.12229
- Sako A, Kitayama J, Kaisaki S, Nagawa H. Hyperlipidemia is a risk factor for lymphatic metastasis in superficial esophageal carcinoma. Cancer Lett. 2004;208:43–49. doi:10.1016/j.canlet.2003.11.010
- Aljada A, Friedman J, Ghanim H, et al. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism. 2006;55:1177–1185. doi:10.1016/j.metabol.2006.04.016
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi:10.1089/met.2008.0034
- Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105. doi:10.1016/j.ypmed.2016.01.022
- Low S, Khoo KCJ, Irwan B, et al. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;143:43–49. doi:10.1016/j.diabres.2018.06.006
- Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana- Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Investig. 2016;46:189–197. doi:10.1111/eci.12583
- Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7:1003–1015.
- Battelli MG, Bortolotti M, Polito L, Bolognesi A. Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol. 2019;21:101070. doi:10.1016/j.redox.2018.101070
- Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–189. doi:10.1017/S002966511100320X
- Khan KH, Yap TA, Yan L, Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer. 2013;32:253–265. doi:10.5732/cjc.013.10057
- Shi H, Zhou L, Yang S, Zhou H. The relationship between Triglyceride and glycose (TyG) index and the risk of gynaecologic and breast cancers. Clin Nutr ESPEN. 2022;51:345–352. doi:10.1016/j.clnesp.2022.08.004
- Yan X, Gao Y, Tong J, Tian M, Dai J, Zhuang Y. Association Between Triglyceride Glucose Index and Non-Small Cell Lung Cancer Risk in Chinese Population. Front Oncol. 2021;11:585388. doi:10.3389/fonc.2021.585388
- Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: a population-based longitudinal study. BMC Endocr Disord. 2020;20:113. doi:10.1186/s12902-020-00581-w
- Sekine Y, Koike H, Nakano T, Nakajima K, Suzuki K. Remnant lipoproteins stimulate proliferation and activate MAPK and Akt signaling pathways via G protein-coupled receptor in PC-3 prostate cancer cells. Clin Chim Acta. 2007;383:78–84. doi:10.1016/j.cca.2007.04.016
- Taïb B, Aboussalah AM, Moniruzzaman M, et al. Lipid accumulation and oxidation in glioblastoma multiforme. Sci Rep. 2019;9:19593. doi:10.1038/s41598-019-55985-z
- Liu H, Liu Z, Jiang B, et al. Prognostic Significance of Hyperglycemia in Patients with Brain Tumors: a Meta-Analysis. Mol Neurobiol. 2016;53:1654–1660. doi:10.1007/s12035-015-9115-4
- van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60:1094–1102. doi:10.1136/gut.2010.225011
- Cedó L, Reddy ST, Mato E, Blanco-Vaca F, Escolà-Gil JCHDL, Potential New LDL. Players in Breast Cancer Development. J Clin Med. 2019;8:853. doi:10.3390/jcm8060853
- Lofterød T, Mortensen ES, Nalwoga H, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer. 2018;18:654. doi:10.1186/s12885-018-4568-2