Abstract
Engraftment syndrome (ES) is an early complication of hematopoietic stem cell transplantation (HSCT) characterized by fever and additional clinical manifestations including rash, diarrhea, lung infiltrates, weight gain, and neurological symptoms. Steroid-resistant ES following HSCT significantly affects the efficacy of transplantation and may even result in patient mortality. As ES essentially represents a cytokine storm induced by engrafted donor cells with interferon-gamma (IFN-γ) playing a central role, we hypothesized that emapalumab (an anti-IFN-γ monoclonal antibody) may be an effective approach to treat steroid-resistant ES. Here, we present a case report of a 14-year-old female patient who received a second haploidentical HSCT due to a relapse of acute myeloid leukemia. Nine days after the transplantation, the patient developed a fever and exhibited a poor response to antimicrobials (ceftazidime/avibactam). A few days later, the patient presented with a new-onset rash, weight gain, and impaired liver function, leading to a diagnosis of ES. Initial immunosuppressive (tacrolimus and mycophenolate mofetil) treatment failed to control the disease. On day 16 post-transplantation, the patient received two infusions of 50 mg of emapalumab. Following the initiation of emapalumab treatment, the patient’s fever returned to normal and ES was effectively controlled. This case report demonstrated that emapalumab had a possible efficacy for steroid-resistant ES and provided a novel therapeutic strategy to treat this clinical complication.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is extensively used to manage various malignant and non-malignant hematological disorders because of its therapeutic potential and improved overall survival rates.Citation1–3 Engraftment syndrome (ES), an early complication of HSCT, is characterized by fever and additional clinical manifestations including rash, diarrhea, lung infiltrates, weight gain, and neurological symptoms.Citation4 ES presents as a non-infectious fever with clinical features resembling acute graft-versus-host disease.Citation5 Currently, glucocorticoid is the standard treatment for ES, exhibiting an effective rate of approximately 80%.Citation5,Citation6 However, no treatment is currently established for patients resistant to steroids.Citation7 Steroid-resistant ES following HSCT significantly affects the efficacy of transplantation and may even result in patient mortality.Citation7,Citation8 Consequently, there is an urgent demand for novel therapeutic strategies to effectively manage severe steroid-resistant ES.
ES essentially represents a cytokine storm induced by engrafting donor cells with interferon-gamma (IFN-γ) playing a central role. Therefore, blockade of IFN-γ may provide a therapeutic benefit. Previous studies have demonstrated favorable disease control by emapalumab (an anti-IFN-γ monoclonal antibody) in hemophagocytic lymphohistiocytosis (HLH).Citation9 Therefore, we hypothesized that emapalumab may be an effective approach to treat steroid-resistant ES. To the best of our knowledge, no reports have been published regarding the use of emapalumab in ES patients. In this study, we present a case of steroid-resistant severe ES occurring after haploidentical allogeneic hematopoietic HSCT (haplo-HSCT), which was effectively controlled by emapalumab treatment.
Case Report
A 14-year-old female patient was diagnosed with acute myeloid leukemia (AML, M4) in June 2021 and received chemotherapy regimens followed by conditioning (decitabine, cytarabine, busulfan, cyclophosphamide, ATG, and semustine) and haplo-HSCT (HLA 8/10, B to O), achieving morphologic response with 0.14% of residual cells and 30% of FLT3-ITD mutations. On post-transplantation day 259, the patient underwent disease relapse, prompting subsequent administration of chemotherapy, targeted therapy, and donor CD34+ stem cell boost, all of which yielded unsatisfactory outcomes. A second haplo-HSCT (HLA 6/10, B to O; CD34+ cells: 10.85×10^6/kg, unmanipulated) was conducted on March 28, 2023, following conditioning (venetoclax, gilteritinib, melphalan, busulfan, and PTCy).
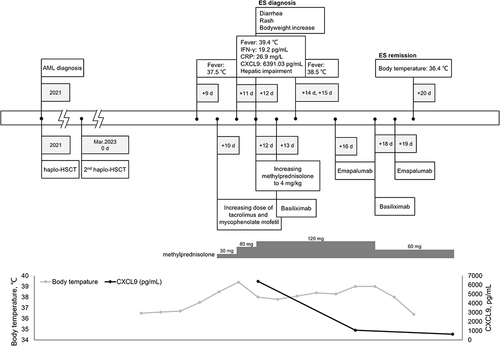
For the second transplantation, we employed a combination of cyclophosphamide (50 mg/kg/d, +3 day, +4 day), cyclosporine (2 mg/kg/d, initiated from −1 day), and mycophenolate mofetil (MMF) (600 mg/m2/d, initiated from −1 day) as a preventive measure against graft-versus-host disease (GVHD). On day 9 post-transplantation, the patient presented with a fever accompanied by an increase in body temperature to 37.5 °C. Subsequently, on day 10, the dosages of tacrolimus and mycophenolate mofetil were increased. On day 11, the patient’s body temperature spiked to 39.4 °C, along with a slight elevation in the IFN-γ level (19.2 pg/mL), an elevated C-reactive protein (CRP) level (26.9 mg/L), significantly increased CXC motif chemokine ligand 9 (CXCL9) level (6391.03 pg/mL), and concurrent hepatic dysfunction. A full timeline presenting clinical presentations and treatments over time is shown in .
Physical examination revealed newly developed erythematous and edematous lips, scattered erythematous rashes on the palms, face, and trunk, lower limb edema, and weight gain of 1.5 kg within 1 week. Antimicrobials (ceftazidime/avibactam) proved ineffective, and blood culture yielded negative results, ruling out infectious fever. The onset of symptoms occurred prior to leukocyte engraftment, excluding acute graft-versus-host disease. On day 12, the patient presented with diarrhea (1000 mL/day) and new erythematous rashes on the palms, face, and trunk, accompanied by weight gain and liver dysfunction (ALT 95.5 U/L, AST 145.6 U/L). Oxygen saturation, renal function, and coagulation were normal and engraftment of white blood cell, hemoglobin, and platelet was not achieved.
The methylprednisolone dosage was increased to 4 mg/kg. Following the diagnostic criteria proposed by Spitzer et al,Citation10 the patient was diagnosed with grade 2 acute ES due to persistent high body temperature, lack of response to glucocorticoid, presence of edema, and experiencing diarrhea. On day 13 and 18, the treatment regimen was augmented with 20 mg of anti-CD25 monoclonal antibody. However, the patient continued to experience fever exceeding 39 °C in the subsequent days. Cytokine analysis revealed a mild increase in IFN-γ which was demonstrated to lead to tissue damage according to previous literature.Citation11 We were concerned that persistently high levels of inflammation and severe organ damage would occur if the increasing level of IFN-γ was not adequately controlled.
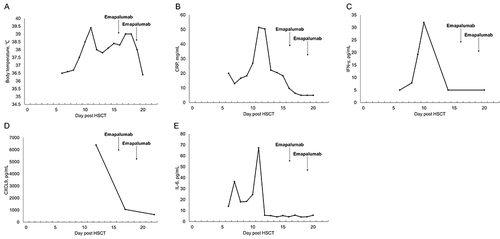
Consequently, the attending physician administered 50 mg of emapalumab on days 16 and 19. On day 20, the patient’s peak body temperature gradually decreased and ES symptoms were effectively controlled. illustrated the dynamic changes in inflammatory markers and body temperature. No significant adverse reactions were observed during emapalumab administration, and there were no indications of severe organ damage or any specific adverse events. Moreover, successful platelet engraftment was achieved on day 11, followed by successful white blood cell engraftment on day 14. Evaluation of bone marrow morphology and residual immunology revealed no abnormalities, confirming achievement of successful allogeneic hematopoietic stem cell transplantation with the second donor’s genotype exhibiting a chimerism rate of 100%. Subsequent follow-up after HSCT demonstrated maintenance of complete donor chimerism in the patient without any signs of graft-versus-host disease or organ dysfunction. Notably, inflammatory marker levels returned to normal, which was accompanied by complete recovery of the patient’s clinical symptoms.
Discussion
This case report highlights the potential efficacy of emapalumab, an IFN-γ-neutralizing antibody, as a promising treatment to control steroid-resistant ES in patients who undergo haplo-HSCT. In severe cases of ES after HSCT, which is challenging to manage, emapalumab treatment is a viable immunomodulatory approach. Subsequent to emapalumab infusion, the patient demonstrated a gradual reduction in body temperature, normalization of inflammatory markers, and alleviation of ES symptoms, indicating successful modulation of the inflammatory response and favorable clinical outcomes.
During HSCT, engraftment of donor cells is often accompanied by early immune reactions. These immune reactions, referred to as ES, typically occur between 4 days before neutrophil engraftment and 1 day after neutrophil engraftment.Citation4,Citation10,Citation12 ES is a common complication in both autologous and allogeneic HSCT, which is characterized by a proinflammatory response that induces local and systemic endothelial damage. This damage is primarily caused by direct and indirect release of proinflammatory cytokines, including interleukin (IL)-1, IFN-γ, and tumor necrosis factor-alpha (TNF-α).Citation11 ES manifests as various symptoms, such as non-infectious fever, maculopapular rash, weight gain, and non-cardiogenic pulmonary edema.Citation11,Citation13–16 In severe cases, ES can lead to multiple organ dysfunction and even mortality.Citation4 The incidence of ES varies significantly with reported occurrence rates ranging from 5% to 79%.Citation17,Citation18 Currently, glucocorticoid is the standard treatment for ES,Citation5,Citation6 achieving an efficacy rate of approximately 80%. However, for patients who do not respond to glucocorticoids, no definitive treatment option is available.Citation7 Patients with severe ES, who are unresponsive to glucocorticoids and lack alternative treatment options, may eventually die.Citation19
Although a skin biopsy was not performed on the patient, we did not consider the symptoms to be indicative of acute GVHD based on timing, as the rash appeared before the engraftment of white blood cells. However, we still used anti-CD25 antibody as a prophylactic treatment for GVHD. The symptoms of the patient, especially body temperature, had no significant improvement meaning the treatment against GVHD seemed not effective. ES pathogenesis primarily involves immune dysregulation, leading to aberrant proliferation of cytotoxic T cells.Citation19–21 Subsequently, this process triggers a cytokine storm characterized by cytokines such as IL-1, IL-6, TNF-α, and IFN-γ with IFN-γ playing a pivotal role.Citation11,Citation22 In this case, evaluation of IL-1 and IL-6 levels was conducted, revealing a normal IL-1 level and a slightly elevated IL-6 level. Consequently, targeted therapies specific to these cytokines were not utilized.
HLH is a severe inflammatory reaction syndrome, and its pathogenesis arises from genetic or acquired immune regulatory dysfunction. Prolonged synapse time between cytotoxic lymphocytes lacking perforin or granule enzymes and target cells leads to excessive production of proinflammatory cytokines. Notably, cytokines such as IFN-γ augment the antigen-presenting ability of antigen-presenting cells, further stimulating T-cell activation and proliferation, thereby triggering the production of additional cytokines. Elevated cytokine levels ultimately culminate in extensive tissue damage and life-threatening hyperinflammatory syndrome.Citation22–25 HLH pathogenesis is typically caused by the breakdown of negative feedback loops, resulting in sustained immune activation and perpetuation of high inflammatory states, known as cytokine storms. IFN-γ plays a critical role in HLH cytokine storms, because it activates the JAK-STAT pathway and regulates the inflammatory immune response.Citation26–29 Emapalumab, which is primarily used for HLH treatment, neutralizes IFN-γ to control cytokine storms and alleviate various organ damage. Considering the similarity in the pathogenesis of ES and HLH, where cytokine storms are pivotal mechanisms, we hypothesized that treatment at the cytokine level warranted consideration. By neutralizing IFN-γ, which is responsible for significant organ damage through an IFN-γ antagonist, treating ES can be realized, thereby enhancing the prognosis of transplant patients.
In the Phase 2/3 clinical trial of emapalumab, the initial dose was 1 mg/kg, administered twice weekly. Pharmacokinetic characteristics of emapalumab were affected by target-mediated drug disposition (TMDD) and the levels of IFN-γ in the body, leading to dose adjustments based on clinical and laboratory parameters during the trial.Citation9 In this patient, a similar dose (50 mg for 31 kg body weight) was administrated twice a week. The rapid decrease in CXCL9 concentration and significant improvement in clinical symptoms suggested effective neutralization of the target, leading to discontinuation of emapalumab. Currently, there are no established criteria for dosage in patients with ES, highlighting the need for further investigation and development of a specific protocol for emapalumab in this population through prospective studies.
CXCL9, a chemokine specifically induced by IFN-γ, selectively binds to CXC chemokine receptor 3 (CXCR3) on various immune cell types, including naïve T, Th1 CD4+ T, effector CD8+ T, NK, and NKT cells. This interaction promotes the development of Th1 inflammation, a crucial immune response against pathogens. Notably, the serum CXCL9 level is a reliable indicator of IFN-γ production by organs, such as the liver and spleen, which are commonly targeted by inflammation.Citation9,Citation30 Unlike IFN-γ, which has a short half-life in blood, CXCL9 exhibits stability and can be easily quantified in serum, making it a valuable surrogate marker for the IFN-γ level in organs. Clinical evidence increasingly supports the utility of CXCL9 as a biomarker for IFN-γ production. Elevated levels of IFN-γ/CXCL9 not only contribute to liver damage and coagulation abnormalities, but also increase the risk of transplant failure.Citation9
The conventional treatment of glucocorticoid aims to suppress lymphocytes to mitigate immune reactions. However, in cases of severe ES where steroid resistance and markedly elevated CXCL9 are present, no definitive treatment is currently available. Once ES is initiated, the production of downstream cytokines becomes a pivotal factor in causing organ damage. In addition to actively inhibiting lymphocytes to prevent further cytokine production, timely clearance of already produced cytokines is also crucial. Animal studies have demonstrated that inhibition of IFN-γ substantially reduces the severity of immune reactions.Citation31 Building upon this discovery, we propose targeting cytokines as a starting point and neutralizing IFN-γ by introducing an IFN-γ antagonist. This approach aims to alleviate the inflammatory damage caused by the cytokine storm in ES and ultimately achieve control over steroid-resistant severe ES with significantly elevated CXCL9 levels.
In this case study, we observed a suboptimal response to conventional glucocorticoid in the patient, who subsequently developed severe ES. To address this, we employed early administration of emapalumab, an IFN-γ antagonist, as a therapy. The outcomes revealed a rapid reduction in body temperature, decreased levels of CXCL9, and effective control of ES. These findings align with the research conducted by Merli et alCitation30 which suggested the safety of anti-IFN-γ monoclonal antibodies in patients who undergo a second hematopoietic stem cell transplantation, even those with generally poor treatment outcomes. Notably, no significant adverse reactions were observed in our case. Nevertheless, it is crucial to acknowledge that this report represents a single case, thereby necessitating caution in drawing definitive conclusions from these data. Furthermore, the application of emapalumab in patients with steroid-resistant ES remains relatively unexplored with limited available data.
Conclusion
In this critically ill patients with steroid-resistant ES, we have observed a substantial increase in the level of CXCL9, a chemokine indicating a high activity level of IFN-γ. In light of these findings, administration of emapalumab, a monoclonal antibody targeting IFN-γ, has emerged as a promising therapeutic strategy. The successful application emapalumab represents a significant breakthrough and a novel treatment for patients with refractory ES, who have exhibited an inadequate response to conventional glucocorticoid. This offers renewed hope for a subset of patients who are resistant to glucocorticoids. Moreover, by effectively managing severe graft reactions in a timely manner, we may be able to reduce the incidence of post-transplantation acute GVHD, thereby paving the way for subsequent treatments.
Abbreviations
CRP, C-reactive protein; CXCL9, CXC motif chemokine ligand 9; CXCR3, CXC chemokine receptor 3; ES, engraftment syndrome; GVHD, graft-versus-host disease; HLH, hemophagocytic lymphohistiocytosis; HSCT, hematopoietic stem cell transplantation; IFN-γ, interferon-gamma; IL, interleukin; MMF, mycophenolate mofetil; TNF-α, tumor necrosis factor-alpha.
Statement of Ethics
This is a case report and institutional approval was not needed. The informed consent was obtained from the patient.
Consent for Publication
A written informed consent was obtained from the patient and her mother for publication of this case report and accompanying clinical data.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- Adhikari J, Sharma P, Bhatt VR. Optimal graft source for allogeneic hematopoietic stem cell transplant: bone marrow or peripheral blood? Future Oncol. 2016;12(15):1823–1832. doi:10.2217/fon-2016-0106
- Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi:10.1182/blood-2011-06-364265
- Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. 2019;37(4):569–582. doi:10.1016/j.det.2019.05.014
- Carreras E, Fernández-Avilés F, Silva L, et al. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant. 2010;45(9):1417–1422. doi:10.1038/bmt.2009.363
- Chang YJ, Pei XY, Huang XJ. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet Haematol. 2022;9(12):e919–e929. doi:10.1016/S2352-3026(22)00293-9
- Sheth V, Jain R, Gore A, Ghanekar A, Saikia T. Engraftment syndrome: clinical features and predictive factors in autologous stem cell transplant. Indian J Hematol Blood Transfus. 2018;34(3):448–453. doi:10.1007/s12288-017-0899-4
- Jin L, Sun Z, Liu H, et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat Commun. 2021;12(1):4137. doi:10.1038/s41467-021-24412-1
- Maqbool S, Nadeem M, Shahroz A, et al. Engraftment syndrome following Hematopoietic stem cell transplantation: a systematic approach toward diagnosis and management. Med Oncol. 2022;40(1):36. doi:10.1007/s12032-022-01894-7
- Locatelli F, Jordan MB, Allen C, et al. Emapalumab in Children With Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med. 2020;382(19):1811–1822. doi:10.1056/NEJMoa1911326
- Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(9):893–898. doi:10.1038/sj.bmt.1703015
- Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: an update unifying the definition and management approach. Biol Blood Marrow Transplant. 2015;21(12):2061–2068. doi:10.1016/j.bbmt.2015.08.030
- Lee Y-H, Lim Y-J, Kim J-Y, Kim Y-D, Lee S-W. Pre-engraftment syndrome in hematopoietic stem cell transplantation. J Korean Med Sci. 2008;23(1):98–103. doi:10.3346/jkms.2008.23.1.98
- Iguchi A, Terashita Y, Sugiyama M, et al. Graft-versus-host disease (GVHD) prophylaxis by using methotrexate decreases pre-engraftment syndrome and severe acute GVHD, and accelerates engraftment after cord blood transplantation. Pediatr Transplant. 2016;20(1):114–119. doi:10.1111/petr.12621
- Khandelwal P, Mellor-Heineke S, Rehman N, et al. Cytokine profile of engraftment syndrome in pediatric hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2016;22(4):690–697. doi:10.1016/j.bbmt.2015.12.016
- Grant A, Chapman LRM, Mitchell R, O’Brien TA. Engraftment syndrome following hematopoietic stem cell transplant: a review of the literature. Clin Transplant. 2020;34(6):e13875. doi:10.1111/ctr.13875
- Isobe M, Konuma T, Kato S, et al. Development of pre-engraftment syndrome, but not acute graft-versus-host disease, reduces relapse rate of acute myelogenous leukemia after single cord blood transplantation. Biol Blood Marrow Transplant. 2019;25(6):1187–1196. doi:10.1016/j.bbmt.2019.02.007
- Konuma T, Kohara C, Watanabe E, et al. Cytokine profiles of pre-engraftment syndrome after single-unit cord blood transplantation for adult patients. Biol Blood Marrow Transplant. 2017;23(11):1932–1938. doi:10.1016/j.bbmt.2017.07.020
- Abongwa C, Abu-Arja R, Rumelhart S, Lazarus HM, Abusin G. Favorable outcome to glucocorticoid therapy for engraftment syndrome in pediatric autologous hematopoietic cell transplant. Pediatr Transplant. 2016;20(2):297–302. doi:10.1111/petr.12652
- Koreth J, Biernacki M, Aldridge J, et al. Syngeneic donor hematopoietic stem cell transplantation is associated with high rates of engraftment syndrome. Biol Blood Marrow Transplant. 2011;17(3):421–428. doi:10.1016/j.bbmt.2010.09.013
- Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–1045. doi:10.1007/s00262-008-0620-4
- Badar T, Khan MA, Szabo A, et al. Incidence and characteristics of engraftment syndrome after autologous hematopoietic cell transplantation in light chain amyloidosis. Amyloid. 2019;26(4):210–215. doi:10.1080/13506129.2019.1645001
- Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol. 2021;12:614704. doi:10.3389/fimmu.2021.614704
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515. doi:10.1016/j.berh.2020.101515
- Merli P, Quintarelli C, Strocchio L, Locatelli F. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr Blood Cancer. 2021;68(4):e28900. doi:10.1002/pbc.28900
- Jenkins MR, Rudd-Schmidt JA, Lopez JA, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med. 2015;212(3):307–317. doi:10.1084/jem.20140964
- Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology. 2019;58(1):5–17. doi:10.1093/rheumatology/key006
- Bauchmuller K, Manson JJ, Tattersall R, et al. Haemophagocytic lymphohistiocytosis in adult critical care. J Intensive Care Soc. 2020;21(3):256–268. doi:10.1177/1751143719893865
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. doi:10.1056/NEJMra2026131
- Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18(9):545–558. doi:10.1038/s41577-018-0029-z
- Merli P, Caruana I, De Vito R, et al. Role of interferon-γ in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2314–2323. doi:10.3324/haematol.2019.216101
- Choi J, Ziga ED, Ritchey J, et al. IFNγR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120(19):4093–4103. doi:10.1182/blood-2012-01-403196