Abstract
Liver metastasis is the main cause of mortality related to colorectal cancer. CXCR4 is necessary for the outgrowth of colon cancer micrometastases. In oncology, it has been demonstrated that several human tumors release lactate dehydrogenase (LDH) into the circulation. CXCR4 gene expression and serum LDH levels are often increased in patients with colorectal cancer. Despite technological advances in cancer therapy, five-year overall survival is still around 50%. Therefore, better treatment needs to be developed. RNA interference (RNAi) is a modern and powerful tool for inhibition of gene expression. However, the rate-limiting step in this technology is effective delivery of RNAi agents. We have investigated a novel strategy of CXCR4 siRNA therapy and its effect on serum LDH levels in a BALB/C mouse model of colorectal cancer metastasis to the liver. Hepatic metastasis was established by injecting a CT26.WT mouse colon carcinoma cell line via the tail vein. Our results demonstrated that CXCR4 siRNA/ dextran-spermine nanoparticles achieved high silencing efficiency with low toxicity. Favorable localization of the nanoparticles was confirmed with CXCR4 gene expression in the liver, that was correlated with serum LDH levels. More research will be needed to determine the effect of CXCR4 silencing on serum LDH levels, which may be a useful marker for predicting liver metastasis in colorectal cancer.
Introduction
Colorectal cancer is the third most common cancer after breast and lung.Citation1 Death from colorectal cancer often results from liver metastases. Despite extensive research into the biology of cancer progression, the molecular mechanisms involved in metastasis of colorectal cancer are not well characterized.Citation2 In cancer patients, serum lactate dehydrogenase (LDH) levels are often increased, and high serum LDH levels have been linked with poor postoperative outcome, as well as failure of radiotherapy and chemotherapy in sarcomas, lymphomas, and carcinomas, including colorectal cancer.Citation3 Recent studies show that chemokines and their receptors are an important factor in organ-selective metastasis and that these are major regulators of cell trafficking and adhesion. Specific chemokines produced and released by target organs attract tumor cells with specific corresponding receptors, resulting in site- and/or organ-specific cancer cell migration and metastasis.Citation4 Among the chemokines, CXCR4 has an important role in the spread of colorectal and breast cancer. Accordingly, silencing of the CXCL12-CXCR4 interaction has shown marked inhibition of metastasis in tumor animal models.Citation5,Citation6
Recently, the technology of RNA silencing, the process triggered by siRNA molecules, is poised to have a major impact on the treatment of human disease, particularly cancer, and can be delivered to a wide range of organs. siRNA is currently being evaluated in clinical trials as a potential therapy for a range of inherited diseases.Citation7,Citation8 However, despite the high therapeutic potential of siRNA, its application in the clinical setting is limited due to problems with stability in blood and methods for effective delivery of chemotherapeutic RNAi agents.Citation9
Recently, we have reported on a novel cationic polymer, dextran-spermine, a new biodegradable polycation based on natural components developed for gene delivery. Effective transfection has been obtained using this polycation for a variety of genes, cell lines, and tissues in vitro and in vivo.Citation11–Citation13 The aim of this study was to investigate the correlation between expression of CXCR4 and serum LDH levels in colorectal cancer metastasis to the liver by using a different strategy for CXCR4 siRNA therapy.
Materials and methods
Materials
HT29, CT26.WT cell lines were purchased from the American Tissue Culture Collection. SiGENOME-ON-TARGET plus SMART pool duplex CXCR4 in vitro (Genbank accession number NM-003467), siRNA1 Sense GAAGCAUGACGGACAAGUAUU; siRNA2.
Sense, GGCCUUAUCCUGCCUGGUAUU; siRNA3 Sense, UAAC UACACCGAGGAAAUGUU; and siRNA4 Sense CAAGCAAGGG UGUGAGUUUUU, and in vivo individual siRNA duplex CXCR4 I (Genbank Accession number BC098322), Sense ACCAACAGUCAGAGGCCAAUU Catalog number LEEIG-000534, siRNA duplex CXCR4 II Sense GGUCAUGGGUU ACCAGAAGUU Catalog number LEEIG-000536 and CXCR4 ON-TARGET plus a siCONTROL nontargeting pool were purchased from Dharmacon RNA Technologies (Lafayette, CO, USA). Dextran-spermine (FI-4/12A) was gifted from the University- Hadassah Medical School, Jerusalem, Israel. Other chemical reagents used in our study were RPMI (Sigma Aldrich, St. Louis, USA), fetal bovine serum (PAA, Yeovil, UK), trypsin- EDTA 1X (PAA), MTS kit (Promega, Madison, USA), Trizol reagent (Invitrogen, Carlsbad, USA), DNAse I kit (Sigma- Aldrich), first-strand cDNA synthesis kit (K1612 Fermentas, Burlington, Canada), Quantitect SYBR Green RT-PCR one-step kit (Qiagen, Hilden, Germany), rabbit polyclonal to CXCR4 (ab2074, Abcam, Cambridge, UK), goat anti-rabbit IgG FITC-labeled (Anaspec, CA, USA), LDH optimized assay kit (Anaspec), and Triton X-100 (Sigma-Aldrich).
Preparation of polysaccharide-oligoamine conjugates
A solution of oxidized polysaccharide 2 g in 200 mL double-distilled water was slowly added over 5–7 hours to a basic solution containing a 1.5 equimolar amount of the corresponding oligoamine dissolved in 100 mL of borate buffer (0.1 M, pH 11). The mixture was gently stirred at room temperature for 24 hours. Reduced amine-based conjugates were obtained after reducing the amine conjugates with excess NaBH4 (2 g) added to the mixture at room temperature for 48 hours. The reduction was repeated with an additional amount of NaBH4 with stirring for 24 hours under the same conditions. The mixture was dialyzed against double-distilled water (6 × 5 L) at 4°C, using 3500 cutoff cellulose tubing (Membrane Filtration Products, Inc, San Antonio, TX). The resulting light-yellow solution was dialyzed, followed by freeze-drying to obtain a yellowish reduced amine-based conjugate with an overall yield of 50%.Citation16,Citation17
Preparation and characterization of CXCR4 siRNA/dextran- spermine nanoparticles
The formation of CXCR4 siRNA/dextran-spermine nanoparticles was performed by mixing CXCR4 siRNA and dextran-spermine at various weight ratios in aqueous solution. Briefly, 5 μg of CXCR4 siRNAs I and II was added to 50 μL of RNase free water and the solution was pipetted up and down 3–5 times, then placed in an orbital mixer for 10 minutes at room temperature. The same volume of RNase-free water containing 5, 10, 15, 20, and 25 μg of dextran-spermine was placed in an orbital mixer for 10 minutes at room temperature. The solution was gently agitated for 30 minutes to form self-assembled siRNA/dextran–spermine complexes. Stability and structural characterization of CXCR4 siRNAs was confirmed by transmission electron microscopy (TEM), a particle size analyzer (Nanophox/Sympatecs GmbH, Clausthal-Zellerfeld, Germany), and a zeta size analyzer (Malvern, Worcestershire, UK). Structural determination of the nanoparticles was done using TEM. For TEM, 100 μL of the sample was kept on a copper grid for five minutes, excess solution was blotted off using filter paper, and the copper grids were air-dried for five minutes.
MTS assay in HT29 and CT26.WT cells
HT29 and CT26.WT cells were seeded into 96-well plates at an initial cell density of 4 × 103 cells/well in a final volume of 100 μL medium per well. After 24 hours, HT29 cells were treated with 30 μg/mL dextran-spermine, dextran-hexamine, and pullulan-spermine. CT26.WT cells were also treated with different concentrations of siRNA control (2000 ng/mL), CXCR4 siRNAs I and II, and CXCR4 siRNA/dextran-spermine (3000 ng/mL). After 48 and 72 hours, an aliquot of 20 μL of combined solution of a tetrazolium compound MTS, was added into each well and incubated for an additional four hours, and the absorbance at 490 nm was measured by Thermo Lab Systems, Cheshire, England.
Transfection and detection of CXCR4 siRNA efficiency
CT26.WT mouse colon carcinoma cells were grown in RPMI-1640 supplemented with 10% fetal bovine serum and incubated at 37°C with 5% CO2. The siRNAs were transfected into HT29 cells at a final concentration of 100 ng/mL using dextran-spermine. To optimize the efficiency of siRNA, CT26.WT cells were transfected with different doses of in vivo naked siRNAs CXCR4 I and II (started from 10, 50, 100, and 200 ng/mL) after 24 hours.
RT-PCR for siRNA optimization
Total RNA was isolated using Trizol reagent and reverse- transcribed to cDNA using a first-strand cDNA synthesis kit according to manufacturer’s instruction. The mouse CXCR4- specific primers for 105 bp were 5′-AAGCAAGGATGTGACTTCGAGAG and 5′-CGAGGAAGGCATAGAGGATGG (GenBank accession number BC098322). Primers for mouse ß-actin 146 bp were 5′-TGTTACCAACTGGGACGACATG- 3′ and 5′-TCAAACATGATCTGGGTCATCTTTTC (Genbank accession number EF095208). The annealing temperature was 57°C and the reaction proceeded for 35 cycles. To control for the presence of intact RNA, real-time (RT) polymerase chain reaction (PCR) for mice ß-actin was performed under the same conditions, as described earlier. Various concentrations of siRNA were tested by RT-PCR from 10, 50, 100, and 200 ng/mL. The PCR products were electrophoresed through 1% agarose gel.
Immunocytochemistry on HT29 cells
HT29 cells were grown on cover slips. After 24 hours, HT29 cells were treated with 100 ng/mL CXCR4 siRNAs, CXCR4 siRNA/dextran-spermine and a nontarget siRNA control. After 72 hours, cells were fixed with phosphate- buffered saline containing 3% paraformaldehyde for 15 minutes at 37°C. After three washes with phosphate-buffered saline, HT29 cells were treated with 0.2% Triton X-100 in phosphate-buffered saline for 10 minutes at 4°C to permeabilize the fixed cells and thereby facilitate access to the antibody. Cells were blocked for 60 minutes at 37°C in blocking buffer containing 2% bovine serum albumin in phosphate-buffered saline. The cells were then incubated with rabbit polyclonal CXCR4 antibody diluted 1:100 in blocking buffer for overnight at 4°C, washed three times in phosphate-buffered saline for 10 minutes, incubated with FITC-conjugated goat-antirabbit IgG at a concentration of 1 μg/mL for 60 minutes at 37°C in the dark, then washed again in phosphate-buffered saline three times for five minutes, rinsed in water, and mounted with mounting medium before examination by fluorescence microscopy.
Animal experiments
Female BALB/C mice aged 7–8 weeks (obtained from the Institute of Medical Research in Malaysia) were divided into six groups, with six mice per group. Group 1 mice were given injections of 1 × 105 CT26.WT mouse colon carcinoma cells transfected with nonspecific control siRNA duplexes, and were subsequently given injections of the control siRNA twice weekly. Group 2 mice were given intravenous injections through the tail vein of 1 × 105 CT26.WT cells transfected with CXCR4 siRNA I and II/dextran spermine (150 ng/g body weight) at 48 hours before injection. The Group 3 mice were injected with CT26.WT cells treated with CXCR4 siRNA I and II 150 ng/g body weight. Mice in Group 2 and Group 3 were not treated after the administration of tumor cells. Group 4 mice were given intravenous injections through the tail vein of 1 × 105 CT26.WT cells transfected with CXCR4 siRNA I and II, and Group 5 were given CXCR4 siRNA I and II/dextran-spermine 48 hours before injection of CT26.WT cells. Mice in Group 4 were treated with CXCR4 siRNA I and II, and mice in Group 5 were treated with CXCR4 siRNA I and II/dextran-spermine twice weekly after injection of CT26.WT cells. Groups 2, 3, 4, and 5 were given CXCR4 siRNA I and II 150 ng/g body weight. Group 6 animals were given nonspecific control siRNA duplexes by twice-weekly injection through the tail vein without prior injection of CT26.WT cells. In Groups A, B, C, D and E, mice were injected with 2 × 104 CT26.WT cells subcutaneously into the lateral abdominal surface near the left side of the pelvis for two purposes, ie, to induce further colorectal cancer with limited effect on important organs, in particular the heart, liver, and lungs, and to ensure growth of tumor cells in vivo. The mice were sacrificed after 35 days.
RNA preparation and RT reverse transcription PCR
Total RNA was isolated from frozen liver tissues (50 mg) using Trizol reagent according to the manufacturer’s instructions. RT quantitative reverse transcriptase PCR was performed using an RT-PCR RotorGene 3000 machine (Corbett, Sydney, Australia), with a Quantitect SYBR Green RT-PCR one-step kit as instructed by the manufacturer. Briefly, 500 ng of RNA was placed into a 20 μL reaction volume containing 0.2 μM of each primer, 10 μL of Syber Green I RT-PCR one-step master mix, and 0.2 μL of reverse transcriptase. A typical protocol included reverse transcription at 50°C for 30 minutes and a denaturation step at 95°C for 15 minutes, followed by 40 cycles with 94°C denaturation for 15 seconds, 57°C annealing for 30 seconds, and 72°C extension for 30 seconds. Melt (72–95°C), hold 45 seconds on the first step, hold five seconds on next steps, Melt A (FAM). To confirm amplification specificity, the PCR products were subjected to a melting curve analysis. Negative controls containing water instead of RNA were run concomitantly to confirm that the samples were not cross contaminated.
Histostaining of liver in BALB/C mice
Hematoxylin and eosin staining was done to evaluate the presence or absence of tumors at the end of the experiments on day 35. Animal organs and tumors were formalin-fixed and paraffin-embedded. Tissue sections were washed twice with xylene for three minutes, rehydrated in ethanol (100% twice for three minutes, 90% for two minutes, 80% for two minutes, 70% for two minutes), and the sections were then stained with hematoxylin and eosin, deparaffinized in xylene, rehydrated, and mounted in accordance with standard procedures.
Serum LDH measurement
Blood samples for serum LDL measurement were obtained by heart puncture at 35 days and allowed to clot. LDL measurements were made using an LDH optimized assay kit (Roche Diagnostics, Mannheim, USA), according to the manufacturer’s instructions. LDH activity was expressed as U/L.
Results
Characterization of CXCR4 siRNA I and II/dextran-spermine nanoparticles
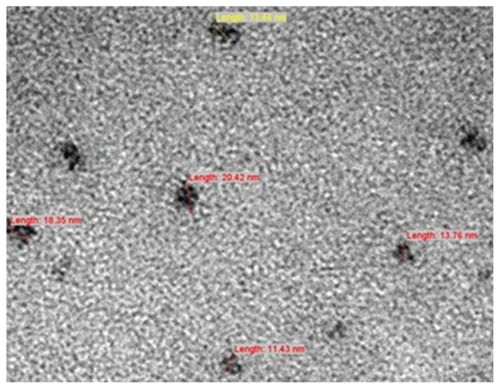
In this study, TEM was used to determine the morphology of the formed nanoparticles after eight hours of preparation using CXCR4 siRNAs I and II/dextran-spermine in various μg ratios from 1:1 to 1:5. At the 1:5 ratio, nanoparticles formed and the particles repelled one another, thereby preventing particles from aggregating. CXCR4 siRNAs/dextran-spermine formed small round particles (). Particle size was automatically calculated using particle sizer analysis/Nanophox after keeping one month at −80°C and freshly preparation. Particle size was obtained (10 × 12.42 nm ± 2.09), (50 × 26.77 nm ± 2.73), and (90 × 57.62 nm ± 2.51). This experiment was done three times and each time was repeated 40 times. The zeta potential of surface charge of three nanoparticles was automatically calculated (39.8 ± 0.2 mV).
Cell viability in HT29 and CT26.WT cells
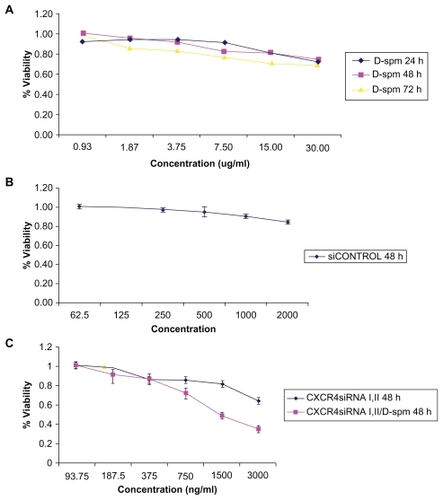
Viability assessed in HT29 cells transfected with dextran-spermine 15 μg/mL after 24 hours was 0.82 ± 0.03, after 48 hours was 0.81 ± 0.0.06, and after 72 hours was 0.71 ± 0.01, (). CT26.WT cells transfected with nontarget control siRNA after 48 hours at a concentration of 2000 ng/mL was 0.84 ± 0.01 (), and CXCR4 siRNAs I and II and CXCR4 siRNAs I and II/dextran-spermine after 48 hours at a concentration of 750 ng/mL were 0.85 ± 0.02 and 0.72 ± 0.04, respectively (). These results indicated that dextran-spermine and CXCR4 siRNAs have no effects on adherent cell growth at 15 μg/mL. This lack of cytotoxicity also implies a limited systemic effect on normal cells in vivo.
Immunocytochemistry on HT29 cells
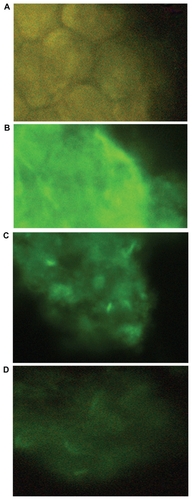
To confirm the RT-PCR experiments, the protein level of CXCR4 in HT29 (as a positive control) was characterized by immunocytochemistry. The results showed that CXCR4 receptors with higher staining in HT29 transfected with the nontarget siRNA control (), moderate staining in HT29 cells transfected with naked CXCR4 siRNAs, and weak staining in HT29 transfected with CXCR4 siRNA/ dextran-spermine complex.
Figure 3 Immunocytochemistry on HT29. Protein level of CXCR4 in HT29 was characterized by immunocytochemistry using rabbit polyclonal CXCR4 antibody and FITC-conjugated goat-antirabbit IgG. A) Negative control with deletion of the primary antibody. B) HT29 transfected with nontarget siRNA control. C) HT29 transfected with CXCR4 siRNA I and II. D) HT29 transfected with CXCR4 siRNA/ dextran-spermine.
CXCR4 expression, serum LDH levels, and histology
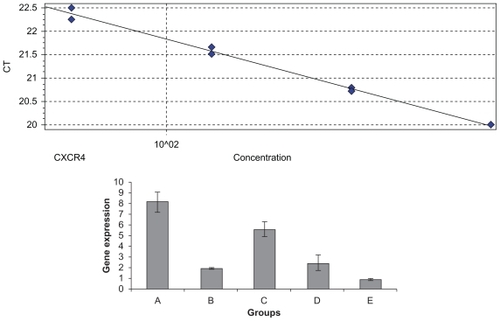
Total RNA was isolated from the liver 35 days after tumor cell injection in the six groups of animals. RT reverse transcription PCR was performed on the RT-PCR machine RotorGene 3000, and analysis of CXCR4 and β-actin expression was performed using the delta-delta CT method. A standard curve was done with a dilution of 1:2. CXCR4 efficiency was 1.34 with an R^2 value of 0.99; β-actin efficiency was 1.04 with an R^2 value of 0.99. Based on one-way analysis of variance and a least significant difference post hoc test, there were significant differences between the groups at the level F = 66.63 (P = 0.000). As illustrated in , mice in Groups A and C showed increased CXCR4 expression level which was higher than for Groups B, C, and E, but no significant differences were found between Group E and Group B (P > 0.05). These results indicated that inhibition with CXCR4 siRNA/dextran-spermine was more efficient than by naked CXCR4 siRNA, and also post-treatment follow transfection of tumor cells more efficiently than just transfection cell treatment with naked CXCR4 siRNA or CXCR4 siRNA/dextran-spermine.
Figure 4 Real-time reverse-transcription polymerase chain reaction was performed on a RotorGene 3000 machine, and data analysis of CXCR4 and β-actin expression used the delta-delta CT method. A standard curve was done with a dilution of 1:2. A) CXCR4 expression (standard curve with efficiency 1.34 and an R^2 value of 0.99. E CXCR4 expression in Groups A–E.
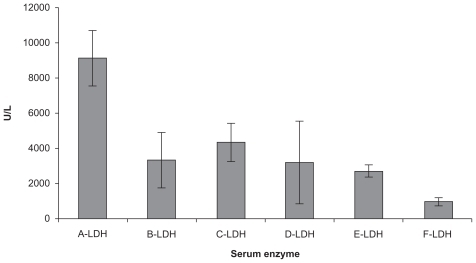
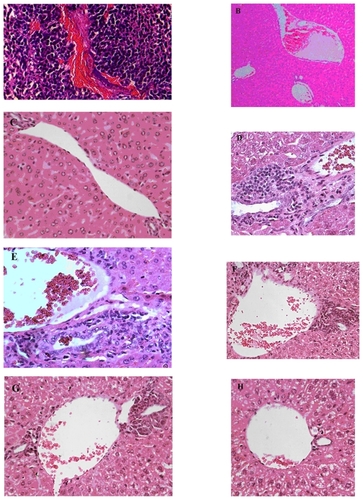
To examine the effects of CXCR4 siRNA naked or nanoparticles on serum LDH levels in the animals, we compared LDH levels between the groups using one-way analysis of variance and an LDH post hoc test (). The results indicated that there were significant differences between the groups (F = 20.27, P = 0.0000). The results indicated that just Group E had received CT26.WT transfected with CXCR4 siRNA/dextran-spermine plus injection of CXCR4 siRNAs I and II/dextran-spermine twice weekly. The level of serum LDH was approximately that of Group F which had not received any cells or treatment. CXCR4 siRNAs I and II/dextran-spermine was more efficient in reduction of CXCR4 expression and bringing LDH levels nearer to normal than naked siRNA. These results were further confirmed by hematoxylin and eosin staining 35 days after tumor cell injection. Hematoxylin and eosin staining of the liver showed that numbers of infiltrative lymphocytes along with cancer cells between the hepatic parenchyma and portal vein were lower in Group E compared with the other groups ().
Figure 6 Histostaining of liver of mice: Effect of CXCR4 siRNAs on inhibition of colorectal cancer metastasis to liver in vivo. A) H and E staining of tumor show growth and angiogenesis in Group A. B) H and E staining of liver (original magnification 10×) in Group F without injection of tumor cells; in this group there were no infiltrative lymphocytes. C) H and E staining (original magnification 40×) of liver in Group F. D) H and E staining of liver (original magnification 40×) in Group A along with subcutaneous injection of tumor cells through tail vein, showing a lot infiltrative lymphocytes between the hepatic parenchyma and portal vein following metastasis of tumor cells to the liver. E) H and E staining (original magnification 40×) of liver in Group B. F) H and E staining (original magnification 40×) of liver in Group C. G) H and E staining (original magnification 40×) of liver in Group D. H) Hematoxylin and eosin staining (original magnification 40×) of liver in Group C. Metastases in Groups A, B, C, D, and E were compared with Group E.
Abbreviation: H and E, hematoxylin and eosin.
Discussion
This study evaluated different methods of siRNA targeting CXCR4 therapy for colorectal metastasis to the liver in vivo. Efficient and safe in vivo delivery of short siRNA which is essential for therapeutic applications has not been established.Citation13 Here dextran-spermine was identified as an effective carrier for siRNA. Two siRNAs were chosen specifically for CXCR4 mRNA so that the sequences were similar between mice and humans, with no similarity to other genes. Eliyahu et al compared the cytotoxicity of dextran-spermine with well established polyplexes and lipoplexes. They reported dextran-spermine being the least cytotoxic.Citation14,Citation15 In this experiment, dextran-spermine was not toxic at 15 μg/mL by MTS and CXCR4 siRNAs at 3000 ng/mL. With respect to the low ratio of 1:5 and low doses of siRNAs used to make the nanoparticles, small size of nanoparticles 57.62 nm ± 2.51 with high zeta potential 39.8 mV ± 0.2. We expected that the nanoparticles could be efficiently internalized into cells with lowest toxicity. Immunocytochemistry of in vitro CXCR4 siRNA/dextran-spermine showed high protein expression in control on HT29 cell membrane, moderate with naked siRNAs I and II, and weak with CXCR4 siRNA/dextran-spermine nanoparticles. Various treatment combinations of siRNAs I and II of CXCR4 with and without dextran-spermine were compared in experimental metastatic animal models of CT26.WT cells. In vivo study demonstrated that self-assembled siRNAs I and II/ dextran-spermine and treatment twice weekly achieved better inhibition of CXCR4 expression than naked siRNAs I and II in vivo (). The highest CXCR4 expression (8.09 ± 0.8 fold) and serum LDH (85.37%) was in Group A mice (injected tumor cells transfected control siRNA) and the lowest CXCR4 expression (2.49 ± 0.04 fold) and serum LDH (27.23%) was in Group E. The level of serum LDH was proportional to CXCR4 expression in the different groups (). There was a significant correlation between CXCR4 expression and LDH level (Pearson correlation coefficient R = 0.861, P = 0. 000). It has been reported that when suspensions of cancer cells are injected via the portal vein or spleen in animals, the cells are rapidly attacked by the host immune system and also suffer from hemodynamic forces.Citation16 In fact, although the majority of injected cancer cells were found to be arrested in the liver sinusoid several minutes after injection, the vast majority of injected cancer cells were disseminated or no longer viable at 24 hours. Finally, only 1% of injected cancer cells survive in the liver.Citation17,Citation18 Hematoxylin and eosin staining of the liver shows that infiltrative lymphocytes along with cancer cells between hepatic parenchyma and portal vein follow CT26.WT cell metastasize to the liver. There were high rates of metastases in Group A, low rates in Groups C and D, and hardly any metastases in Groups B and E.
In this study we used different strategies for CXCR4 silencing. Combining CXCR4 siRNAs I and II with nanoparticle technology inhibits CXCR4 gene expression more than naked CXCR4 siRNAs I and II. Inhibition of CXCR4 expression at the mRNA level by a combination of two siRNAs with dextran-spermine was correlated with serum LDH in a model of colorectal cancer. In conclusion, dextran-spermine demonstrated improved transfection efficiency in siRNA therapy. More research is needed to determine the effect of CXCR4 silencing on serum LDH levels, which may be a useful marker to predict liver metastasis in colorectal cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- ISD onlineCancer incidence, mortality and survival data2010 http://www.isdscotland.org/isd/1696,htmlAccessed 2010
- GeogheganJGScheeleJTreatment of colorectal liver metastasesBr J Surg.19998615816910100781
- BeckPRBelfieldASpoonerRJBlumgartLHWoodCBSerum enzymes in colorectal cancerCancer.1979431772177636219
- TachibanaKHirotaSIizasaHThe chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tractNature.19983935915949634237
- RuffiniPAMorandiPCabiogluNManipulating the chemokine-chemokine receptor network to treat cancerCancer.20071092392240417503430
- LiangZYoonYVotawJSilencing of CXCR4 blocks breast cancer metastasisCancer Res.20056596797115705897
- ElbashirSMDuplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cellsNat Rev Genet.2001411494498
- DykxhoornDMLiebermanJThe silent revolution: RNA interference as basic biology, research tool, and therapeuticAnnu Rev Med.20055640142315660519
- CaplenNJParrishSImaniFFireAMorganRASpecific inhibition of gene expression by small doublestranded RNAs in invertebrate and vertebrate systemsProc Natl Acad Sci USA.2001989742974711481446
- AzzamTEliyahuHShapiraLLinialMBarenholzYDombAJPolysaccharide-oligoamine based conjugates for gene deliveryJ Med Chem.2002451817182311960493
- AzzamTEliyahuHMakovitzkiADombAJDextran-spermine conjugate: An efficient vector for gene deliveryMacromolecular Symposia.2003195247261
- HosseinkhaniHAzzamTTabataYDombAJDextran-spermine polycation: An efficient non-viral vector for in vitro and in vivo gene transfectionGene Ther.20041119420314712304
- NishinaKUnnoTUnoYEfficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherolMol Ther.20081673474018362929
- EliyahuHMakovitzkiAAzzamTZlotkinAJosephAGazitDNovel dextran-spermine conjugates as transfecting agents: Comparing water-soluble and micellar polymersGene Ther.20051249450315565162
- HagitEAvivaJTonyAYechezkelBAbrahamJDDextran-spermine-based polyplexes – Evaluation of transgene expression and of local and systemic toxicity in miceBiomaterials.2006271636164516221492
- WeissLThe hemodynamic destruction of circulating cancer cellsBiorheology.1987241051153651584
- FidlerIJMetastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridineJ Natl Cancer Inst.1970457737825513503
- Barbera-GuillemESmithIWeissLCancer-cell traffic in the liver. II. Arrest, transit and death of B16F10 and M5076 cells in the sinusoidsInt J Cancer.1993532983018425768