Abstract
Several fixed-dose combinations (FDCs) of long-acting bronchodilators (a long-acting muscarinic antagonist [LAMA] plus a long-acting β2-agonist [LABA]) are available for the treatment of COPD. Studies of these FDCs have demonstrated substantial improvements in lung function (forced expiratory volume in 1 second) in comparison with their respective constituent monocomponents. Improvements in patient-reported outcomes (PROs), such as symptoms and health status, as well as exacerbation rates, have been reported compared with a LABA or LAMA alone, but results are less consistent. The inconsistencies may in part be owing to differences in study design, methods used to assess study end points, and patient populations. Nevertheless, these observations tend to support an association between improvements in forced expiratory volume in 1 second and improvements in symptom-based outcomes. In order to assess the effects of FDCs on PROs and evaluate relationships between PROs and changes in lung function, we performed a systematic literature search of publications reporting randomized controlled trials of FDCs. Results of this literature search were independently assessed by two reviewers, with a third reviewer resolving any conflicting results. In total, 22 Phase III randomized controlled trials of FDC bronchodilators in COPD were identified, with an additional study including a post-literature search (ten for indacaterol–glycopyrronium once daily, eight for umeclidinium–vilanterol once daily, three for tiotropium–olodaterol once daily, and two for aclidinium–formoterol twice daily). Results from these studies demonstrated that the LAMA–LABA FDCs significantly improved lung function compared with their component monotherapies or other single-agent treatments. Furthermore, LABA–LAMA combinations also generally improved symptoms and health status versus monotherapies, although some discrepancies between lung function and PROs were observed. Overall, the safety profiles of the FDCs were similar to placebo. Further research is required to examine more closely any relationship between lung function and PROs in patients receiving LABA–LAMA combinations.
Introduction
Appropriate pharmacological management of COPD involves treatment with inhaled bronchodilators to reduce airflow limitation and hyperinflation. Most patient groups identified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy can be managed using long-acting inhaled bronchodilators (long-acting muscarinic antagonists [LAMAs] and long-acting β2-agonists [LABAs]), with or without inhaled corticosteroids.Citation1 Fixed dose combinations (FDCs) provide potent bronchodilation versus single agents,Citation2 with some advantage in terms of convenience and simplicity compared with combinations administered via separate inhalers. Beta agonists (BAs) and muscarinic antagonists (MAs) target different pathways to promote smooth-muscle relaxation and inhibit pulmonary constriction. Combining bronchodilators with different modes of action appears to be additive, providing greater efficacy versus component monotherapies.Citation3 Randomized controlled trials (RCTs) of LABA–LAMA combinations via separate inhalers have generally shown improved lung function versus component monotherapies.Citation4–Citation12
COPD is characterized by persistent airflow limitation, with forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio and percentage predicted FEV1 widely used as pathophysiological markers.Citation1 However, COPD is multidimensional, with pulmonary, extrapulmonary, and systemic effects. Outcomes in addition to FEV1 are needed to assess disease burden and treatment efficacy.Citation13 Spirometry is central to COPD diagnosis, but does not measure COPD burden in terms of health status.Citation14 Additionally, spirometry is not always performed, and symptoms and exacerbation history can play important roles in treatment initiation and management.Citation15 It is therefore important that spirometry is accompanied by assessments using patient-reported outcome (PRO) measures, such as breathlessness, physical functioning, and health status.Citation14 Minimal clinically important differences (MCIDs) for these assessments and other COPD outcomes have been reviewed by Jones et al.Citation14 Although a few studies and reports have examined associations between improved lung function (mainly FEV1) and PROs in COPD,Citation16–Citation21 the relationship between these efficacy measures is often weak, particularly for LAMAs and LABA–LAMA combinations. Here, we examine the evidence for the use of FDC bronchodilators in COPD, assess effects on PROs, and evaluate relationships between PROs and changes in lung function.
Materials and methods
This systematic literature search (not registered) was performed in accordance with the general principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).Citation22 The literature search identified primary, English-language, RCT publications of fixed-combination bronchodilators reporting treatment effects on lung function and/or PROs in comparison with placebo, bronchodilator monotherapy, or inhaled corticosteroid–LABA combinations in patients with COPD (Table S1). Data sources included a ProQuest search of Biosis, Biosis previews, Embase and Medline databases (January 1, 2006 to July 31, 2014), and abstracts from principal respiratory congresses (January 1, 2009 to May 20, 2015; Table S2). These selected search dates ensured that all relevant publications on fixed-combination bronchodilators were captured. Following the publication-database searches and during preparation of this manuscript (August 2015 onward), additional relevant articles became available, and thus these were added to the literature-search results.
All search results were extracted and gathered by a single party. Titles and abstracts were then scrutinized in parallel by two independent reviewers, and papers were categorized as relevant (where both reviewers categorized a paper as “relevant”), not relevant (where both reviewers judged a paper as “not relevant”), or potentially relevant (where one reviewer judged a paper as “relevant” and the other judged the same paper as “not relevant”). Irrelevant publications/studies comprised review papers, unapproved treatment doses, nonclinical trials, incorrect drug, or incorrect disease. Conflicting results were resolved by a third reviewer, who provided input as to whether the abstract was of potential relevance based on the same criteria as the first reviewers. To reduce the risk of omitting relevant studies/papers, all relevant and potentially relevant results were subsequently reviewed by the authors, who had the final decision regarding which publications to take to the next review level. Where relevance was not discernible from abstracts, full copies of author-confirmed relevant/potentially relevant articles were further assessed by two reviewers and conflicts resolved by a third reviewer. Data from the literature describing treatment differences with the FDC and comparator are summarized – according to end point – using least-squares mean (LSM) and 95% confidence interval (95% CI), odds ratio (OR), rate ratio, or hazard ratio (HR).
Results
Systematic literature-search results
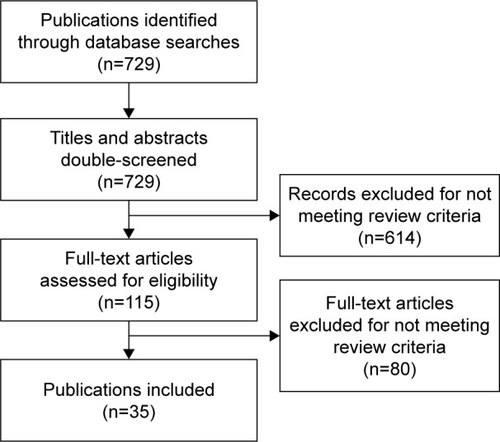
The searches yielded 729 records, from which 35 primary publications were relevant (). Literature searches were supplemented with information from ClinicalTrials.gov, and author expertise/knowledge (eg, if authors were aware that important publications were missing from search results).Citation23 Between the time of the predefined search end (July 2014 for published manuscripts and May 2015 for congress abstracts) and the drafting of this manuscript (August 2015 onwards), additional FDC studies were being published, and are thus included in this review.Citation23–Citation34
Figure 1 Flowchart of systematic literature search.
Abbreviations: LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Trials of fixed-dose dual-combination bronchodilators
FDC bronchodilators approved or in advanced clinical development for COPD include: indacaterol–glycopyrronium once daily (OD; QVA149; Ultibro® Breezhaler®; Novartis International AG, Basel, Switzerland), umeclidinium–vilanterol 110/50 µg OD (Laventair/Anoro® Ellipta®; GlaxoSmithKline PLC, London, UK), tiotropium–olodaterol OD (Spiolto® Respimat®; Boehringer Ingelheim, Ingelheim, Germany), aclidinium–formoterol twice daily (bis in die [BID]; Duaklir® Genuair®; AstraZeneca PLC, London, UK) and glycopyrrolate–formoterol (PT003; AstraZeneca).
Indacaterol–glycopyrronium OD is approved in >70 countries. Of 13 large Phase III trials of indacaterol–glycopyrronium, publications are available for ten (SHINE, ILLUMINATE, BRIGHT, ENLIGHTEN, SPARK, BLAZE, BEACON, LANTERN, QUANTIFY, and FLAME), all of which report lung function and PRO data and are included in this review ().Citation2,Citation24–Citation26,Citation35–Citation40 These active-comparator and placebo-controlled trials ranged from 3 to 64 weeks in duration.
Table 1 Clinical trials of FDC bronchodilator therapies evaluating treatment effects on lung function and/or patient-reported outcome
Umeclidinium–vilanterol 62.5/25 µg OD is approved in the US and EU (higher doses are not reviewed here). Findings from 12 Phase III trials had been reported in publications or conference abstracts at the time of the literature search, including: five 24-week studies,Citation23,Citation32,Citation41 seven 12-week studies,Citation27,Citation33,Citation42,Citation43 and one 52-week safety study (125/25 µg).Citation45 Lung-function and PRO data have been fully reported for six of the eight trials listed in .Citation23,Citation27,Citation32,Citation41
Tiotropium–olodaterol (5/5 µg; lower doses are not reviewed) OD has been approved in more than 20 European countries, the US, Canada, and Australia since May 2015. Results from two 1-year studies with tiotropium–olodaterol 5/5 µg (included in this review; ) have been reported and include data on lung function and health status versus the monocomponents.Citation30 Results from an additional Phase III trial evaluating lung function and volume (VIVACITO) have been published (),Citation31 two Phase III trials have been presented as abstracts,Citation34,Citation45 one further Phase III study has been completed (ClinicalTrials.gov NCT1536262) and four are ongoing (ClinicalTrials.gov NCT2006732, NCT1964352, NCT1969721, and NCT2085161).
Aclidinium–formoterol (400/12 µg BID) is approved in the EU. Findings from two of four Phase III trials have been fully reported comparing the combination therapy versus monocomponents or placebo, and are included in this paper ().Citation28,Citation29,Citation46 Results from a 24-week Phase III study comparing aclidinium–formoterol with salmeterol–fluticasone combination (SFC) BID had been published in abstract form at the time of the literature search.Citation47 For glycopyrrolate–formoterol (in late-stage development), only Phase II congress abstracts are available.Citation48–Citation50 Three Phase III studies are ongoing (ClinicalTrials.gov NCT1854645, NCT1854658, and NCT1970878).
In this review, we focus on the 23 aforementioned published Phase III RCTs and listed in (supplemented with results presented at major respiratory congresses, where applicable): ten with indacaterol–glycopyrronium OD, eight with umeclidinium–vilanterol OD, three with tiotropium–olodaterol OD, and two with aclidinium–formoterol BID. The remaining primary publications from the literature search were excluded, due to duplicate publications of the same results (eg, where a primary publication superseded several congress abstracts).
Patient population and study design
Patient populations, inclusion criteria, treatment blinding, and other characteristics differed between trials (). The majority of indacaterol–glycopyrronium OD studies enrolled symptomatic patients with moderate-to-severe airflow limitation (GOLD 2008, 2009, or 2010 classification), except for SPARK and FLAME, which enrolled patients with severe-to-very-severe or moderate-to-very-severe disease, respectively, and one or more exacerbations in the past year.Citation2,Citation24,Citation26,Citation35–Citation40 The eight umeclidinium–vilanterol OD trials enrolled patients with moderate-to-severe or moderate-to-very-severe COPD who were symptomatic.Citation23,Citation27,Citation32,Citation41 Patients in the tiotropium–olodaterol OD studies had moderate-to-very-severe COPD.Citation30,Citation31 The aclidinium–formoterol BID studies were conducted in patients with moderate-to- severe COPD.Citation28,Citation29
Lung function
Across eight trials (3–64 weeks), indacaterol–glycopyrronium OD provided significant LSM treatment differences in trough FEV1 of 60–80 mL versus tiotropium 18 µg, 70–80 mL versus indacaterol 150 µg or glycopyrronium 50 µg alone, 68 mL versus tiotropium + formoterol 18/12 µg, 62–72 mL versus SFC 50/500 µg BID, and 189–200 mL versus placebo ().Citation2,Citation24–Citation26,Citation35,Citation36,Citation39,Citation40 Preliminary data suggest that the extent of FEV1 improvement may vary: in a post hoc analysis of SHINE, data from patients in the spirometry subset who received indacaterol–glycopyrronium OD (n=399) showed that 39.8% had an increase in FEV1 of ≥200 mL between baseline and week 26, 23.8% achieved ≥300 mL, and 13.1% had an increase of ≥400 mL.Citation51
Table 2 Lung function: margin of efficacy of fixed combinations versus comparators in fully published studies
In three Phase III studies, LSM treatment differences in trough-FEV1 change from baseline to week 24 with umeclidinium–vilanterol 62.5/25 µg OD were 60–112 mL versus tiotropium 18 µg, 52 mL versus umeclidinium 62.5 µg, 22 mL versus umeclidinium 125 µg (not statistically significant), 90–95 mL versus vilanterol 25 µg, and 167 mL versus placebo.Citation23,Citation32,Citation41 In two 12-week studies, umeclidinium–vilanterol 62.5/25 µg produced greater increases in trough FEV1 versus individual components.Citation33 In another two 12-week studies, umeclidinium–vilanterol 62.5/25 µg resulted in significant improvements in FEV1 0–24 hours and trough FEV1 compared with 50/250 μg BID.Citation27
At week 24 of the two 1-year studies, tiotropium–olodaterol 5/5 μg OD increased trough FEV1 by 82–88 mL versus olodaterol 5 μg and by 50–71 mL versus tiotropium 5 μg.Citation30 A 6-week incomplete crossover study showed improvements in 24-hour lung function with tiotropium–olodaterol 5/5 µg versus components or placebo.Citation31
Aclidinium–formoterol (400/12 μg BID) increased week 24 trough FEV1 significantly versus placebo (143 mL) and formoterol (85 mL) in the ACLIFORM study, but the smaller difference (~25 mL) versus aclidinium BID was not statistically significant.Citation28 Similar results were observed in the AUGMENT trial, with a significant difference for the combination versus formoterol (45 mL), but not aclidinium (28 mL).Citation29
Symptoms
Improvements in dyspnea and other symptoms were seen with fixed-dose LABA–LAMA therapies versus monotherapies and for indacaterol–glycopyrronium OD versus SFC BID. (, ).Citation2,Citation24,Citation25,Citation38,Citation39 Indacaterol–glycopyrronium significantly improved transition dyspnea index (TDI) scores in SHINE and ILLUMINATE versus placebo, open-label tiotropium, and SFC.Citation2,Citation39 In BLAZE, indacaterol–glycopyrronium significantly improved self-administered computerized total TDI score versus placebo (LSM treatment difference 1.37, P<0.001) and blinded tiotropium (LSM treatment difference: 0.49, P=0.021).Citation38 The proportion of patients achieving the MCID (≥1-point) for TDI score was also significantly increased versus blinded tiotropium in BLAZE (OR 1.78, P<0.05) and versus SFC in ILLUMINATE (OR 1.56, P<0.05; ).Citation2,Citation39 In QUANTIFY, a similar reduction in dyspnea was observed with indacaterol–glycopyrronium versus tiotropium + formoterol, and significantly more patients achieved clinically relevant improvements in TDI total score with indacaterol–glycopyrronium (49.6%) versus tiotropium + formoterol (42.4%, P=0.033).Citation25
Table 3 Symptoms: margin of efficacy of fixed combinations versus comparators in published studies
Figure 2 Differences between monotherapy and combination bronchodilators or placebo in TDI patient-response rates in published studies.
Abbreviations: BID, bis in die (twice daily); SFC, salmeterol–fluticasone propionate; TDI, transition dyspnea index.
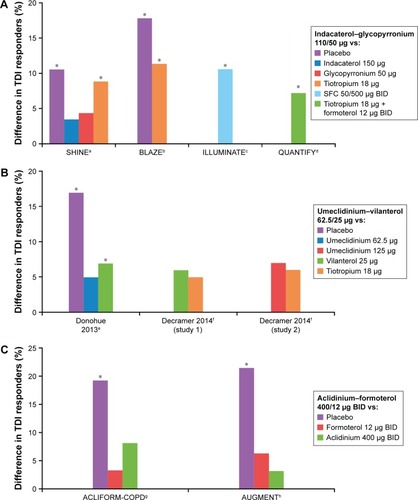
In LANTERN, a comparable improvement with indacaterol–glycopyrronium OD and SFC BID was demonstrated for TDI focal score and St George’s Respiratory Questionnaire (SGRQ) total score from baseline after 26 weeks; the percentage of patients achieving the MCID for both end points was higher with indacaterol–glycopyrronium versus SFC.Citation24 Compared with its component monotherapies, indacaterol–glycopyrronium was associated with numerical improvements in TDI score and percentage of TDI responders at week 26 in SHINE.Citation2 At week 12, improvement in TDI score with indacaterol–glycopyrronium was significantly greater than with glycopyrronium (LSM treatment difference 0.41, P=0.03).
Three indacaterol–glycopyrronium OD studies evaluated patient-diary data and reported significantly improved symptom scores versus indacaterol, glycopyrronium, tiotropium, or placebo ().Citation2,Citation36,Citation38 In the shorter BRIGHT trial, change in mean daily symptom score from baseline to week 3 was numerically greater for indacaterol–glycopyrronium versus tiotropium and placebo.Citation35 In ILLUMINATE, differences in scores for most symptoms were comparable for indacaterol–glycopyrronium and SFC BID.Citation39
In three 24-week studies, umeclidinium–vilanterol 62.5/25 µg OD significantly improved TDI focal and Shortness of Breath with Daily Activity (SOBDA) scores versus placebo, with numerical improvements versus mono-components and tiotropium.Citation23,Citation41 The proportion of patients achieving the MCID for TDI score was significantly increased in patients receiving umeclidinium–vilanterol versus placebo (OR 2, P<0.001) and vilanterol (OR 1.4, P<0.05)Citation41 in one study ().Citation23 LSM changes from baseline to week 24 in SOBDA scores were clinically significant (≥0.1 unit) for umeclidinium–vilanterol, vilanterol 25 µg, umeclidinium 62.5 and 125 µg, and tiotropium 18 µg.Citation23,Citation41 SOBDA responder rates were reported for one trial, and were significantly higher for umeclidinium–vilanterol (OR 1.8, P<0.01) and its monocomponents (umeclidinium 52.5 µg OR 1.7, P<0.01; vilanterol 25 µg OR 1.6, P<0.05) versus placebo. In two 12-week studies, there was no significant difference in TDI focal scores between umeclidinium–vilanterol 62.5/25 µg and salmeterol–fluticasone propionate 50/250 µg.Citation27 Exercise-associated dyspnea (Borg) was reduced with umeclidinium–vilanterol 62.5/25 µg compared with placebo in one of two studies; active–placebo differences were not significant for the individual components.Citation33 In combined results from two 1-year studies, tiotropium–olodaterol OD increased TDI total score versus monocomponents (week 24) by approximately 0.4 points with the higher dose and by a similar margin (0.3–0.4 points) with the lower dose.Citation30
Symptoms were evaluated using a number of end points in the two 24-week aclidinium–formoterol BID studies.Citation28,Citation29 For TDI total score, aclidinium–formoterol 400/12 µg BID achieved a significant, >1-point improvement versus placebo (and a higher proportion of TDI responders), but the differences versus the monotherapies were not significant. For Evaluating Respiratory Symptoms (E-RS) score, the combination was significantly better than placebo (both studies) and the monotherapies (one study). Aclidinium–formoterol 400/12 µg improved nighttime symptom scores versus placebo (one study) or aclidinium BID (one study); early morning symptom scores were improved versus placebo and aclidinium (both studies), assessed by questionnaires for both.Citation28,Citation29
Rescue-medication use
Rescue-medication usage provides a surrogate measure of symptom control, and was reported in most of the published indacaterol–glycopyrronium OD and umeclidinium–vilanterol OD Phase III trials (). Indacaterol–glycopyrronium treatment consistently led to significantly less rescue-medication use per day than LABA or LAMA monotherapy or LABA–inhaled corticosteroids in each trial with active comparators.Citation2,Citation26,Citation35,Citation38–Citation40 In LANTERN, rescue-medication use was comparable between the indacaterol–glycopyrronium and SFC BID groups.Citation24 Daily rescue-medication use was similar or numerically slightly lower with umeclidinium–vilanterol versus either umeclidinium or vilanterol monotherapy, significantly lower versus tiotropium in two of three trials, and significantly lower versus SFC in one of two trials.Citation23,Citation27,Citation32,Citation33,Citation41 Rescue-medication use remained at approximately two puffs/day with tiotropium–olodaterol OD over the course of 52 weeks; at the end of the studies, this was 0.3–0.4 puffs/day less than with olodaterol and 0.7–0.8 puffs/day less than with tiotropium.Citation30 In the two 24-week studies with aclidinium–formoterol 400/12 µg BID, rescue-medication use was significantly lower compared with placebo and aclidinium BID, but not compared with formoterol.Citation28,Citation29
Table 4 Rescue-medication use: margin of efficacy of fixed combinations versus comparators in published studies
Exacerbations
The effects of FDC therapy on exacerbation rates and time to first exacerbation are summarized in .
Table 5 Exacerbations: margin of efficacy of fixed combinations versus comparators in published studies that included exacerbations as an efficacy outcome
The effect of indacaterol–glycopyrronium OD on exacerbation rate was examined as the primary end point in both SPARK and FLAME, and exacerbation rates have also been reported from ILLUMINATE, LANTERN, and QUANTIFY.Citation24–Citation26,Citation39,Citation40,Citation52 In SPARK, indacaterol–glycopyrronium significantly reduced rates of moderate-to-severe (primary end point, rate ratio 0.88; P=0.038) and all exacerbations (LSM treatment difference 0.85, P<0.01) versus glycopyrronium.Citation40 Compared with open-label tiotropium, rates of moderate-to-severe exacerbations were 10% lower with indacaterol–glycopyrronium (P=0.096), and rates of all exacerbations were 14% lower (P<0.01). In comparison with SFC BID in a post hoc analysis of data from ILLUMINATE, rates of moderate-to-severe exacerbations (rate ratio 0.8, not significant [NS]) and all exacerbations (rate ratio 0.69, NS) were numerically lower with indacaterol–glycopyrronium.Citation52 In LANTERN, indacaterol–glycopyrronium significantly reduced the rate of moderate or severe exacerbations by 31% (P=0.048) over SFC.Citation24 Furthermore, in the recent FLAME study, indacaterol–glycopyrronium significantly reduced the rates of all exacerbations (primary end point) by 11% (P=0.003) and of moderate-to-severe exacerbations by 17% (P<0.001) compared with SFC; findings were consistently in favor of indacaterol–glycopyrronium when patients were analyzed according to their baseline disease characteristics, including baseline eosinophil count (<2% or ≥2%).Citation26 This study also found that compared with SFC, indacaterol–glycopyrronium was associated with longer times to first exacerbation, representing reduced risks of 16% for all exacerbations (P<0.001), 22% for moderate-to-severe exacerbations (P<0.001), and 19% for severe exacerbations (P=0.046). Finally, QUANTIFY showed a comparable percentage of patients experiencing at least one moderate or severe exacerbation and a comparable time to first moderate or severe exacerbation between the two treatment groups (indacaterol−glycopyrronium vs tiotropium + formoterol).Citation25
Currently, there are no studies evaluating exacerbation risk as a primary end point in patients receiving umeclidinium–vilanterol OD. The data available from analysis of secondary end points indicate that umeclidinium–vilanterol significantly increased time to first exacerbation versus placebo (HR 0.5, P<0.001),Citation41 but not compared with vilanterol 25 µg (HR 0.7, NS) or umeclidinium 125 µg (HR 1, NS).Citation23
Time to first exacerbation was comparable for combination therapy versus tiotropium alone in two trialsCitation23 and significantly greater in a third study (HR 0.5, P=0.044).Citation32 In the combined results of the two 52-week studies with tiotropium–olodaterol OD, there was only a “trend” for improvement in exacerbations with both doses of the combination versus the monotherapy components.Citation30 Over the 24 weeks of the ACLI-FORM study, using the health care resource-utilization definition of exacerbations, aclidinium–formoterol BID 400/12 µg was not significantly different from placebo or its separate components; with the EXACT (EXAcerbations of COPD Tool) definition, a significant difference was demonstrated versus placebo, but not compared with the components.Citation28
Exacerbations were not reported as an efficacy outcome in the AUGMENT study.Citation29
Health status
Indacaterol–glycopyrronium OD significantly improved health status, assessed using the SGRQ (). In SPARK, indacaterol–glycopyrronium improved SGRQ total score versus glycopyrronium (all P<0.01) and open-label tiotropium (all P<0.05; 12–64 weeks).Citation40 In SHINE, improvement in SGRQ with indacaterol–glycopyrronium was superior to open-label tiotropium (P=0.009) and placebo (P=0.002) and comparable to component monotherapies.Citation2 In a 26-week study, indacaterol–glycopyrronium and SFC BID provided similar improvements in health status.Citation39 However, in FLAME, significant improvements over time in SGRQ total score were observed for indacaterol–glycopyrronium compared with SFC, with treatment differences that ranged from −1.2 points to −1.8 points over the time points measured between weeks 12 and 52 (all P<0.01).Citation26 The SGRQ responder rate for the MCID (reduction of ≤4 units from baseline)Citation53 was also significantly greater with indacaterol–glycopyrronium versus SFC in FLAME (OR 1.3, P<0.001)Citation26 and versus glycopyr-ronium (OR 1.62, P=0.00013) and open-label tiotropium (OR 1.48, P=0.0017) at all time points except week 64 in SPARK.Citation40 In QUANTIFY, indacaterol–glycopyrronium was noninferior to tiotropium + formoterol for improvement in SGRQ score; the percentage of patients achieving a MCID was significantly in favor of indacaterol–glycopyrronium (50.1% vs 42.5%, P=0.038) in the per-protocol set.Citation25 Similarly, in LANTERN comparable improvements with indacaterol–glycopyrronium versus SFC were observed for all SGRQ analyses (weeks 12 and 26).Citation24
Table 6 Health status: margin of efficacy of fixed combinations versus comparators in published studies
Significant improvements in SGRQ total score mean change from baseline (P≤0.001) and percentages of SGRQ responders (OR 2, P≤0.001) were reported for umeclidinium–vilanterol 62.5/25 µg OD versus placebo in three 24-week studies.Citation41 Across three of four trials, health-status improvement was not significantly different for umeclidinium–vilanterol versus monotherapy with tiotropium, vilanterol, or umeclidinium (SGRQ total scores or percentage of SGRQ responders).Citation23,Citation41 The fourth trial reported significant improvement in SGRQ total score from baseline (P<0.006) and percentage of SGRQ responders (OR 1.4, P=0.022) for umeclidinium–vilanterol versus tiotropium.Citation32 Improvements in SGRQ from baseline were not significantly different between umeclidinium–vilanterol 62.5/25 µg and salmeterol–fluticasone propionate 50/250 µg in two 12-week studies.Citation27
In combined results from two 1-year studies, tiotropium–olodaterol 5/5 µg OD significantly improved SGRQ total score at week 24 by 1.2 and 1.7 units versus its respective components. Proportions of SGRQ responders were significantly increased for all the combination-versus-component comparisons, apart from tiotropium–olodaterol 2.5/5 µg versus tiotropium 2.5 µg. In the 24-week ACLIFORM and AUGMENT studies, aclidinium–formoterol BID improved SGRQ total score and percentage of responders significantly compared with placebo in one study, but did not achieve significant differences against its components in either study.Citation28,Citation29
Safety
To date, the most extensive safety data available for FDC bronchodilators comes from indacaterol–glycopyrronium OD trials. Overall, indacaterol–glycopyrronium was well tolerated across the studies, and had a similar safety profile to placebo in individual trials and analyses of pooled data.Citation2,Citation36,Citation39,Citation40,Citation54–Citation56 The incidence of adverse events (AEs) and serious AEs (SAEs) reported with indacaterol–glycopyrronium treatment was comparable to that of placebo, indacaterol, glycopyrronium, tiotropium (± formoterol) or SFC BID.Citation2,Citation24–Citation26,Citation36,Citation39,Citation40 Interestingly, the FLAME trial reported a significant reduction in the incidence of pneumonia with indacaterol–glycopyrronium compared with SFC (3.2% vs 4.8%, respectively; P=0.02).Citation26 In an analysis of pooled data from 11,404 patients, the HR for indacaterol–glycopyrronium versus placebo showed no significant increase in the overall risk for death (HR [95% CI] 0.93 [0.34–2.54]), cardiocerebrovascular events (0.6 [0.29–1.24]), major adverse cardiovascular events (MACEs; 1.04 [0.45–2.42]), pneumonia (1.1 [0.54–2.25]), COPD exacerbations (0.6 [0.4–0.91]), or atrial flutter/fibrillation (1.03 [0.49–2.18]).Citation54
Over 24 weeks, umeclidinium–vilanterol 62.5/25 µg OD was well tolerated, and the incidence of AEs and serious AEs was similar for combination therapy versus placebo and monocomponents.Citation41,Citation57 The rate of class-effect AEs associated with anticholinergic (eg, dry mouth) and BA (eg, tachycardia) agents was similar to that observed for placebo.Citation41,Citation57 In two 12-week studies, umeclidinium–vilanterol 62.5/25 µg and SFC 250/50 µg were both well tolerated and had similar AE profiles.Citation27 In a pooled analysis of data from eight trials of umeclidinium–vilanterol 62.5/25 µg and 125/25 µg, no increased risk of MACE was observed with active treatment versus placebo.Citation58 Small numerical imbalances in cardiac ischemia were reported in some studies, but not others. As the imbalances were not dose-related, they were not considered drug-related. The incidence of cardiovascular AEs of special interest was comparable for umeclidinium–vilanterol, mono-components, and placebo.
In the two 1-year tiotropium–olodaterol OD studies, the frequency of AEs was largely comparable between the combination- and individual component-treatment groups. The rates of MACE and cardiac events did not differ significantly between the combination and the individual component groups.Citation30 Similarly, AE reporting (including MACE and Holter monitoring) in the two aclidinium–formoterol BID studies was generally comparable across all treatment groups.Citation28,Citation29
In a 2013 preliminary report from a retrospective cohort study of mortality in more than 5,000 patients with COPD, LAMA–LABA combination therapy reduced both all-cause (HR 0.53 [95% CI 0.34–0.84]) and cardiovascular mortality (HR 0.39 [95% CI 0.17–0.9]).Citation59 Reductions in both mortality types were also observed with LAMA–LABA–inhaled corticosteroids, LABA–inhaled corticosteroids, and LAMA-only treatment.
Discussion
We identified 23 published Phase III RCTs of FDC bronchodilators in COPD. The data demonstrated that fixed-dose LAMA–LABA combinations significantly improved lung function compared with component mono-therapies or single agents.Citation2,Citation23,Citation30–Citation32,Citation35,Citation36,Citation39–Citation41 Indacaterol–glycopyrronium OD, umeclidinium–vilanterol OD, and tiotropium–olodaterol OD also provided significant improvements over component monotherapies and/or tiotropium in several PROs.Citation2,Citation23,Citation30,Citation32,Citation35,Citation38,Citation40,Citation41 Compared with its components, aclidinium–formoterol BID improved symptoms (one study),Citation28 but did not improve health status.Citation28,Citation29 Indacaterol–glycopyrronium and umeclidinium–vilanterol significantly improved lung function compared with SFC BID.Citation26,Citation27,Citation39 Indacaterol–glycopyrronium also improved exacerbation rates in LANTERN and FLAME (), reduced dyspnea in ILLUMINATE, and led to reductions in use of rescue medication in ILLUMINATE and FLAME compared with SFC.Citation24,Citation26,Citation27,Citation39 The safety profiles of the FDC agents were similar to placebo and incidence of pneumonia significantly reduced with indacaterol–glycopyrronium versus SFC in FLAME.Citation2,Citation23,Citation26,Citation30,Citation32,Citation36,Citation39–Citation41,Citation54,Citation56
Several studies have examined the relationship between improvements in lung function following LABA or LAMA monotherapy and improvements in other outcomes, such as SGRQ total score, TDI, exacerbation rate, and rescue-medication use. However, although significant or clinically relevant correlations appear at group levels, they tend to be only moderate, weak, or too weak to be useful at individual levels.Citation16–Citation19,Citation21 This may be because some patients have very poor health despite only mild lung-function impairments or vice versa.Citation17 Indeed, the health impact of COPD is not necessarily mediated entirely through expiratory airflow limitation; a better correlate may instead be exercise performance.Citation17 The analyzed studies may also have been too short in duration to capture meaningful changes in exacerbations or health status, and only a few studies were available for some outcomes.Citation18 Finally, the Hawthorne effect may also have played a role, as changes in FEV1 of 0 still resulted in a 2.5-point reduction in SGRQ score in some cases.Citation18
Likewise, in trials of combination-bronchodilator therapy versus components, improvement in FEV1 was not always mirrored by improved PROs. For example, significant improvement in dyspnea for umeclidinium–vilanterol OD versus monocomponents occurred only for vilanterol (in one of three trials), despite improvements in FEV1.Citation23,Citation41 Possible reasons for this include insufficient sensitivity/specificity in instruments assessing PROs. Additionally, such measurements as inspiratory capacity may be more strongly related to dyspnea and COPD pathophysiology than FEV1.Citation60 Therefore, it may be useful to examine correlations between other outcomes instead, in larger sample sizes or longer-duration studies. Findings may still be somewhat limited though, as these end points are often only secondary, meaning power may be lacking.
Patient-selection criteria represent an important limitation of RCTs. Most trials recruit subjects from highly selective populations likely to represent less than 5% of “real-life” patients. As such, the extrapolation of RCT data is limited.Citation61 Populations are generally chosen to demonstrate the primary end point (usually lung function). Clinical trial participants tend to be less symptomatic than general patient populations, and clinical trials may exclude patients likely to benefit the most from treatment (as a maximum level of benefit may be reached sooner). Additionally, the most symptomatic patients in control arms may discontinue study treatment to obtain greater symptom relief. In contrast, real-life studies are likely to involve broader populations and treat each study arm to a similar level. Roche et al suggested a new framework to categorize the approach taken in clinical trials from highly controlled efficacy RCT management to usual clinical care.Citation61 The positioning of studies on this scale can be useful as a descriptive classification.Citation61 Future COPD trials may need to include more real-life patient populations and ecology of care. In addition, composite end points, such as lack of exacerbations and improved health status, may provide greater insight into the true benefits of treatment.
Additional studies of fixed-combination bronchodilators are needed to characterize further the relationship between FEV1 and PROs with these agents, as well as defining optimal strategies for their use in clinical practice. Should therapy be initiated with a single bronchodilator and then stepped up to a LABA–LAMA combination and/or triple therapy with LABA–LAMA plus another agent as needed, or should treatment commence with a LABA–LAMA in certain patients?
In conclusion, our review of a systematic literature search indicates that fixed-dose LABA–LAMA combinations significantly improved lung function compared with their component monotherapies. In general, LABA–LAMA combinations also improved other outcomes, including symptoms and health status, compared with the monotherapies, although some discrepancies between lung function and PROs were apparent. Further research is needed to explore the relationship between lung-function outcomes and PROs in patients receiving LABA–LAMA combinations.
Author contributions
All authors contributed to the concept and objectives of the review and provided guidance on the literature search, presentation, and discussion of the findings, as well as critically reviewing the article. In addition, all authors reviewed and approved the final manuscript.
Acknowledgments
The authors were assisted in the preparation of the manuscript by Molly Heitz and Sarah Filcek, professional medical writers at CircleScience, an Ashfield company, part of UDG Healthcare PLC. Medical writing support was funded by Novartis Pharma AG (Basel, Switzerland).
Supplementary materials
Table S1 Search strategy and results for published manuscripts and congress abstracts
Table S2 Congress abstract search strategy and results
Disclosure
AØ has received payment for lectures/speaking from Boehringer Ingelheim, GlaxoSmithKline, Meda, Sandoz, and Pfizer. He has advisory board membership with Boehringer Ingelheim, Novartis and Teva. DP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmith Kline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from UK National Health Service, British Lung Foundation, Aero-crine, AKL Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Zentiva, and Theravance; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Novartis and Mundipharma; payment for travel/accommodation/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals, and AstraZeneca; funding for patient enrolment or completion of research from Chiesi, Teva Pharmaceuticals, Zentiva, and Novartis; stock/stock options from AKL Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; and is peer reviewer for grant committees of the Medical Research Council, Efficacy and Mechanism Evaluation programme, and HTA. Neither MT nor any member of his close family has any shares in pharmaceutical companies. In the last 3 years, he has received honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies marketing respiratory and allergy products: Aerocrine, AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline and Teva. MT has received honoraria for attending advisory panels with Aero-crine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, and Novartis. He has received sponsorship to attend international scientific meetings from AstraZeneca, GlaxoSmithKline, and Mundipharma; and has received funding for research projects from Almirall and GlaxoSmithKline. Neither TW nor his close family members have any shares in pharmaceutical companies. In the last 3 years, TW has received honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies marketing respiratory and allergy products: Almirall, Astra Zeneca, Boehringer Ingelheim, Chiesi, Mundipharma, Novartis, GlaxoSmithKline, and Teva. He has received honoraria for attending advisory panels with Astra Zeneca, Boehringer Ingelheim, Chiesi, MSD, and Novartis, as well as receiving funding for research projects from Novartis. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global Strategy for the Diagnosis, Management, and Prevention of COPDBethesda (MD)GOLD2016 Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed October 23, 2015
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- CazzolaMMolimardMThe scientific rationale for combining long-acting β2-agonists and muscarinic antagonists in COPDPulm Pharmacol Ther201023425726720381630
- van NoordJAAumannJLJanssensEEffects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPDChest2006129350951716537846
- BertonDCReisMSiqueiraACEffects of tiotropium and for-moterol on dynamic hyperinflation and exercise endurance in COPDRespir Med201010491288129620580216
- TashkinDPPearleJIezzoniDVargheseSTFormoterol and tiotropium compared with tiotropium alone for treatment of COPDCOPD200961172519229704
- VogelmeierCKardosPHarariSGansSJStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med2008102111511152018804362
- WangJJinDZuoPWangTXuYXiongWComparison of tiotropium plus formoterol to tiotropium alone in stable chronic obstructive pulmonary disease: a meta-analysisRespirology201116235035821138499
- TashkinDPDonohueJFMahlerDAEffects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPDRespir Med2009103451652419208459
- TashkinDPLittnerMAndrewsCPTomLinsonLRinehartMDenis-MizeKConcomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trialRespir Med2008102447948718258423
- HananiaNABootaAKerwinETomLinsonLDenis-MizeKEfficacy and safety of nebulized formoterol as add-on therapy in COPD patients receiving maintenance tiotropium bromide: results from a 6-week, randomized, placebo-controlled, clinical trialDrugs20096991205121619537837
- van NoordJAAumannJLJanssensECombining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptomsRespir Med20101047995100420303247
- JonesPWAgustiAGOutcomes and markers in the assessment of chronic obstructive pulmonary diseaseEur Respir J200627482283216585091
- JonesPWBeehKMChapmanKRDecramerMMahlerDAWedzichaJAMinimal clinically important differences in pharmacological trialsAm J Respir Crit Care Med2014189325025524383418
- AisanovZBaiCBauerleOPrimary care physician perceptions on the diagnosis and management of chronic obstructive pulmonary disease in diverse regions of the worldInt J Chron Obstruct Pulmon Dis2012727128222563246
- JonesPWDonohueJFNedelmanJPascoeSPinaultGLassenCCorrelating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysisRespir Res20111216122206353
- JonesPWHealth status measurement in chronic obstructive pulmonary diseaseThorax2001561188088711641515
- WestwoodMBourbeauJJonesPWCerulliACapkun-NiggliGWorthyGRelationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic reviewRespir Res2011124021477298
- HarunaAOgaTMuroSRelationship between peripheral airway function and patient-reported outcomes in COPD: a cross-sectional studyBMC Pulm Med2010101020205936
- WatsonLSchoutenJPLöfdahlCGPrideNBLaitinenLAPostmaDSPredictors of COPD symptoms: does the sex of the patient matter?Eur Respir J200628231131816707516
- DonohueJFJonesPBartelsCRelationship between change in trough FEV1 and COPD patient outcomes: pooled analysis of 23 clinical trials in patients with COPDEur Respir J201546Suppl 59PA1013
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- DecramerMAnzuetoAKerwinEEfficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trialsLancet Respir Med20142647248624835833
- ZhongNWangCZhouXLANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPDInt J Chron Obstruct Pulmon Dis2015101015102626082625
- BuhlRGessnerCSchuermannWEfficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to severe COPD (QUANTIFY): a randomised, non-inferiority studyThorax201570431131925677679
- WedzichaJABanerjiDChapmanKRIndacaterol-glycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- DonohueJFWorsleySZhuCQHardakerLChurchAImprovements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbationsRespir Med2015109787088126006754
- SinghDJonesPWBatemanEDEfficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised studyBMC Pulm Med20141417825404569
- D’UrzoADRennardSIKerwinEMMergelVLeslbaumARCaractaCFEfficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD studyRespir Res20141512325756831
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- BeehKMWestermanJKirstenAMThe 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary diseasePulm Pharmacol Ther201532535925956072
- Maleki-YazdiMRKaelinTRichardNZvarichMChurchAEfficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: Results of a 24-week, randomized, controlled trialRespir Med2014108121752176025458157
- MaltaisFSinghSDonaldACEffects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trialsTher Adv Respir Dis20148616918125452426
- O’DonnellDCasaburiRDe SousaDEffects of 6 weeks’ treatment with once-daily tiotropium and olodaterol fixed-dose combination on inspiratory capacity and exercise endurance in patients with COPD: the MORACTO studiesAm J Respir Crit Care Med20151911A3972
- BeehKMKornSBeierJEffect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT studyRespir Med2014108458459224534204
- DahlRChapmanKRRudolfMSafety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN studyRespir Med2013107101558156723867808
- DahlRJadayelDAlagappanVKChenHBanerjiDEfficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON studyInt J Chron Obstruct Pulmon Dis2013850150824159259
- MahlerDADecramerMD’UrzoADual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: BLAZE studyEur Respir J20144361599160924176997
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med201311516024321804
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med20131319920924429126
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mg in COPDRespir Med2013107101538154623830094
- DonohueJFSinghDMunzuCKilbrideSChurchAMagnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trialsRespir Med2016112657426797016
- SinghDWorsleySZhuCQHardakerLChurchAUmeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trialBMC Pulm Med2015159126286141
- DonohueJFNiewoehnerDBrooksJO’DellDChurchASafety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled studyRespir Res2014157825015176
- MaltaisFIturraJBKirstenAEffects of 12 weeks of once-daily tiotropium and olodaterol fixed-dose combination on exercise endurance in patients with COPDEur Respir J201444Suppl 58P283
- DonohueJFSoongWWuXShresthaPLeiALong-term safety of aclidinium bromide/formoterol fumarate fixed-dose combination: results of a randomized 1-year trial in patients with COPDRespir Med2016116414827296819
- VogelmeierCPaggiaroPLDorcaJEfficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD studyEur Respir J20164841030103927492833
- ReisnerCRoseEStromSFixed combination of glycopyrrolate and formoterol MDI (GFF-MDI) demonstrates superior inspiratory capacity (IC) compared to tiotropium DPI (Tio) following 7 days dosing, in a randomized, double-blind, placebo-controlled phase 2B study in patients with COPDEur Respir J201138Suppl 55P879
- ReisnerCRennardSFogartyCPearl Therapeutics’ combination LAMA/LABA MDI (GFF-MDI, PT003) provides a significant benefit on home peak expiratory flow rate (PEFR) and reduces the need for rescue albuterol use compared to its components administered alone, Spiriva Handihaler, and Foradil Aerolizer in a randomized, double-blind, placebo-controlled phase 2B study in patients with COPDPoster presented at: American Thoracic Society (ATS) International ConferenceMay 18–23, 2012San Francisco, CA
- ReisnerCGotfriedMDenenbergMBLow doses of Pearl Therapeutics’ LAMA/LABA combination MDI (GFF MDI, PT003) provide superior bronchodilation compared to components and to open-label Spiriva HandiHaler in a randomized, double-blind, placebo-controlled Phase IIB study in patients with COPDPoster presented at: American Thoracic Society (ATS) International ConferenceMay 17–22, 2013Philadelphia, PA
- ChapmanKRBatemanEDOlssonPChenHBanerjiDFogelRA significant proportion of patients with COPD show marked improvements in lung function with QVA149 (high responders): a post-hoc analysis of the SHINE studyChronic Obstr Pulm Dis201522159
- BatemanEDVogelmeierCChenHBanerjiDComparison of COPD exacerbations with once-daily QVA149 versus twice-daily salmeterol/fluticasone combination: the ILLUMINATE studyChest20141453409A
- JonesPWSt. George’s Respiratory Questionnaire: MCIDCOPD200521757917136966
- WedzichaJADahlRBuhlRPooled safety analysis of the fixed-dose combination of indacaterol and glycopyrronium (QVA149), its monocomponents, and tiotropium versus placebo in COPD patientsRespir Med2014108101498150725135743
- FergusonGBarnesNMehtaRD’AndreaPChenHBanerjiDCardio- and cerebro-vascular safety profile of QVA149 in patients with COPD: a pooled analysisAm J Respir Crit Care Med20131871A1488
- Van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patientsCOPD20107641842721166630
- US Food Drug AdministrationNDA 203-975 umeclidinium and vilanterol inhalation powder for the long-term, once-daily maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD)2013 Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/pulmonary-allergydrugsadvisorycommittee/ucm367411.pdfAccessed October 23, 2015
- NaccarelliGFinkleJChopraBBrooksJHarrisSChurchACardiovascular safety of umeclidinium/vilanterol in COPD: results from eight randomized clinical trialsPoster presented at: American Thoracic Society (ATS) International ConferenceMay 16–21, 2014San Diego, CA
- ManoharanAShortPMAndersonWJLipworthBJImpact of long-acting bronchodilator therapy on mortality in COPD: a real-life retrospective cohort studyThorax201368Suppl 3A179A180
- Di MarcoFMilic-EmiliJBoveriBEffect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPDEur Respir J2003211869412570114
- RocheNReddelHKAgustiAIntegrating real-life studies in the global therapeutic research frameworkLancet Respir Med2013110e29e3024461762
- AsaiKMinakataYHirataKQVA149 once-daily is safe and well tolerated and improves lung function and health status in Japanese patients with COPD: the ARISE studyEur Respir J201342Suppl 57694s
- D’UrzoARennardSKerwinEOne-year efficacy of aclidinium/formoterol fixed-dose combination in COPD patients: the AUGMENT COPD studyPoster presented at: European Respiratory Society (ERS) International CongressSeptember 6–10, 2014Munich
