Abstract
Purpose
Choosing the appropriate time to switch to noninvasive positive-pressure ventilation (NPPV) plays a crucial role in promoting successful weaning. However, optimal timing for transitioning and weaning patients from mechanical ventilation (MV) to NPPV has not been clearly established. In China, the pulmonary infection control (PIC) window as a switching point for weaning from MV has been performed for many years, without definitive evidence of clinical benefit. This study aimed to summarize the evidence for NPPV at the PIC window for patients with respiratory failure from COPD.
Methods
A comprehensive search for randomized controlled trials (RCTs) was performed. The trials were all parallel studies comparing the PIC window weaning strategy versus conventional weaning strategy in treatment of patients with respiratory failure due to COPD.
Results
Sixteen studies of 647 participants were eligible. When compared with conventional weaning strategy, early extubation followed by NPPV at the point of PIC window significantly reduced the mortality rate (risk ratios [RRs] 0.36, 95% confidence interval [CI] 0.23 to 0.57) and ventilator-associated pneumonia (VAP) (RR 0.28, 95% CI 0.19 to 0.41); it also decreased the duration of invasive ventilation (weighted mean difference [WMD] −7.68 days, 95% CI −9.43 to −5.93) and total duration of ventilation (WMD −5.93 days, 95% CI −7.29 to −4.58), which also shortened the lengths of stay in an intensive care unit (WMD −8.51 days, 95% CI −10.23 to −6.79), as well as length of stay in hospital (WMD −8.47 days, 95% CI −8.61 to −7.33).
Conclusion
The results showed that the PIC window as a switching point for sequential ventilation in treatment of respiratory failure in COPD patients may be beneficial. It might yield not only relevant information for caregivers in China but also new insights for considering the PIC window by physicians in other countries.
Introduction
COPD remains a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the US and is projected to rank fifth in 2020 in disease caused throughout the world.Citation1 In China, respiratory diseases (of which COPD is a significant component) are the third leading cause of death in rural areas and the fourth leading cause of death in urban areas, accounting for 1 million deaths and over 5 million disabilities each year.Citation2 Approximately 80% of COPD exacerbates due to pulmonary infection, as well as some severe respiratory failure often requiring endotracheal intubation (ETI) and mechanical ventilation (MV).Citation3 ETI and MV can help to drain sputum and reduce the respiratory workload, partially or even completely, so as to control bronchial pulmonary infection. Respiratory muscle fatigue, hyperinflation, and malnutrition are common in COPD patients, which may require prolonged MV.Citation3 Prolonged MV has been associated with the development of complications, for example, upper airway pathology, sinusitis, and ventilator-associated pneumonia (VAP). VAP is associated with increased morbidity and mortality of VAP in the intensive care unit (ICU), which would be ~30% or higher.Citation4 Minimizing the duration of artificial airway placement is an important goal of critical care.
Early withdrawal of invasive MV (IMV) followed by noninvasive positive-pressure ventilation (NPPV) is a new strategy for avoiding or reducing the duration of invasive mechanical support for intubated patients with respiratory failure. Choosing an appropriate time to transfer from IMV to NPPV is the key for performing sequential MV successfully. The invasive-noninvasive sequential ventilation defined as early extubation is conducted before conventional criteria for weaning and followed by NPPV. To neglect or delay the switching point for sequential ventilation can certainly miss the optimal opportunity.Citation3 However, the appropriate switching point has been controversial. So far, no generally accepted boundary for a standard switch point has been defined.
In clinical practice, the pulmonary infection control (PIC) window has been the switch point for transferring from IMV to NPPV, so the time for early extubation can be more accurately judged; improved therapy efficacy was achieved by Wang et al. When the PIC window occurs, a patient’s condition will become stable and improved if ventilation support is provided, especially for measures to resolve fatigue to the respiratory muscles.Citation3 Timely extubation followed by NPPV with the appearance of the PIC window could manage the problem of patient’s fatigue involving respiratory muscles and ventilator insufficiency simultaneously, thereby avoiding lower airway infection and VAP. In China, the PIC window has been used as the switching point for sequential ventilation in the treatment of COPD respiratory failure exacerbation for many years, despite the lack of definitive evidence of clinical benefit.
Moreover, the conclusions of these trials are inconsistent, so that the safety of this intervention remains uncertain. In order to comprehensively estimate the efficacy and safety of this weaning strategy, a systematic review and meta-analysis were conducted to summarize and analyze the results of randomized controlled trials (RCTs), comparing the PIC window weaning strategy versus the conventional weaning strategy in the treatment of patient with respiratory failure due to COPD. This meta-analysis suggests that the PIC window as a switching point for sequential ventilation could significantly reduce mortality, VAP, length of ICU and hospital stay, and duration of MV compared to conventional weaning strategy.
Methods
This systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.Citation5
Criteria for the PIC window
The standard of the PIC window was determined by the following items: 1) significantly decreased radiographic infiltrations; 2) significantly reduced quantity of sputum, thinning, and decreased density of sputum; 3) at least one of these accompanying signs: body temperature decreased to <37.5°C, leukocyte count <10×109/L or 2×109/L less than before; and 4) adjustment of ventilator settings to 10–12 times per minute for synchronous intermittent mechanical ventilation (SIMV) and 10–12 cmH2O (1 cmH2O =0.0198 kPa) for pressure support ventilation (PSV).
Search strategy
This study aimed to identify all RCTs to assess the efficacy of PIC in the treatment of patients with respiratory failure due to COPD exacerbation. The electronic search strategy applied standard filters for the identification of RCTs. The following databases were searched: MEDLINE, EMBASE, Cochrane databases, Chinese electronic databases (eg, Wan Fang Database), CNKI (China National Knowledge Infrastructure) Database, and Current Controlled Trials from its inception to February 2016. The search included the following: pulmonary infection control window; continuous positive airway pressure; bilevel positive airway; non-invasive ventilation; COPD; lung disease; pulmonary disease; airway obstruction; obstructive pulmonary disease; emphysema; acute exacerbation; respiratory failure linked with RCT OR controlled clinical trial, in various combinations. There were no limits regarding the language of publication. Cross-references from original articles and reviews were checked, and sometimes authors were contacted to obtain additional unpublished data. Similarly a search of relevant trials from the clinical trial registry was performed to identify the existence of unpublished data. Trials published solely in abstract form were excluded.
Selection of studies
The specific inclusion criteria were as follows: 1) COPD exacerbation due to pulmonary infection; 2) receiving IMV due to respiratory failure; 3) the PIC window appearing after antibiotic use, IMV, and comprehensive therapy; 4) RCTs comparing the PIC window as a weaning strategy (extubation and NPPV via face or nasal mask, immediately upon appearance of the PIC window) versus the conventional weaning strategy (IMV was received continuously after the PIC window by using the conventional weaning technique); and 5) primary outcomes: mortality during hospital admission, VAP; secondary outcome measures: length of ICU stay, length of hospital stay, duration of MV, and adverse events associated with weaning.
Data collection and quality assessment
Initial selection was performed by distributing references among pairs of independent reviews. Titles and abstracts were independently reviewed by two reviewers (YYL and QCL) to identify their potential relevance for further assessment. Data from all the studies included in this analysis were obtained during the end of extension phases of the trial. Any disagreement appeared during the process was resolved through discussion and team consensus. In the case of unpublished reports or multiple published data, the most recent versions were extracted. After obtaining the full text, the authors independently assessed all the studies for inclusion based on the predefined criteria. If studies had partly overlapped subjects, the one with a larger sample size was selected. The quality of each trial was evaluated by using the Cochrane five-risk of bias domains tool.Citation6
Data analysis
For dichotomous outcomes, risk ratios (RRs) with 95% CI were calculated. For continuous outcomes, a weighted mean difference (WMD) and 95% CI were calculated. I2 values of ≥50% indicated a substantial level of heterogeneity.Citation7 When found, a sensitivity analysis was performed to determine the source of heterogeneity. Subgroup analyses were conducted, and the meta-analysis was reanalyzed, including risk of bias (low vs high), sample size (≤40 vs >40), disease severity (moderate vs severe respiratory failure), and experimental strategy (face mask vs face/nasal mask). Publication bias was carried out by the funnel plot and assessed by Egger’s test.Citation8 All the analyses were performed with Review Manager (Version 5.1, The Cochrane Collaboration, Copenhagen, Denmark) and Stata (Version 12.0; Stata Corporation, College Station, TX, USA). A P-value of <0.05 was considered to be statistically significant.
Results
Study selection
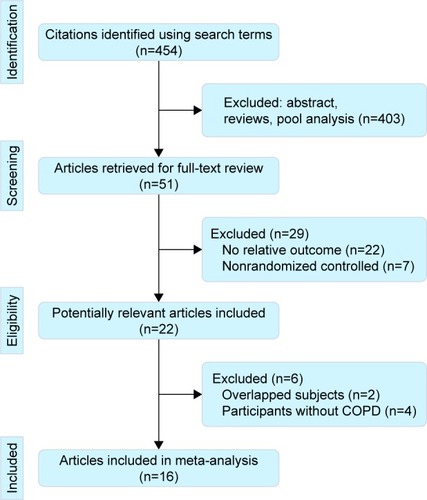
The electronic database search identified 454 citations. Of these, the first screening excluded 403 citations based on abstracts or titles, leaving 51 articles for full-text review. In these articles, 29 studies were excluded due to lack of relative outcomes and nonrandomized controls. After a detailed review, 6 studies were excluded: 2 for duplicated publications and 4 for did not evaluating NPPV in COPD patients. Thus, 16 trials were included in the meta-analysis,Citation3,Citation9–Citation23 with detailed steps of the study selection process shown in .
Study description
The level of PSV, the respiratory rate of SIMV, and tidal volume needed adjustment according to the patient’s durability, ventilation, and blood gas analysis in all trials. During MV, comprehensive therapy was performed: including administration of antibiotics, dissolution of sputum, drainage of airway secretion, dilatation of bronchi, recovery of electrolyte disturbance, and nutrient support. Once the PIC window had appeared, each patient was randomly assigned to the noninvasive ventilation (NIV) group or the control group. All the trials compared NPPV with conventional MV therapy using full face masks. However, nasal masks were also used in 5 trials.Citation13,Citation14,Citation20–Citation23 The continuous positive airway pressure in these trials ranged from 3.5 to 12.0 cmH2O. The inspiratory positive airway pressure ranged from 14.5 to 20 cmH2O, and expiratory positive airway pressure was set at 5 cmH2O in most trials. The main study characteristics are summarized in . In general, the methodological quality was acceptable. All the trial reports described the use of appropriate randomization methods, mainly computer-generated randomization lists (Figure S1).
Table 1 Characteristics of included studies
Primary outcomes
Mortality during hospital admission
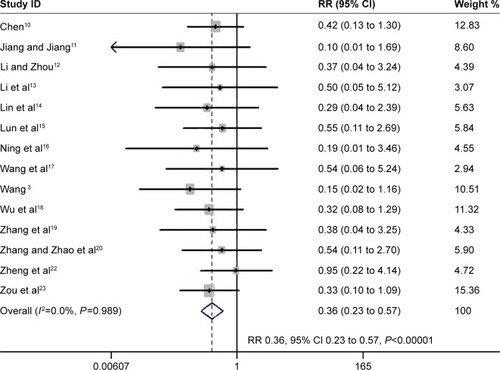
Fourteen studies evaluated whether the PIC window is a weaning strategy for reduced mortality.Citation3,Citation10–Citation23 The meta-analysis associated it with significantly decreased mortality (RR 0.36, 95% CI 0.23 to 0.57, P<0.00001; I2=0%, P=0.989) ().
VAP
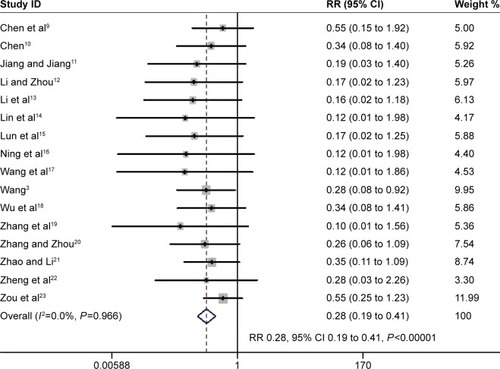
The proportion of participants developing VAP was reported in 16 trials involving 647 participants.Citation3,Citation9–Citation23 The pooled estimate demonstrated a beneficial effect of the PIC window as a weaning strategy in reducing VAP (RR 0.28, 95% CI 0.19 to 0.41, P<0.00001; I2=0%, P=0.966) ().
Secondary outcomes
Duration of MV
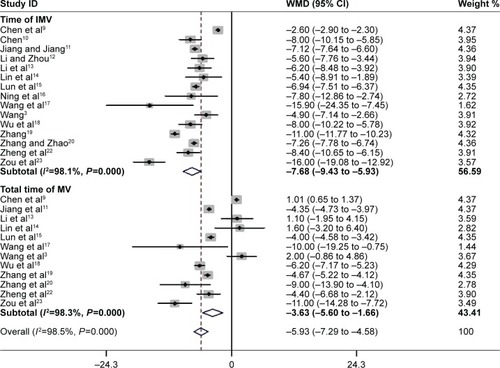
Fifteen studies assessed mean duration of IMV.Citation3,Citation9–Citation23 Pooled data using a random-effects model found strong evidence for the PIC window as a weaning strategy when compared to the conventional weaning strategy (WMD −7.68 days; 95% CI −9.43 to −5.93 days, P<0.00001; I2=98.1%, P=0.000) (). Twelve trials with 521 participants reported the total time of MV.Citation3,Citation9,Citation11,Citation13–Citation23 The summary estimate found a significant decrease in the total time of MV (WMD −5.93 days; 95% CI −7.29 to −4.58 days, P=0.000; I2 =98.3%, P=0.000) ().
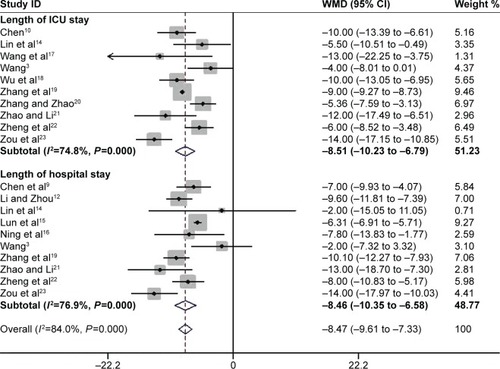
Length of ICU stay and hospital stay
Ten trials involving 429 participants evaluated ICU length of stay.Citation3,Citation10,Citation14,Citation17–Citation23 The PIC window provided a significant benefit toward shortening it (WMD −8.51 days, 95% CI −10.23 to −6.79 days, P=0.000; I2=74.8%, P=0.000). Ten studies with 442 participants reported on the length of hospital stay.Citation3,Citation9,Citation12,Citation14–Citation16,Citation18,Citation20–Citation23 Pooled data demonstrated a reduction in hospital stay, favoring the PIC window as a weaning strategy (WMD −8.47 days; 95%CI −9.61 to −7.33 days, P<0.0001; I2=76.9%, P=0.000) ().
Adverse events associated with weaning
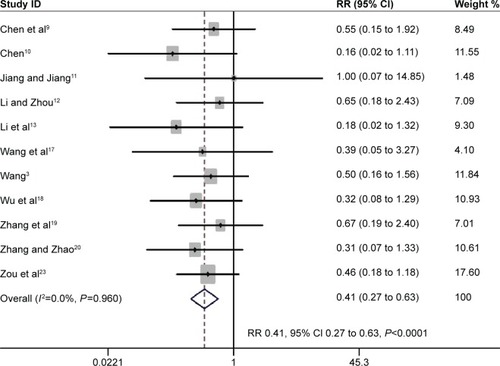
The rate of reintubation was reported separately from the proportion of weaning failures in 11 trials with 478 participants.Citation3,Citation9–Citation13,Citation17–Citation23 The pooled estimate supported a significant reduction in reintubation rate with noninvasive weaning (RR 0.41, 95% CI 0.27 to 0.63, P<0.0001; I2=0%, P=0.96) ().
Sensitivity analysis
There was substantial heterogeneity in the continuous outcomes (duration of endotracheal mechanical intubation, total duration of MV, and length of ICU and hospital stay). Sensitivity analysis was performed to determine the source of heterogeneity (Figure S2). It was found that the study by Wang et al is a partial source of heterogeneity,Citation3 mainly due to a larger sample size (n=90) versus other studies. However, omitting this study did not significantly alter the pooled WMD values.
Subgroup analysis
To exclude the effect of confounding factors, such as risk of bias and disease severity, subgroup analysis was introduced. Subgroup analyses are summarized in Table S1, which found that the conclusions remained robust for methodological changes, demonstrating that data of the present study are reliable.
Publication bias
Publication bias was detected by Begg’s and Egger’s tests. Funnel plot of the studies evaluated primary outcomes (mortality and VAP), which appeared to be symmetrical through visual examination. The data suggested that there was no evidence of publication bias (P>0.05) (Figure S3).
Discussion
This study identified 16 trials comparing the PIC window as a switch point for NPPV versus the conventional weaning strategy in patient treatment with respiratory failure due to COPD exacerbations. The PIC window weaning strategy showed significant improvement in mortality, VAP, length of ICU and hospital stay, and duration of MV compared to conventional weaning strategy. Together, the present data suggested that the PIC window might be a new tool for screening patients potentially ready to be extubated and immediately placed under NPPV.
Choosing an appropriate time to transfer from invasive ventilation (IV) to NIV is crucial in performing sequential MV successfully.Citation24,Citation25 The clinician’s concern is optimum timing regarding the condition of the patient to wean from MV. Unfortunately, there is no consensus or relevant guidelines that yield a distinct conclusion. The PIC window has been defined as a prompt stage of controlled pulmonary infection following artificial airway establishment, sputum drainage, and antibiotic administration.Citation1 Wang et al proposed this stage as the optimum timing to replace IV with NIV, so that ventilatory insufficiency and respiratory muscle fatigue might resolve, while lower airway infection and VAP were avoided.Citation1 However, the PIC window for transitioning patients from MV to NIV for weaning has not been clearly established. According to this meta-analysis, it has been showed that the PIC window as a switching point for sequential ventilation may be beneficial for respiratory failure in COPD patients due to pulmonary infection. This may result in helpful information for clinicians to identify optimum timing for withdrawal.
The standard test for extubation readiness is the spontaneous breathing trial (SBT), performed by using the T-tube by disconnecting the patient from the ventilator and providing additional oxygen.Citation26 Cabello et al compared 3 trial modalities before extubation in difficult-to-wean patients. They found that the patient effort was higher during a T-tube trial than during a pressure support trial.Citation27 The number of patients who were extubated after 48 hours was similar when the weaning trial was performed with the T-tube or pressure support trial.Citation28 Two studies have demonstrated that some patients who failed a T-tube trial could immediately succeed with a pressure support trial.Citation27,Citation29 SBT is usually granted to patients who pass the weaning assessment. Despite its benefits among COPD patients, NIV should not be indicated for all patients failing SBT; they may be exposed to extubation failure due to substantial comorbidities.Citation3 The main difference between the PIC widow weaning strategy and the SBT weaning strategy is optimal switching time to NPPV for further weaning in patients with COPD undergoing IPPV. The appearance of the PIC window indicates that pulmonary infections are under control. At this stage, drainage of airway secretions is minor, and the endotracheal tube may not be absolutely necessary, although respiratory muscle fatigue becomes relatively more significant in the development of respiratory failure. Thus, NPPV was continued to relieve fatigue of the respiratory muscle as well as ventilatory insufficiency. A recent study showed that in COPD patients with respiratory failure, weaning by SBT (assisted with NPPV) is suggested under insignificant pulmonary infection, while replacement by NPPV at the PIC window is encouraged for significant pulmonary infection.Citation30 Thus, this window may serve as an alternative weaning tool in COPD patients with pulmonary infection. However, a comparison of data obtained from the application of NPPV at the PIC window or at SBT after meeting simple weaning criteria is still missing.Citation31–Citation35 Additional well-designed, adequately powered RCTs are needed to compare the efficacy of the PIC window weaning strategy with the SBT weaning strategy in treatment of COPD patients with respiratory failure.
Limitations
Several limitations of this study should be taken into account. First, due to substantial heterogeneity among studies reporting continuous outcomes (time of IMV, total time of MV, and length of ICU and hospital stay), caution must be used while interpreting these results. Second, in China, ~80% of COPD patients with exacerbations are due to pulmonary infection.Citation3 This may be the main reason for the PIC window being used primarily. However, one should note that bronchial pulmonary infection accounted for 50%–70% of acute exacerbation of COPD occurrences worldwide.Citation2 Thus, it may provide new insights for physicians considering the PIC window weaning strategy in other countries.
Conclusion
In summary, these data suggested that the PIC window reduced the need for intubation and mortality without increasing the risk of weaning failure in treatment of respiratory failure in COPD patients. It might not only yield relevant information for caregivers in China but also new insights for considering the PIC window by physicians in other countries.
Supplementary materials
Figure S1 Risk of bias analysis.
Notes: (A) Risk-of-bias summary: the authors’ judgments about each risk-of-bias item for the each included studies. (B) Risk-of-bias graph: the authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
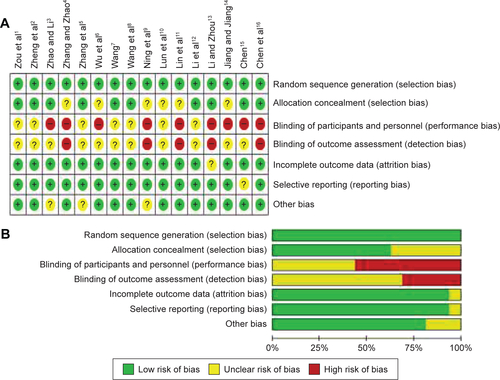
Figure S2 Sensitivity analysis.
Notes: (A) Time of IMV. (B) Total time of MV. (C) Length of ICU stay. (D) Length of hospital stay.
Abbreviations: IMV, intermittent mandatory ventilation; MV, mandatory ventilation; ICU, intensive care unit; CI, confidence interval.
Figure S3 Publication bias.
Notes: The primary outcomes mortality (A and B) and ventilator-associated pneumonia (C and D) were detected by Begg’s and Egger’s tests.
Abbreviations: SE, standard error; OR, odds ratio.
Table S1 Results of subgroup analyses from a meta-analyses of randomized controlled trials
References
- ZouSHZhouRChenPApplication of sequential noninvasive following invasive mechanical ventilation in COPD patients with severe respiratory failure by investigating the appearance of pulmonary-infection-control-windowZhong Nan DaXueXueBao Yi Xue Ban200631120124
- ZhengRQLiuLYangYProspective randomized controlled clinical study of sequential non-invasive following invasive mechanical ventilation in patients with acute respiratory failure induced by COPDChinese J Emerg Med2005142129
- ZhaoJMLiGXSequential non-invasive following short-term invasive mechanical ventilation initiated at the point of PIC windowMed J West China201224271275
- ZhangXYZhaoYClinical research of sequential invasive-noninvasive mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease with respiratory failureJ ClinPulm Med201217420423
- ZhangJXGaoXLLiuSHAnalysis of sequential mechanical ventilation in chronic obstructive pulmonary disease patients with severe respiratory failureInt J Respir200727246251
- WuQWangHXZhengSYEffect of sequential non-invasive following short-term invasive mechanical ventilation (MV) on patients with AECOPDAppl J Gen Pract20075945949
- WangCCollaborating Research Group for Noninvasive Mechanical Ventilation of Chinese Respiratory SocietyPulmonary infection control window in treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi-centred studyChin Med J20051181589159416232342
- WangCShangMHuangKSequential non-invasive mechanical ventilation following short-term invasive mechanical ventilation in COPD induced hypercapnic respiratory failureChin Med J (Engl)2003116394312667385
- NingKDongYCWangYTValue of the right time to the shift of invasive and noninvasive ventilation-clinical characteristics to the treatment of AECOPD with respiratory failureChinese J Gen Pract20108711712
- LunYHYanJWLinRPApplication of sequential non-invasive following short-term invasive mechanical ventilation in patients with AECOPDChinese J N Clin Med20092144148
- LinWXYangJMLinQSequential mechanical ventilation in patients with chronic obstructive pulmonary disease caused by severe respiratory failureShan Dong Med J200848102103
- LiJLChangSXLuCLStudy on clinical application of bi-level positive airway pressure after removal of endotracheai intubation for Chronic Obstructive Pulmonary Disease complicated with type II respiratory failureClin Med China201228712714
- LiJHZhouZXSequential non-invasive following short-term invasive mechanical ventilation in COPD patients with severe respiratory failurePractGeriatr200822368372
- JiangYPJiangLDResearch on bi-level noninvasive positive pressure ventilation in the treatment of invasive-noninvasive sequential ventilationMod Prev Med20083513711375
- ChenGEffective of sequential non-invasive following short-term invasive mechanical ventilation (MV) on AECOPD patientsMed Innovat Res20085910
- ChenHHuangFLiangYXApplied research of invasive-noninvasive mechanical ventilation for chronic obstructive pulmonary disease complicating acute respiratory failureLing Nan J Emerg Med201015275277
Disclosure
The authors report no conflicts of interest in this work.
References
- GOLD CommitteeGlobal Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2015 Available from: http://www.goldcopd.org/Accessed November 20, 2015
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large, population-based surveyAm J Respir Crit Care Med200717675376017575095
- WangCCollaborating Research Group for Noninvasive Mechanical Ventilation of Chinese Respiratory SocietyPulmonary infection control window in treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi-centred studyChin Med J20051181589159416232342
- LadeiraMTVitalFMAndrioloRBAndrioloBNAtallahANPeccinMSPressure support versus T-tube for weaning from mechanical ventilation in adultsCochrane Database Syst Rev20145CD006056
- MoherDLiberatiATetzlaffJPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementOpen Med20093e123e13021603045
- ValeCLTierneyJFBurdettSCan trial quality be reliably assessed from published reports of cancer trials: evaluation of risk of bias assessments in systematic reviewsBMJ2013346f179823610376
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ200332755756012958120
- EggerMDavey SmithGSchneiderMBias in meta-analysis detected by a simple, graphical testBMJ19973156296349310563
- ChenHHuangFLiangYXApplied research of invasive-noninvasive mechanical ventilation for chronic obstructive pulmonary disease complicating acute respiratory failureLing Nan J Emerg Med201015275277
- ChenGEffective of sequential non-invasive following short-term invasive mechanical ventilation (MV) on AECOPD patientsMed Innovat Res20085910
- JiangYPJiangLDResearch on bi-level noninvasive positive pressure ventilation in the treatment of invasive-noninvasive sequential ventilationMod Prev Med20083513711375
- LiJHZhouZXSequential non-invasive following short-term invasive mechanical ventilation in COPD patients with severe respiratory failurePract Geriatr200822368372
- LiJLChangSXLuCLStudy on clinical application of bi-level positive airway pressure after removal of endotracheai intubation for Chronic Obstructive Pulmonary Disease complicated with type II respiratory failureClin Med China201228712714
- LinWXYangJMLinQSequential mechanical ventilation in patients with chronic obstructive pulmonary disease caused by severe respiratory failureShan Dong Med J200848102103
- LunYHYanJWLinRPApplication of sequential non-invasive following short-term invasive mechanical ventilation in patients with AECOPDChinese J N Clin Med20092144148
- NingKDongYCWangYTValue of the right time to the shift of invasive and noninvasive ventilation-clinical characteristics to the treatment of AECOPD with respiratory failureChinese J Gen Pract20108711712
- WangCShangMHuangKSequential non-invasive mechanical ventilation following short-term invasive mechanical ventilation in COPD induced hypercapnic respiratory failureChin Med J (Engl)2003116394312667385
- WuQWangHXZhengSYEffect of sequential non-invasive following short-term invasive mechanical ventilation (MV) on patients with AECOPDAppl J Gen Pract20075945949
- ZhangJXGaoXLLiuSHAnalysis of sequential mechanical ventilation in chronic obstructive pulmonary disease patients with severe respiratory failureInt J Respir200727246251
- ZhangXYZhaoYClinical research of sequential invasive-noninvasive mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease with respiratory failureJ Clin Pulm Med201217420423
- ZhaoJMLiGXSequential non-invasive following short-term invasive mechanical ventilation initiated at the point of PIC windowMed J West China201224271275
- ZhengRQLiuLYangYProspective randomized controlled clinical study of sequential non-invasive following invasive mechanical ventilation in patients with acute respiratory failure induced by COPDChinese J Emerg Med2005142129
- ZouSHZhouRChenPApplication of sequential noninvasive following invasive mechanical ventilation in COPD patients with severe respiratory failure by investigating the appearance of pulmonary-infection-control-windowZhong Nan Da Xue Xue Bao Yi Xue Ban20063112012416562692
- BurnsKEMeadeMOPremjiANoninvasive positive-pressure ventilation as a weaning strategy for intubated adults with respiratory failureCochrane Database Syst Rev201312CD004127
- NavaSAmbrosinoNCliniENoninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary diseaseAnn Intern Med19981287217289556465
- ThilleAWCortés-PuchIEstebanAWeaning from the ventilator and extubation in ICUCurr Opin Crit Care201319576423235542
- CabelloBThilleAWRoche-CampoFPhysiological comparison of three spontaneous breathing trials in difficult-to-wean patientsIntensive Care Med2010361171117920352189
- EstebanAAliaIGordoFExtubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative GroupAm J Respir Crit Care Med19971564594659279224
- EzingeardEDiconneEGuyomarc’hSWeaning from mechanical ventilation with pressure support in patients failing a T-tube trial of spontaneous breathingIntensive Care Med20063216516916283162
- SongYChenRZhanQThe optimum timing to wean invasive ventilation for patients with AECOPD or COPD with pulmonary infectionInt J Chron Obstruct Pulmon Dis20161153554227042042
- RamFSPicotJLightowlerJWedzichaJANon-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20041CD004104
- YanHYYangYWuYLClinical analysis of optimal timing for application of noninvasive positive pressure ventilation in treatment of AECOPD patientsEur Rev Med Pharmacol Sci2014182176218125070824
- ReddyRMGuntupalliKKReview of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2007244145218268918
- RoseLSchultzMJCardwellCRAutomated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children (review)Cochrane Database Syst Rev20146CD009235
- LadeiraMTVitalFMAndrioloRBPressure support versus T-tube for weaning from mechanical ventilation in adultsCochrane Database Syst Rev20145CD006056