Abstract
Introduction
Plasminogen activator inhibitor-1 (PAI-1), a major inhibitor of fibrinolysis, is associated with thrombosis, obesity, insulin resistance, dyslipidemia, and premature aging, which all are coexisting conditions of chronic obstructive pulmonary disease (COPD). The role of PAI-1 in COPD with respect to metabolic and cardiovascular functions is unclear.
Methods
In this study, which was nested within a prospective cohort study, the serum levels of PAI-1 were cross-sectionally measured in 74 stable COPD patients (Global Initiative for Chronic Obstructive Lung Disease [GOLD] Stages I–IV) and 18 controls without lung disease. In addition, triglycerides, high-density lipoprotein cholesterol, fasting plasma glucose, waist circumference, blood pressure, smoking status, high-sensitive C-reactive protein (hs-CRP), adiponectin, ankle–brachial index, N-terminal pro-B-type natriuretic peptide, and history of comorbidities were also determined.
Results
The serum levels of PAI-1 were significantly higher in COPD patients than in controls, independent of a broad spectrum of possible confounders including metabolic and cardiovascular dysfunction. A multivariate regression analysis revealed triglyceride and hs-CRP levels to be the best predictors of PAI-1 within COPD. GOLD Stages II and III remained independently associated with higher PAI-1 levels in a final regression analysis.
Conclusion
The data from the present study showed that the serum levels of PAI-1 are higher in patients with COPD and that moderate-to-severe airflow limitation, hypertriglyceridemia, and systemic inflammation are independent predictors of an elevated PAI-1 level. PAI-1 may be a potential biomarker candidate for COPD-specific and extra-pulmonary manifestations.
Introduction
The coexistence of cardiovascular diseases (CVDs) and metabolic disorders is frequent in patients with chronic obstructive pulmonary disease (COPD).Citation1 The prevalence of both these conditions is highest in patients with moderate-to-severe COPD in particular and drops in patients with very severe COPD.Citation2 Accordingly, CVD is a leading cause of death in the earlier stage of the disease.Citation3 Thrombosis, in turn, is the most frequent underlying mechanism of the three major CVDs: ischemic heart disease, stroke, and venous thromboembolism,Citation4 which raises the question whether blood markers involved in the pathogenesis of thrombosis are linked to COPD.
Plasminogen activator inhibitor-1 (PAI-1) is a member of the superfamily of serine protease inhibitors and the principal inhibitor of fibrinolysis within the plasminogen activator system.Citation5,Citation6 High concentration of PAI-1 plays a pivotal role in the pathogenesis of arterial and venous thrombosis and, therefore, precedes the occurrence of thrombotic events.Citation7,Citation8 In addition, higher level of PAI-1 is associated with obesity, insulin resistance, diabetes, hyperlipidemia, and premature aging.Citation5,Citation9,Citation10 All these conditions are prevalent in COPD,Citation11,Citation12 and a potential influence of PAI-1 on an enhanced thrombogenesis in COPD has been hypothesized.Citation13 Indeed, a recent case–control study demonstrated that PAI-1 polymorphisms related to a higher PAI-1 expression are associated with COPD.Citation14 Furthermore, a study conducted on patients with predominantly mild-to-moderate COPD found that higher serum PAI-1 levels are related to airflow limitation.Citation15 Whether the observed elevation in PAI-1 levels in COPD is independent of metabolic and cardiovascular functions is unknown.
In order to elucidate the clinical value of PAI-1 as a potential biomarker in COPD, the serum levels of PAI-1 and a broad spectrum of potential confounding factors for PAI-1 were analyzed in patients with mild-to-very severe COPD and in control subjects.
Methods
This cross-sectional study is nested within a prospective COPD cohort study conducted at the Pulmonary Research Institute, the LungenClinic Grosshansdorf (Grosshansdorf, Germany). Details regarding the COPD population and the methodology of the study have been published elsewhere.Citation16–Citation18 In the present study, 74 stable outpatients with mild-to-very severe COPD (ie, n=16, 20, 18, and 20 with Global Initiative for Chronic Obstructive Lung Disease [GOLD] Stages I, II, III, and IV, respectively) and 18 nonsmoking controls without any lung disease from the 3-year follow-up visit were included. The study was approved by the Ethics Committee of the Medical Association of Schleswig-Holstein, Bad Segeberg, Germany (III/EK 116/05[I]; 185/08[I]), and all the participants provided written informed consent. In this study, the serum levels of PAI-1 were analyzed by using enzyme-linked immunosorbent assay (ELISA; Human Serpin E1/PAI-1 DuoSet ELISA; R&D Systems, Wiesbaden-Nordenstadt, Germany; detection range =0.312–20 ng/mL); metabolic and cardiovascular functions as possible confounders of PAI-1, including triglyceride levels, high-density lipoprotein (HDL) cholesterol, fasting plasma glucose, waist circumference, blood pressure, the presence of the metabolic syndrome (based on the latter five variables according to the criteria of the International Diabetes FederationCitation19), smoking status, and history of coronary artery disease and diabetes, were determined; high-sensitivity C-reactive protein (hs-CRP) was measured as an established marker of systemic inflammation and adiponectin, which is involved in anti-inflammatory, anti-diabetic, and anti-atherogenic processes;Citation20 in addition, global cardiac function by serum levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and the presence of atherosclerosis by the ankle–brachial index (ABI) were also determined as previously described.Citation16,Citation18
First, the differences between groups were analyzed univariately by using unpaired t-test, χ2 test, and analysis of variance (ANOVA) with least significant difference post hoc analysis. Then, a multivariate linear regression analysis was conducted with PAI-1 as the dependent variable and COPD as a predictor, and adjustments for possible confounders, such as age, sex, body mass index (BMI), impaired fasting glucose (ie, ≥100 mg/dL),Citation19 dyslipidemia (ie, triglycerides >150 mg/dL or HDL <40 mg/dL in male and <50 mg/dL in female),Citation19 cardiac dysfunction (ie, NT-proBNP >125 pg/mL),Citation21 atherosclerosis (ie, ABI ≤0.90),Citation22 as well as history of diabetes, hypertension, and coronary artery disease, were performed. A second multivariate regression analysis with PAI-1 as the dependent variable aimed at evaluating the best independent predictors for PAI-1 within COPD. Therefore, a stepwise approach was chosen with a backward elimination. Only the variables that showed at least a trend for statistical significance on the bivariate level, that is, P<0.10, were included in the model. Next, whether COPD severity stages were still independently associated with PAI-1 within the final model was proved. Last, the interaction effects of GOLD stages with the remaining predictors of the final model were tested by using separate two-way ANOVAs.
Results
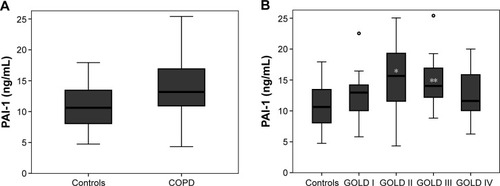
Impaired fasting glucose, dyslipidemia, cardiac dysfunction, hypertension, and history of diabetes (P>0.05, respectively; ) did not differ between the patients with COPD and controls. The frequency of atherosclerosis and history of coronary artery disease increased in patients with COPD compared with controls (P<0.05). Serum levels of PAI-1 were significantly higher in COPD patients than in controls (). Stratified by severity stages, PAI-1 levels were highest in GOLD Stages II and III (). Smoking status and statin use had no effect on the level of PAI-1 in patients with COPD (P=0.51 and 0.50, respectively). In a multivariate linear regression analysis, the presence of COPD remained an independent predictor for higher PAI-1 levels after adjustments for age, sex, BMI, impaired fasting glucose, dyslipidemia, cardiac dysfunction, hypertension, diabetes, atherosclerosis, and history of coronary artery disease (regression coefficient B for COPD patients versus controls =2.9, 95% confidence interval [CI] =0.34–5.41, P=0.027).
Table 1 Characteristics of patients with COPD and controls without any lung disease
Figure 1 Serum levels of PAI-1 (A) in patients with COPD and controls without any lung disease (P=0.015) and (B) according to COPD severity.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; PAI-1, plasminogen activator inhibitor-1.
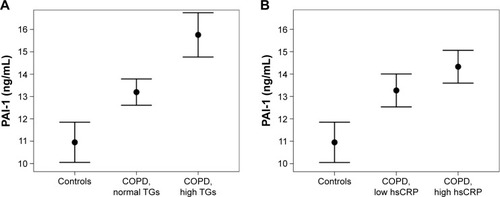
A next step was aimed at identifying the predictors for PAI-1 within COPD. Significant correlations of PAI-1 with HDL cholesterol, triglycerides, hs-CRP, and adiponectin (P<0.05, respectively; ) were found on the bivariate level. The association between age and PAI-1 showed a trend for significance (P=0.058). In a multivariate regression analysis with a backward elimination, log-triglycerides (3.3, 95% CI =0.8–5.9, P=0.012) and log-hs-CRP (0.83, 95% CI =0.03–1.63, P=0.043) were found to be the best independent predictors for PAI-1 in COPD. visualizes the results from the multivariate regression analysis, that is, mean PAI-1 levels in COPD patients stratified according to triglycerides and hs-CRP in comparison with controls.
Table 2 Bivariate associations of PAI-1 with lung function, metabolic, cardiac, and inflammatory markers in patients with COPD
Figure 2 Mean serum PAI-1 levels in controls and COPD patients according to (A) normal or elevated triglyceride levels (cutoff: 150 mg/dL) and (B) low or high hs-CRP (cutoff: median hs-CRP, ie, 3.0 mg/L).
Abbreviations: COPD, chronic obstructive pulmonary disease; hs-CRP, high-sensitive C-reactive protein; PAI-1, plasminogen activator inhibitor-1; TGs, triglycerides.
In a final model we studied whether COPD severity stages add additional information as predictors for PAI-1 independent of triglycerides and hs-CRP within the total study cohort. It was found that GOLD Stages II and III were independently associated with PAI-1 (). There were no significant interaction effects of GOLD stages either on triglycerides or on hs-CRP.
Table 3 Independent predictors for the level of PAI-1 – a multivariate linear regression analysis
Discussion
The main finding of the present study was that serum levels of PAI-1 are higher in patients with COPD independent of metabolic and cardiovascular disorders. Furthermore, hyper-triglyceridemia and systemic inflammation were found to be the best independent predictors for higher PAI-1 levels in COPD patients, whereas the presence of moderate-to-severe airflow limitation remained to be independently associated with PAI-1.
PAI-1 is a multifunctional protein associated with thrombosis as well as with metabolic conditions, such as obesity, dyslipidemia, insulin resistance, and diabetes,Citation5,Citation7,Citation9,Citation10 which all are important concomitant conditions of COPD.Citation1,Citation12 There are two very recent studies showing an association between elevated PAI-1 levels and COPD, but considered neither comorbidities of COPD nor the whole spectrum of airflow limitation, from mild to very severe stage.Citation14,Citation15 The present study is the first to demonstrate that higher serum PAI-1 levels in COPD are independent of a broad spectrum of possible confounders, in particular established variables indicating metabolic and cardiovascular dysfunction. Interestingly, this study found the highest PAI-1 levels in patients with GOLD Stages II and III. This observation is in good agreement with the finding of the previous study showing a significant association between higher levels of PAI-1 and airflow limitation in patients with predominantly mild-to-moderate COPD.Citation15 Moreover, it was also found that triglycerides and hs-CRP are the best predictors for elevated PAI-1 levels in COPD patients. In general, an elevation in triglycerides and hs-CRP plays an important role for CVD.Citation23,Citation24 Moreover, an association of PAI-1 levels with serum lipids and inflammatory markers has been found,Citation9,Citation25 although the mechanistic link behind this remains not fully understood.Citation5,Citation26 In patients with COPD, hypertriglyceridemia and systemic inflammation are also found frequently and predict mortality.Citation27–Citation29 The present study demonstrated an independent association of high PAI-1 serum levels with moderate-to-severe airflow limitation, hypertriglyceridemia, and systemic inflammation.
COPD is characterized by airway and lung abnormalities and is frequently complicated by concomitant cardiovascular and metabolic disorders.Citation1 Local pathological changes of COPD include parenchymal tissue destruction, resulting in emphysema, and disrupted repair and defense mechanisms, resulting in small airway fibrosis.Citation1 PAI-1 is synthesized by a variety of cells including vascular endothelial cells, macrophages, and fibroblasts and not only is involved in the pathogenesis of thrombosis but also activates tissue repair.Citation30–Citation32 Transiently increased levels of PAI-1 may protect extracellular matrix proteins from proteolytic degradation, inducing matrix remodeling and enhancing wound healing.Citation33,Citation34 It is well documented that tissue homeostasis is maintained by the balance of extracellular matrix synthesis and degradation depending on the cellular proteolytic activities of urokinase-type/tissue-type plasminogen activator and plasmin, which mostly rely on the activity of PAI-1.Citation33,Citation35 According to the data from the present study, the independent elevation of PAI-1 in patients with COPD may indicate a disease-specific mechanism. We speculate that higher PAI-1 levels may to some extent reflect processes of airway remodeling in COPD. These processes start early in the course of the disease and are less present in advanced COPD,Citation36 which might be the reason why the PAI-1 level is highest in moderate COPD. This concept is in good agreement with the findings of Hogg et al showing that indicators of small airway remodeling are increased in moderate COPD in comparison with controls and declined in very severe COPD again.Citation36 In line with this result, the plasminogen activator system has already been identified as a potential treatment target for chronic respiratory diseases including COPD.Citation37
On the other hand, the pivotal role of PAI-1 in the pathogenesis of thrombosis, in general, seems to be clear,Citation5,Citation7–Citation10 which allows to propose that higher levels of PAI-1 in patients with moderate-to-severe COPD (GOLD Stages II and III) may be associated with an increased risk for cardiovascular events. This idea is supported by the observation that CVD and cardiovascular mortality are highest in patients with moderate-to-severe COPD and drops in very severe COPD.Citation2,Citation3 Overall, PAI-1 is recognized as one of the central molecules linking the metabolic syndrome/obesity to the increased CVD risk.Citation26,Citation38 Beyond its function as a tissue-remodeling activator, PAI-1 is also an adipocytokine, which is upregulated along with fat accumulation and, therefore, is associated with insulin resistance, dyslipidemia, and obesity.Citation5,Citation9,Citation26,Citation38 Metabolic comorbidities are, in turn, especially prevalent in COPD patients with GOLD Stages II and III.Citation2 Altogether, PAI-1 may serve as a cross-talk molecule between airway remodeling during the course of COPD, accompanied metabolic comorbidities, and a subsequent increased risk for cardiovascular events, especially in moderate COPD.
The present study has some limitations. First, this is a cross-sectional study and therefore does not allow any interpretation on causality. The elevation of PAI-1 in COPD could also represent a bystander effect. However, it was indicated that PAI-1 may play a plausible role in COPD, and this is the first study conducted in a well-characterized COPD cohort to demonstrate a clear association of PAI-1 with COPD independent of a broad range of possible confounders. Second, the sample size, especially in the control group, is small. Despite these limitations, a significant difference in PAI-1 between COPD patients and controls was found. Of note, the control subjects were free from lung diseases but not explicitly healthy and, therefore, were well matched in terms of metabolic comorbidities. Furthermore, for remaining differences in some cardiovascular conditions, statistical adjustments were performed. Nevertheless, further studies are needed to confirm these observations and to evaluate the role of PAI-1 for cardiovascular outcomes in COPD.
Conclusion
The results of this study showed that PAI-1 levels are higher in patients with COPD, independent of metabolic disorders and cardiovascular function. Hypertriglyceridemia and systemic inflammation may play a role for elevated levels of PAI-1 in COPD, even though they do not explain this elevation solely, as moderate-to-severe airflow limitation is independently associated with higher levels of PAI-1. These findings suggest PAI-1 as a potential biomarker candidate that indicates a link between airway remodeling, metabolic comorbidities, and an increased cardiovascular risk.
Acknowledgments
This study was supported by the German Center for Lung Research (Deutsches Zentrum für Lungenforschung [DZL]) and Sir Bo Hjelt.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med201318734736522878278
- Dal NegroRWBonadimanLTurcoPPrevalence of different comorbidities in COPD patients by gender and GOLD stageMultidiscip Respir Med2015102426246895
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J2006281245125717138679
- ISTH Steering Committee for World Thrombosis DayThrombosis: a major contributor to global disease burdenThromb Res201413493193825312343
- CesariMPahorMIncalziRAPlasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditionsCardiovasc Ther201028e72e9120626406
- LoskutoffDJSawdeyMMimuroJType 1 plasminogen activator inhibitorProg Hemost Thromb19899871152492381
- SobelBEIncreased plasminogen activator inhibitor-1 and vasculopathy. A reconcilable paradoxCirculation1999992496249810330378
- Juhan-VagueIPykeSDAlessiMCJespersenJHaverkateFThompsonSGFibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and DisabilitiesCirculation199694205720638901651
- Juhan-VagueIAlessiMCMavriAMorangePEPlasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular riskJ Thromb Haemost200311575157912871293
- YamamotoKTakeshitaKKojimaTTakamatsuJSaitoHAging and plasminogen activator inhibitor-1 (PAI-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderlyCardiovasc Res20056627628515820196
- CavaillesABrinchault-RabinGDixmierAComorbidities of COPDEur Respir Rev20132245447524293462
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest200913517318019136405
- TapsonVFThe role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary diseaseProc Am Thorac Soc20052717716113472
- EssaESEl WahshRAAssociation between plasminogen activator inhibitor-1-675 4G/5G insertion/deletion polymorphism and chronic obstructive pulmonary diseaseCOPD20161375675927073968
- WangHYangTLiDElevated circulating PAI-1 levels are related to lung function decline, systemic inflammation, and small airway obstruction in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2016112369237627713627
- WatzHWaschkiBBoehmeCClaussenMMeyerTMagnussenHExtrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional studyAm J Respir Crit Care Med200817774375118048807
- WaschkiBKirstenAMHolzODisease progression and changes in physical activity in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201519229530626020495
- WaschkiBKirstenAHolzOAngiopoietin-like protein 4 and cardiovascular function in COPDBMJ Open Respir Res20163e000161
- AlbertiKGZimmetPShawJThe metabolic syndrome–a new worldwide definitionLancet20053661059106216182882
- WoutersEFAdiponectin: a novel link between adipose tissue and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201318852252323992584
- McMurrayJJAdamopoulosSAnkerSDESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESCEur J Heart Fail20121480386922828712
- AboyansVCriquiMHAbrahamPMeasurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart AssociationCirculation20121262890290923159553
- LibbyPTriglycerides on the rise: should we swap seats on the seesaw?Eur Heart J20153677477625548060
- StrangFSchunkertHC-reactive protein and coronary heart disease: all said–is not it?Mediators Inflamm2014201475712324808639
- CrutchleyDJMcPheeGVTerrisMFCanossa-TerrisMALevels of three hemostatic factors in relation to serum lipids. Monocyte procoagulant activity, tissue plasminogen activator, and type-1 plasminogen activator inhibitorArteriosclerosis198999349392511826
- AlessiMCJuhan-VagueIPAI-1 and the metabolic syndrome: links, causes, and consequencesArterioscler Thromb Vasc Biol2006262200220716931789
- DahlMVestboJLangePBojesenSETybjaerg-HansenANordestgaardBGC-reactive protein as a predictor of prognosis in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200717525025517053205
- FabbriLMRabeKFFrom COPD to chronic systemic inflammatory syndrome?Lancet200737079779917765529
- TanniSEZamunerATCoelhoLSValeSAGodoyIPaivaSAAre metabolic syndrome and its components associated with 5-year mortality in chronic obstructive pulmonary disease patients?Metab Syndr Relat Disord201513525425353094
- IdellSCoagulation, fibrinolysis, and fibrin deposition in acute lung injuryCrit Care Med200331S213S22012682443
- KucharewiczIKowalKBuczkoWBodzenta-LukaszykAThe plasmin system in airway remodelingThromb Res20031121715013265
- ParkJAFredbergJJDrazenJMPutting the squeeze on airway epitheliaPhysiology (Bethesda)20153029330326136543
- GhoshAKVaughanDEPAI-1 in tissue fibrosisJ Cellular Physiol201222749350721465481
- VassalliJDSappinoAPBelinDThe plasminogen activator/plasmin systemJ Clin Invest199188106710721833420
- VisseRNagaseHMatrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistryCirc Res20039282783912730128
- HoggJCMcDonoughJEGosselinkJVHayashiSWhat drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease?Proc Am Thorac Soc2009666867220008873
- SchuligaMWestallGXiaYStewartAGThe plasminogen activation system: new targets in lung inflammation and remodelingCurr Opin Pharmacol20131338639323735578
- BarnardSAPietersMDe LangeZThe contribution of different adipose tissue depots to plasma plasminogen activator inhibitor-1 (PAI-1) levelsBlood Rev20163042142927233154
