Abstract
Background and objective
Pulmonary tuberculosis (TB) is a risk factor for chronic obstructive pulmonary disease (COPD); however, few clinical studies have investigated treatment effectiveness in COPD patients with destroyed lung by TB. The Indacaterol effectiveness in COPD patients with Tuberculosis history (INFINITY) study assessed the efficacy and safety of once-daily inhaled indacaterol 150 µg for the treatment of Korean COPD patients with destroyed lung by TB and moderate-to-severe airflow limitation.
Methods
This was a multicenter, double-blind, parallel-group study, in which eligible patients were randomized (1:1) to receive either once-daily indacaterol 150 µg or placebo for 8 weeks. The primary efficacy endpoint was change from baseline in trough forced expiratory volume in 1 s at Week 8; the secondary endpoints included changes in transition dyspnea index score and St George’s Respiratory Questionnaire for COPD score at Week 8. Safety was evaluated over 8 weeks.
Results
Of the 136 patients randomized, 119 (87.5%) completed the study treatment. At Week 8, indacaterol significantly improved trough forced expiratory volume in 1 s versus placebo (treatment difference [TD] 140 mL, P<0.001). Statistically significant improvement in transition dyspnea index score (TD =0.78, P<0.05) and numerical improvement in St George’s Respiratory Questionnaire for COPD score (TD =−2.36, P=0.3563) were observed with indacaterol versus placebo at Week 8. Incidence of adverse events was comparable between the treatment groups.
Conclusion
Indacaterol provided significantly superior bronchodilation, significant improvement in breathlessness and improved health status with comparable safety versus placebo in Korean COPD patients with destroyed lung by TB and moderate-to-severe airflow limitation.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease characterized by progressive inflammation of the airways and persistent airflow limitation.Citation1 COPD is considered as one of the major causes of morbidity and mortality worldwide.Citation1 Evidence from epidemiologic studies shows that tuberculosis (TB) can be a significant contributor for the development of COPD, particularly in TB-endemic areas.Citation2,Citation3 A recent meta-analysis showed that TB and COPD are strongly correlated in countries with higher TB incidence rates such as South Africa and the PhilippinesCitation3 (annual TB incidence rates of 1,003/100,000 and 265/100,000 population, respectively). Pulmonary TB can cause a destructive change in pulmonary parenchyma and over a period of time, it may lead to chronic respiratory obstruction, mainly COPD. This condition is termed as “destroyed lung by TB”.Citation4 Bronchial and parenchymal structural changes resulting in reduction in lung volume loss, bronchiectasis, bronchovascular distortion and fibrotic bands are common radiologic findings in lungs destroyed by TB.Citation5 Previous reports have suggested that a TB-destroyed lung is associated with chronic airflow limitation (forced expiratory volume in 1 s [FEV1]/forced vital capacity <0.7); thus, coexistence of COPD should be considered in the differential diagnosis.
There is no specific treatment guideline for COPD patients with destroyed lung by TB,Citation6 and there are no data based on controlled studies on the treatment efficacy in COPD patients with destroyed lung by TB. We, therefore, conceptualized the Indacaterol effectiveness in COPD patients with Tuberculosis history (INFINITY) study to evaluate the efficacy and safety of a long-acting β2-agonist (LABA) indacaterol in COPD patients with destroyed lung by TB. Indacaterol, an inhaled, once-daily (o.d.) LABA, was the choice as the active treatment in our study due to the following attributes: provides 24 h bronchodilation with an effect significantly greater than that of salmeterol and similar to that of tiotropium.Citation7,Citation8 Indacaterol has been shown to provide clinically and statistically significant improvement in health status, quality of life and reduction in exacerbation rate compared to salmeterol and tiotropium, with a safety profile similar to that of placebo.Citation9,Citation10 Here, we report the efficacy and safety outcomes from the INFINITY study.
Methods
Study design
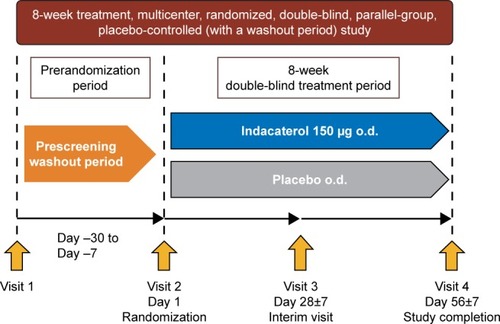
The study was a multicenter, randomized, double-blind, placebo-controlled, 8-week trial conducted in South Korea (). The first patient was enrolled on February 25, 2013 and the last patient completed the study on September 1, 2014. After screening, eligible patients were randomized in a 1:1 ratio (by interactive response technology) to treatment with double-blind indacaterol 150 µg o.d. or placebo o.d. (morning) delivered through the Breezhaler® device (Novartis Pharma AG, Stein, Switzerland). A salbutamol inhaler was provided as rescue medication. Institutional review boards and ethics committees at the participating centers approved the study (see Table S1 for a full list of the institutional review board and ethics committee for each center). This study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and local regulatory requirements. All participants provided written informed consent for this study. Clinical trial registration number: NCT1778062; URL: www.ClinicalTrials.gov.
Patients
Patients were aged ≥19 years, had moderate-to-severe COPD (stage II or III, as defined in the Global Initiative for Chronic Obstructive Lung Disease 2010 criteria) and were required to have a post-bronchodilator FEV1 of at least 30% and <80% of the predicted normal and a post-bronchodilator FEV1/forced vital capacity ratio <0.70. All patients had a history of TB with no change in the chest imaging test over the past 1 year. They were required to have at least one finding of destructed pulmonary parenchyma in the chest X-ray, among the following: 1) lung volume loss, 2) bronchovascular distortion, 3) fibrosis, 4) bronchiectasis, and the sum of all legion volumes equivalent to over one-third of one lung confirmed by a radiologist or a pulmonology specialist. Both smokers and nonsmokers were eligible to enter the trial. Key exclusion criteria included patients with a history of asthma, respiratory infection within the previous 6 weeks and a history of COPD worsening that required hospitalization within the previous 6 weeks.
Assessments
Spirometry (FEV1) was performed at randomization and at Weeks 4 and 8 in the morning (between 8 and 11 am). Dyspnea was evaluated using the baseline dyspnea index at baseline and the transition dyspnea index (TDI) at Weeks 4 and 8. St George’s Respiratory Questionnaire for COPD (SGRQ-C) was used to evaluate the changes in health status of the patients at baseline and at Weeks 4 and 8. Safety was assessed by recording adverse events (AEs) and serious AEs (SAEs) throughout the study; in addition, electrocardiograms, hematology, clinical chemistry, urinalysis, physical condition and vital signs (pulse and blood pressure) were assessed. The primary objective of this study was to show superiority of indacaterol in trough FEV1 (defined as the mean of FEV1 values at 23 h 10 min and 23 h 45 min post-dose) versus placebo at Week 8. The secondary objectives included assessment of TDI focal score and SGRQ-C total score at Week 8 for indacaterol versus placebo. We also assessed the incidence of acute exacerbation and safety over 8 weeks of treatment period. COPD assessment test (CAT) score at Week 8 was investigated as an exploratory objective.
Statistical analyses
A sample size of 132 patients (indacaterol, 66; placebo, 66) was intended for randomization and this number was sufficient to detect a 120 mL difference in trough FEV1 at Week 8 between indacaterol and placebo at 5% significance level with an estimated 80% power, based on previous phase III indacaterol studies. However, patients with TB history could have been excluded from previous COPD trials with indacaterol, so there may be no prior data in these study populations. A standard deviation of 225 mL and 15% dropout rates were assumed.
Intent-to-treat (ITT) population and per protocol (PP) population were utilized for all efficacy analyses. The ITT set included all patients who received at least one dose of the investigational drug and had primary efficacy endpoint after administration of the investigational drug, and the PP set was a subset of the ITT population without any major protocol deviations (study drug compliance <80% or >120%, inclusion or exclusion criteria violation, etc). All safety analyses were performed on the safety population, which included all patients who received at least one dose of the study medication. Last observation carried forward was used to impute missing values.
Mean, standard deviation, minimum and maximum were calculated for continuous variables, and frequency and proportion were calculated for categorical variables. All the test statistics used for this data analysis were the results of the two-sided test, and the statistical significance level was 0.05. Analysis of covariance was used to test the difference between the two groups. All statistical analyses were performed using SAS release 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
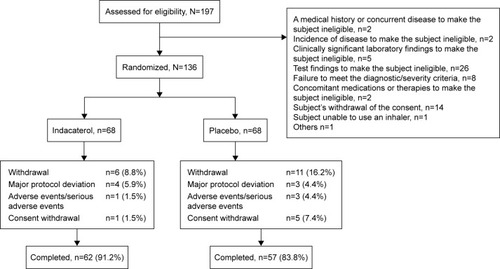
Of 197 patients screened, 136 were randomized, 119 completed the study and 17 discontinued the study treatment. Reasons for study discontinuation were major protocol deviation (n=7), AEs/SAEs (n=4) and consent withdrawal (n=6). One patient in the placebo group was excluded from the ITT set due to “failure to perform” primary efficacy evaluation, and 94 patients (indacaterol, n=51; placebo, n=43) were included in the PP set ().
Demographics and baseline characteristics
The demographic and baseline clinical characteristics were similar between indacaterol and control groups (). However, the proportion of male population was slightly higher in the indacaterol treatment group. There was no report of exacerbations in the previous year (92.7%) for a majority of the patients. The most common radiologic findings in both groups were lung volume loss (84.6%), followed by fibrosis (64.7%) and bronchiectasis (51.5%).
Table 1 Baseline demographic and clinical characteristics
Efficacy
Spirometry
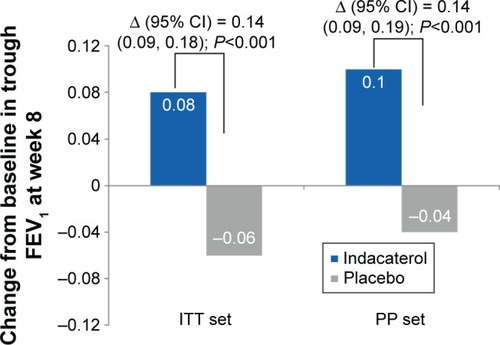
Trough FEV1 at Week 8 (24 h post-dose) was significantly improved with indacaterol compared with placebo, with treatment differences of 0.14 L (P<0.001; ). Patients treated with indacaterol showed statistically significantly improved FEV1 versus placebo, regardless of the smoking status (treatment differences in the range of 0.13–0.16 L) and also the previous use of fixed-dose combination of inhaled β2-agonist and inhaled corticosteroids (ICS; treatment differences in the range of 0.11–0.15 L; ).
Table 2 Change from baseline in trough FEV1 at week 8 (A) and analysis of change from baseline in FEV1 at week 8 by smoking status and previous use of fixed-dose combination of inhaled β2-agonist and ICS (B)
Figure 3 Change from baseline in trough FEV1 at Week 8 (ITT set and PP set).
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 s; ITT, intent-to-treat; LS, least squares; PP, per protocol; SE, standard error.
Dyspnea, health status and patient symptoms
Indacaterol treatment resulted in significant improvement in TDI focal score at Week 8 with a treatment difference of 0.78 and 0.81 (ITT set and PP set, respectively) compared with placebo (P=0.0465; ). At Week 8, the proportion of patients with a clinically important increase of ≥1 in TDI score was 44.1% with indacaterol versus 27.3% with placebo in the ITT set (P=0.042); this percentage was 47.1% with indacaterol versus 18.6% with placebo in the PP set (P=0.004), as shown in .
Table 3 Change from baseline in TDI focal scores at Week 8 (A) and change from baseline in SGRQ-C scores at Week 8 (B)
Figure 4 (A) Proportion of patients with a clinically important improvement from baseline in TDI total score (≥1 point; ITT and PP population). (B) Proportion of patients achieving MCID in St George’s Respiratory Questionnaire score.
Abbreviations: ITT, intent-to-treat; MCID, minimal clinically important difference; PP, per protocol; TDI, transition dyspnea index.
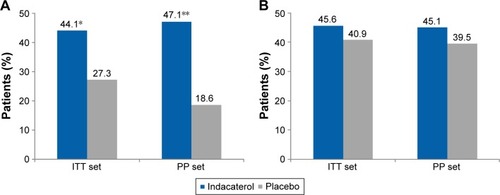
The mean SGRQ-C total score change from baseline was greater with indacaterol than with placebo, and the treatment difference was not statistically significant (−2.35, P=0.356 and −4.79, P=0.118 from ITT set and PP set, respectively; ). The percentages of patients with a clinically important improvement from baseline SGRQ-C total score of 4 units were numerically higher in the indacaterol-treated group compared with placebo in both ITT (45.6% versus 40.9%; P=0.585) and PP (45.1% versus 39.5%; P=0.587; ) sets.
The CAT score slightly increased from baseline by 0.25 points with indacaterol, and also with placebo by 1.80 points. The difference between the two groups was not statistically significant (P=0.192; ).
Table 4 Change from baseline in COPD assessment test scores
Safety
shows the overall incidence of AEs and those reported most frequently. COPD worsening, upper respiratory tract infection and nasopharyngitis were the common AEs in both groups. COPD worsening occurred with a high frequency in the placebo group. The proportions of patients with SAEs in the indacaterol and placebo groups were 4.4% and 1.5%, respectively. All SAEs were assessed to be not related to the study drug by the investigator. There were no deaths during the study.
Table 5 Incidence of frequent adverse events and serious adverse events in treatment groups (safety set)
Discussion
Our study provides evidence that indacaterol improves the lung function and symptom control in COPD patients with destroyed lung by TB. Treatment with indacaterol showed significantly higher trough FEV1 and increased TDI scores than treatment with placebo at Week 8. To the best of our knowledge, this is the first prospective, randomized controlled study evaluating the efficacy and safety of a long-acting bronchodilator in a specific COPD subpopulation.
COPD patients are reported to have a threefold increased risk of developing TB, compared with the general population.Citation11 COPD and TB also share several risk factors such as smoking, diabetes and nutritional imbalance. Approximately >50% of the patients in this study had a smoking history; since smoking is a well-known risk factor for COPD, it may be difficult to attribute COPD in patients from this study to either smoking or TB. Nevertheless, a study in Colombia reported that the correlation between TB and airway obstruction was stronger than that observed between cigarette smoking and airway obstruction.Citation12
Interestingly, certain treatments for COPD may contribute to the related risk for TB. A Taiwanese study revealed that use of high-dose ICS (fluticasone treatment >500 mg/day) was an independent risk factor for the development of active TB in patients with COPD (hazard ratio, 4.74; 95% confidence interval: 1.01, 22.37; P=0.014).Citation13 In another Korean study by Kim et al, ICS use increased the risk of pulmonary TB and the risk was greater in patients who had radiologic sequelae of prior pulmonary TB.Citation14
To date, no treatment guidelines are available for patients with TB-destroyed lung and based on previous reports that TB can cause chronic airflow limitation,Citation15,Citation16 long-acting muscarinic antagonist or LABA + ICS have been used occasionally.Citation6,Citation17 Inhaled bronchodilators are recommended by the guidelines as the cornerstone of COPD treatment; however, there have been no studies to evaluate treatment options and outcomes in patients with concomitant TB sequelae. This can be a concern to the physicians in countries with higher prevalence rates of TB.
Indacaterol, an o.d. β2-agonist delivered by a single-dose dry powder inhaler, has been shown to provide effective control of symptoms, better lung function and quality of life compared with placebo and some of the other available bronchodilators, with a favorable safety profile.Citation18 In our study, trough FEV1 of patients in the indacaterol group was significantly improved after 8 weeks of treatment compared with those in placebo group, with the treatment difference 140 mL exceeding the minimum clinically important difference. This result was consistent regardless of the smoking status or previous ICS + LABA medication use and similar to that observed in prior indacaterol phase III clinical trials, compared to placebo.Citation19,Citation20
The bronchodilator effect of indacaterol was also accompanied by an improvement of symptom control in terms of dyspnea. However, the other secondary and exploratory variables, SGRQ-C total score and CAT, did not reach statistical significance, which may be due to the limited number of patients and short study duration. Safety and tolerability were similar between indacaterol and placebo, as shown by previous clinical trial data. The major strength of our study is the uniqueness of the study population, which was not included in large COPD clinical trials previously but represents a specific phenotype seen in TB-endemic areas. In addition, our prospective study design was robust, since previously reported studies were analyses using retrospective chart review or without any control arm. However, the present study was conducted for 8 weeks, considering ethical concerns with patients in the placebo group; so, there are limitations to generalize the short-term results from this study.
In recent years, there has been increased evidence of the benefits of dual bronchodilation (LABA/long-acting muscarinic antagonist fixed-dose combination) compared with a single bronchodilator in COPD patients with moderate- to-severe airflow limitation. This study is valuable as it demonstrated the efficacy and safety of o.d. indacaterol 150 µg in COPD patients with TB-destroyed lung, and we may expect future studies with dual bronchodilators in these patients for a long-term treatment period.
Conclusion
The INFINITY study demonstrated that the inhalation of indacaterol has a significant effect on spirometric parameters in COPD patients with destroyed lung by TB. Indacaterol provided significantly superior bronchodilation, significant improvement in breathlessness, and showed a tendency to improve the health status, with comparable safety versus placebo in Korean COPD patients with destroyed lung by TB and moderate-to-severe airflow limitation. These findings support the benefits of indacaterol in the treatment management of these patients. This study will add value to treatment of COPD patients with TB history. Although it is difficult to ascertain the exact risk factor for COPD patients with destroyed lung by TB, the role of both smoking and TB in causing airflow obstruction cannot be ruled out. In light of the increasing incidence and detrimental effects of COPD and TB combined, it is imperative to have an effective treatment that provides better lung function and improved quality of life to COPD patients with destroyed lung by TB. Indacaterol, with its proven efficacy and safety in this study and as reported previously, can be a clinically efficacious treatment option for these patients.
Acknowledgments
The authors thank patients and staff at the participating centers in the study. The authors also thank Chiranjit Ghosh, PhD (professional medical writer; Novartis) for assistance in the preparation of this paper. These people received no compensation apart from their usual salary for their contributions. This study was funded by Novartis Korea Ltd., Seoul, South Korea. The sponsor funded the design/concept/analysis of the study and manuscript development. All investigators received fees from Novartis for conducting the study. The study results were previously presented as a poster at the APSR 2015 congress.
Supplementary material
Table S1 Full list of institutional review boards and ethics committees which approved this study
Disclosure
Song Kim is a Novartis employee. The other authors report no conflicts of interest in this work.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease2016 Available from: http://goldcopd.org/Accessed May 5, 2016
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J200628352353216611654
- ByrneALMaraisBJMitnickCDLeccaLMarksGBTuberculosis and chronic respiratory disease: a systematic reviewInt J Infect Dis20153213814625809770
- HassanISAl-JahdaliHHObstructive airways disease in patients with significant post-tuberculous lung scarringSaudi Med J20052671155115716047080
- KimHYSongKSGooJMLeeJSLeeKSLimTHThoracic sequelae and complications of tuberculosisRadiographics200121483985811452057
- RheeCKYooKHLeeJHClinical characteristics of patients with tuberculosis-destroyed lungInt J Tuberc Lung Dis2013171677523232006
- DonohueJFFogartyCLötvallJINHANCE Study InvestigatorsOnce-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropiumAm J Respir Crit Care Med2010182215516220463178
- KornSKerwinEAtisSAmosCOwenRLassenCINSIST study groupIndacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week studyRespir Med2011105571972621367594
- JonesPWBarnesNVogelmeierCLawrenceDKramerBEfficacy of indacaterol in the treatment of patients with COPDPrim Care Respir J201120438038821785813
- CopeSDonohueJFJansenJPComparative efficacy of long-acting bronchodilators for COPD: a network meta-analysisRespir Res20131410024093477
- InghammarMEkbomAEngströmGCOPD and the risk of tuberculosis – a population-based cohort studyPLoS One201054e1013820405056
- YakarHIGunenHPehlivanEThe role of tuberculosis in COPDInt J Chron Obstruct Pulmon Dis20171232332928176901
- ShuCCWuHDYuMCTaiwan Anti-Mycobacteria Investigation (TAMI) Group. Use of high-dose inhaled corticosteroids is associated with pulmonary tuberculosis in patients with chronic obstructive pulmonary diseaseMedicine (Baltimore)2010891536120075705
- KimJHParkJSKimKHJeongHCKimEKLeeJHInhaled corticosteroid is associated with an increased risk of TB in patients with COPDChest201314341018102423079688
- WillcoxPAFergusonADChronic obstructive airways disease following treated pulmonary tuberculosisRespir Med19898331951982595036
- MenezesAMHallalPCPerez-PadillaRLatin American Project for the Investigation of Obstructive Lung Disease (PLATINO) Team. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin AmericaEur Respir J20073061180118517804445
- YumHKParkINEffect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lungTuberc Respir Dis (Seoul)201477416717125368662
- SethHDSultanSGotfriedMHRole of indacaterol, a once-daily bronchodilator, in chronic obstructive pulmonary diseaseJ Thorac Dis20135680681424409359
- ChapmanKRRennardSIDograAOwenRLassenCKramerBINDORSE Study InvestigatorsLong-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled studyChest20111401687521349928
- FeldmanGSilerTPrasadNINLIGHT 1 study group. Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week studyBMC Pulm Med2010101120211002