Abstract
Computed tomography scan images have been used to identify different radiological COPD phenotypes based on the presence and severity of emphysema, bronchial wall thickening, and bronchiectasis. Bronchiectasis is defined as an abnormal dilation of the bronchi, usually as a result of chronic airway inflammation and/or infection. The prevalence of bronchiectasis in patients with COPD is high, especially in advanced stages. The identification of bronchiectasis in COPD has been defined as a different clinical COPD phenotype with greater symptomatic severity, more frequent chronic bronchial infection and exacerbations, and poor prognosis. A causal association has not yet been proven, but it is biologically plausible that COPD, and particularly the infective and exacerbator COPD phenotypes, could be the cause of bronchiectasis without any other known etiology, beyond any mere association or comorbidity. The study of the relationship between COPD and bronchiectasis could have important clinical implications, since both diseases have different and complementary therapeutic approaches. Longitudinal studies are needed to investigate the development of bronchiectasis in COPD, and clinical trials with treatments aimed at reducing bacterial loads should be conducted to investigate their impact on the reduction of exacerbations and improvements in the long-term evolution of the disease.
Introduction
Patients with COPD may present with different clinical characteristics, prognoses, and response to treatment.Citation1 This has resulted in increased efforts to identify subgroups of patients that share similar characteristics – the so-called clinical phenotypes – in order to provide more individualized and effective therapy.Citation2,Citation3 Some studies have attempted to determine these phenotypes by investigating the morphological findings observed in lung computed tomography (CT) scans.Citation4 In this respect, the presence of pulmonary emphysema,Citation4 bronchial wall thickening,Citation5 and bronchiectasisCitation6 have been proposed as three of the main morphological findings likely to provide relevant information about different phenotypes of COPD.Citation4,Citation7
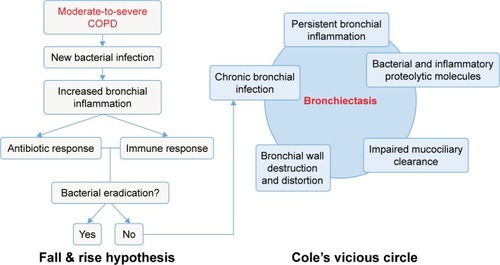
Bronchiectasis is defined as irreversible and generally progressive dilation of the airways. Some 30 years ago, ColeCitation8 proposed a pathogenic vicious circle originating from chronic bronchial infection caused by potentially pathogenic microorganisms (PPMs) and the consequent chronic inflammation which results in remodeling of the airways and damage to local defense mechanisms, which in turn facilitate the persistence of PPMs in the bronchial tree despite the administration of treatment. Chronic bronchial infection is also frequently found in patients with COPD and would provide a link between the two diseases.Citation9
The relationship between bronchiectasis and COPD has generated several questions. Is there any real increased prevalence of bronchiectasis in patients with COPD? Does the presence of bronchiectasis have an impact on the clinical characteristics, prognosis, or response to treatment in COPD, to the extent that it can be considered a distinct clinical phenotype? Should bronchiectasis in patients with COPD be seen as merely a comorbidity, or as a consequence of the disease’s natural history? Is there a causal relationship between COPD and bronchiectasis? If this is the case, what are the pathophysiological mechanisms responsible for this relationship? And, finally, what is the role of chronic bronchial infection and exacerbations in this relationship?
COPD and bronchiectasis: under-and misdiagnosis
COPD and bronchiectasis are very prevalent in the general population. In Spain up to 10% of the adult population has COPDCitation10 and 1.5% of men older than 65 years have bronchiectasis;Citation11 therefore, they may both coexist. Although the diagnosis of COPD is based on physiology and that of bronchiectasis on morphology, both diseases may result in similar lung function abnormalities and non-specific respiratory symptoms. As a consequence, there is a strong possibility of misdiagnosis in favor of COPD, because spirometry is more widely available than CT scans and physicians usually think primarily about COPD when confronted with a smoker with cough, sputum production, and airflow obstruction.Citation12 In this respect, O’Brien et alCitation13 observed that approximately one third of patients referred from primary care with a COPD diagnosis had bronchiectasis demonstrated by CT scan, even with normal spirometric values. Furthermore, in COPD patients with pulmonary hypertension, the diagnostic criterion for bronchiectasis (based on the demonstration of a bronchial lumen diameter greater than the diameter of the adjacent vessel) may be misleading.Citation14
Prevalence of bronchiectasis in COPD
The prevalence of bronchiectasis in patients with COPD has been analyzed in several studies, with conflicting results ranging from 4% to 72% ().Citation4,Citation15–Citation28 The methodologies used in these studies were very diverse. They differed in the characteristics of the patients included (different severity, non-consecutive inclusion, or inclusion during exacerbations), the use of different CT diagnostic criteria for bronchiectasis, or the study’s objective (related or unrelated to bronchiectasis analysis). These differences may partly explain the disparities found in the reported prevalence. Moreover, most of these studies excluded patients with previously known bronchiectasis and those with bronchiectasis in only one pulmonary segment, as this circumstance can be found in a significant percentage of elderly people in the general population, or in smokers with no airway obstruction.Citation29 Recently, Tan et alCitation30 found that 19.9% of healthy individuals had bronchiectasis compared with 35.1% of severe COPD patients of the same age. This means that the real prevalence of bronchiectasis in COPD patients might be overestimated, and other CT characteristics such as bronchial wall thickening should be used to differentiate real bronchiectasis in COPD from bronchiectasis associated with aging.Citation14
Table 1 Characteristics of the studies analyzing the prevalence and outcomes related to the presence of bronchiectasis in COPD patients
In patients with COPD the characteristics found to be associated with a higher prevalence of bronchiectasis include advanced age,Citation22 male gender,Citation25 and a history of previous exacerbations.Citation13,Citation15,Citation18,Citation19,Citation25 The bronchiectasis observed in patients with COPD is usually cylindrical (72%–90%), predominantly in both pulmonary bases (52%–81%), bilateral (52%–67%), and with moderate scores in radiological analyses of severity.Citation13–Citation17,Citation21,Citation23 Interestingly, one consistent finding is the relationship between the increased severity of COPD and the higher prevalence of bronchiectasis.Citation16,Citation20–Citation22,Citation24,Citation25
Impact of bronchiectasis on COPD
The impact of bronchiectasis on the natural history of COPD has been analyzed in two recent meta-analysesCitation31,Citation32 and can be summarized as follows ().
Table 2 Characteristics of patients with COPD and bronchiectasis compared with COPD patients without bronchiectasis
Clinical aspects
The presence of bronchiectasis in COPD has been associated with a lower body mass index,Citation22 older age,Citation22,Citation25 a greater production and purulence of sputum,Citation17 a greater number of comorbidities,Citation25 and a higher body mass index, airflow obstruction, dyspnea, and exercise index.Citation22 However, the most widely recognized association is a greater frequency and severity of exacerbations, identified for the first time by Patel et al,Citation17 and confirmed in subsequent studies.Citation16,Citation22–Citation26
Microbiological aspects
The variable most consistently associated with the presence of bronchiectasis in patients with COPD is chronic bronchial infection by PPMs (odds ratio [OR] between 3.76–7.33), particularly Pseudomonas aeruginosa (OR between 3.5–4.75),Citation31,Citation32 along with the existence of a greater bacterial load.Citation17
Functional aspects
There is also significant agreement that the presence of bronchiectasis is associated with more severe bronchial obstruction.Citation13,Citation15,Citation21–Citation23
Inflammatory aspects
The presence of bronchiectasis has been associated with an increase in both local and systemic inflammation.Citation17,Citation23,Citation24 Patel et alCitation17 observed that patients with bronchiectasis presented an increase in the concentrations of IL-8 and IL-6 in sputum as a result of the greater bacterial load. Similarly, two studies observed an increase in C-reactive protein and erythrocyte sedimentation rate in peripheral blood.Citation23,Citation24
Prognostic aspects
Only a few studies have analyzed the relationship between the presence of bronchiectasis and poor outcomes in patients with COPD. A prospective study on 201 consecutive patients with stable COPD followed-up for 48 months concluded that the presence of bronchiectasis was associated with greater mortality (OR: 2.45; P=0.02), independent of age, the presence of comorbidities, or the severity of airway obstruction;Citation23 these results were confirmed by Mao et al.Citation27 In contrast, another two studies – one performed on 406 COPD patients during a severe exacerbationCitation25 and another on 338 patients with no previous exacerbationsCitation26 – did not find any association between the presence of bronchiectasis and greater mortality. However, the meta-analysis by Du et alCitation32 concluded that the mortality risk in COPD is significantly increased in the presence of bronchiectasis with an OR of 1.96 (95% confidence interval =1.04–3.70).
Searching for causality: is this possible?
A further advance in our understanding of the interaction between COPD and bronchiectasis would be to know whether bronchiectasis in COPD patients develops as a result of COPD or its determining factors, such as smoking, presence of chronic bronchial infection, and exacerbations.
Even though some etiological classifications of bronchiectasis include COPD as one of its causes, so far no longitudinal study has demonstrated any causal relationship, although Bradford Hill’s classic etiologic criteriaCitation33 could provide the basis for an argument in favor of this possibility.
Strength of association
Two recent meta-analyses concluded that the presence of PPMs (OR: 7.33 and 3.76, respectively), colonization by P. aeruginosa (OR: 3.50 and 4.75, respectively), and a greater number of exacerbations (OR: 1.97 and 1.54, respectively)Citation31,Citation32 were the variables most strongly associated with the presence of bronchiectasis in COPD. Although it is still unknown whether chronic bronchial infection and frequent exacerbations occur before or after the development of bronchiectasis in patients with COPD, the results of these meta-analyses support the pathophysiological hypothesis that development of bronchiectasis in COPD is associated with chronic bronchial infection and frequent exacerbations.
Time sequence
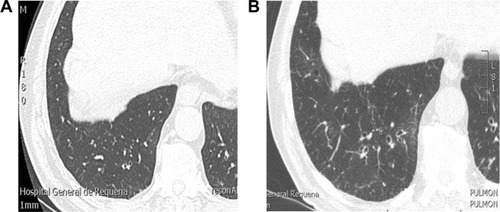
This would be based on the observation of “de novo” bronchiectasis in COPD patients in whom it was not previously present. shows a patient who, in 2007, presented with severe COPD (forced expiratory volume in 1 second [FEV1]: 49% predicted), chronic bronchitis, and frequent exacerbations but had no bronchiectasis detected by high-resolution computed tomography (HRCT) scan at that time. The same patient participated in a new study 8 years later, when his FEV1 was 29% predicted, revealing bibasal cylindrical bronchiectasis. In spite of an exhaustive etiological study, it was impossible to identify any cause for bronchiectasis other than COPD itself. Longitudinal studies involving repeated HRCT are required to confirm a time sequence that would support a causal relationship between the two diseases.
Dose-response effect
The dose-response effect could be applied to the higher prevalence of bronchiectasis observed in patients with a greater severity of COPD.Citation16,Citation20–Citation22,Citation24,Citation25
Consistency
Consistency refers to the percentage of studies that reach the same conclusions. In this respect, most studies have observed not only a high prevalence of bronchiectasis, but also a similar pattern, comprising cylindrical, bilateral, and bibasal bronchiectasis.Citation15–Citation17,Citation23 Furthermore, bronchiectasis seems to be found more frequently in older patientsCitation22 and heavier smokers, which would concur with the hypothesis that bronchiectasis occurs as a consequence of years of bronchial inflammation or infection.
Biological plausibility
Subjects with COPD present different forms of impaired immunity that facilitate the survival and proliferation of PPMs in the lower airways. In fact, microbiological cultures of sputum are positive for PPMs in about 40%–70% of patients with stable COPD.Citation9,Citation34–Citation36 This chronic bronchial infection unleashes a persistent bronchial inflammation and these two together progressively damage the bronchial wall through the release of bacterial and inflammatory proteolytic products, leading to the formation of bronchiectasis, in accordance with the classic postulates of Cole,Citation8 described previously. The reduced activity of many antimicrobials as a result of the chronic bronchial infection and the difficult diffusion to the bronchial secretions resulting from the presence of bronchiectasis, makes it much more difficult to eradicate the PPMs, and so the process becomes chronic, thereby increasing the frequency and severity of the exacerbations in a patient with COPD. This sequence of events is illustrated in .
Figure 2 Pathophysiological hypothesis of the development of bronchiectasis in patients with COPD.
Analogy
This refers to the existence of similar examples that would explain the relationship that needs to be demonstrated. The origin of bronchiectasis in patients with cystic fibrosis could be a valid example in this respect.Citation37
Experimental evidence
There is no evidence to date of any delay to, or non-appearance of bronchiectasis in patients with COPD as a result of prophylactic treatment for exacerbations or long-term anti-inflammatory or antibiotic treatment, although this would undoubtedly be one of the main objectives of future studies.
Bronchiectasis-COPD overlap: comorbidity or a distinct clinical phenotype?
COPD can exist without bronchiectasis and bronchiectasis is often not present alongside chronic airflow obstruction; however, when bronchiectasis of unknown etiology is demonstrated in a patient with COPD it is difficult to accept that it is merely a comorbidity that appeared by chance. Tobacco smoking is the main etiological factor for COPD, and it also impairs lung defense mechanisms and facilitates chronic and acute infection. The role of infection in the cascade of events that accelerate the progression of COPD in smokers, and how it can be a key player in the development of bronchiectasis, has already been addressed. It is therefore reasonable to assume that in some patients the two diseases, COPD and bronchiectasis, may be clinical manifestations of the same process, justifying the overlapping term or clinical phenotype COPD-bronchiectasis.Citation38–Citation40 It is interesting to observe that a significant proportion of patients with bronchiectasis included in clinical trials were also smokers with chronic airflow obstruction.Citation41 This highlights the strong relationship between these two entities, but is it a case of bronchiectasis with airflow obstruction, or COPD with bronchiectasis? Hurst et alCitation40 highlighted the importance of differentiating between patients with bronchiectasis who present with not fully reversible airflow obstruction and COPD patients presenting the anatomical abnormalities of bronchiectasis. This second case would fulfil the criteria of a phenotype of the COPD disease spectrum.Citation40
The diagnosis of the COPD-bronchiectasis phenotype will have clinical implications for more frequent chronic bronchial infection, impairment in respiratory symptoms (cough and sputum), more frequent and severe exacerbations, and impaired health-related quality of life. These signs and symptoms are very similar to those of the COPD-infective phenotype and there must be a huge overlap between them.Citation3,Citation42 It can be speculated that the COPD-bronchiectasis phenotype may be a sub-phenotype or an evolution of the infective phenotype of COPD (), characterized by chronic bronchial infection by PPMs and frequent bacterial exacerbations.Citation42 Some patients with COPD and infective phenotype may have developed bronchiectasis, but others may be in the initial stages of the disease and would be susceptible to therapeutic strategies aimed at suppressing bacterial growth and avoiding bacterial persistence, in order to prevent the development of bronchiectasis. Unfortunately, no longitudinal studies are yet available to confirm this sequence of events.
Figure 3 Relationship between COPD-bronchiectasis overlap phenotype and infective and chronic bronchitis and exacerbator phenotypes.
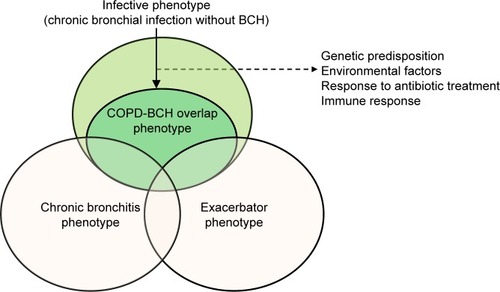
The COPD-bronchiectasis phenotype would also overlap with the chronic bronchitis and frequent exacerbator phenotypesCitation3 (). In contrast to the previously mentioned phenotypes, the COPD-bronchiectasis phenotype is stable and can be objectively diagnosed via imaging.Citation43 Some national guidelines have included the COPD-bronchiectasis phenotype as an important clinical phenotype that need to be considered in the treatment of COPD.Citation44
Bronchiectasis in COPD: therapeutic consequences
Although the presence of bronchiectasis in patients with COPD is usually associated with chronic bronchial infection and more frequent exacerbations, mostly of a bacterial etiology,Citation25,Citation31,Citation32 this infective component is not adequately covered by the usual treatment for COPD based on inhaled therapy with bronchodilators, with or without inhaled corticosteroids (ICS). Some recent studies have demonstrated that even patients on triple therapy may suffer from frequent exacerbations.Citation45 One of the reasons would be the lack of appropriate treatment for the infective component of the disease. An HRCT scan of the thorax must be performed in patients with COPD and frequent exacerbations, and when bronchiectasis is identified, they must receive treatment for both COPD and bronchiectasis, in the latter case mainly aimed at controlling chronic and acute infection.
There have not been any trials of therapies for COPD complicated by bronchiectasis. In most pharmacologic clinical trials on COPD the presence of significant bronchiectasis is an exclusion criterion; therefore, no recommendations can be formulated on the basis of solid evidence, although special attention must be paid to anti-inflammatory and antibiotic therapies.
The efficacy of ICS in COPD is controversial, particularly in patients with frequent bacterial exacerbations and/or low concentrations of bronchial or blood eosinophils, as may be the case in patients with associated bronchiectasis. Furthermore, the use of ICS may be associated with an increase in the bronchial bacterial load in patients with COPD and chronic bronchial infection;Citation46 in fact, ICS are not indicated as maintenance treatment in bronchiectasis,Citation47 and the last update of the GOLD strategy indicates that in the case of associated bronchiectasis, ICS may not be indicated in patients with bacterial colonization or recurrent lower respiratory tract infections.Citation48 Therefore, unless patients present with high blood eosinophil levels and/or clinical signs of bronchial hyper-responsiveness,Citation49 ICS should not be used, or only used at the lowest possible dosage. One alternative may be the use of macrolides or roflumilast, which is effective in neutrophilic inflammation and in patients with chronic cough and sputum production,Citation50 both characteristics of bronchiectasis in COPD.
There have been therapeutic trials on bronchiectasis that include adult smokers with airflow obstruction that is not fully reversible and similar to COPD.Citation41 From these trials we can speculate that the use of long-term macrolides or inhaled antibiotics could be beneficial in reducing exacerbations in these patients.Citation50 Other strategies such as physiotherapy must also be considered.Citation47
What do we need from future studies on this topic?
To analyze the true prevalence of bronchiectasis in COPD patients, large studies of consecutive stable COPD patients from international registries should be performed. Bronchiectasis diagnosis should be made by means of HRCT scans interpreted with uniform radiological criteria, taking into account the existence of bronchial dilations mimicking bronchiectasis in the elderly.
The causal relationship between COPD and bronchiectasis requires longitudinal studies to be adequately assessed. These studies should include evaluation of the lungs by repeated HRCT scans to verify the development of “de novo” bronchiectasis in subjects with COPD, and they should investigate the related factors that predispose to this association. Longitudinal studies should also confirm the prognostic impact of bronchiectasis on outcomes such as exacerbations, hospitalizations, and mortality in COPD.
Another interesting aspect for future research is the study of specific biomarkers linking COPD and bronchiectasis, especially those related to neutrophilic inflammation, COPD severity, exacerbations, bronchial wall thickening, or increased susceptibility to chronic bronchial infection.Citation51 The composition and relevance of the lung microbiome and its changes with the evolution of the disease, or with treatment, provide another area for future research.Citation52
Finally, therapeutic trials should be conducted on patients with COPD and bronchiectasis to investigate the efficacy and safety of specific treatments such as mucolytics, phosphodiesterase IV inhibitors, long-term macrolides, and inhaled antibiotics, particularly in more severe patients with a high risk of exacerbations and chronic bronchial infection.
Conclusion
The prevalence of bronchiectasis in patients with COPD is high. Some of the etiological factors for bronchiectasis are present in patients with COPD, and may be responsible for the development of bronchiectasis in susceptible individuals. It is not clear why some patients with COPD develop bronchiectasis and others do not, but the presence of a chronic bronchitis phenotype may determine an increased risk of chronic bronchial infection and recurrent infective exacerbations, which perpetuate the vicious circle of infection, inflammation, and tissue destruction. The presence of bronchiectasis in COPD is associated with more frequent and severe exacerbations, impaired quality of life, and possibly reduced survival. Longitudinal studies are needed to investigate the development of bronchiectasis in COPD, and clinical trials with treatments aimed at reducing bacterial loads should be conducted to investigate their impact on the reduction of exacerbations and improvements in the long-term evolution of the disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- HanMKAgustiACalverleyPMChronic obstructive pulmonary disease phenotypes: the future of COPDAm J Respir Crit Care Med2010182559860420522794
- MiravitllesMCalleMSoler-CataluñaJJClinical phenotypes of COPD: identification, definition and implications for guidelinesArch Bronconeumol2012483869822196477
- BafadhelMUmarIGuptaSThe role of CT scanning in multidimensional phenotyping of COPDChest2011140363464221454400
- MairGMaclayJMillerJJAirway dimensions in COPD: relationship with clinical variablesRespir Med2010104111683169020541384
- Martinez-GarciaMÁSelmaMJNavarroCMuñoz-ReinaABronchiectasis phenotype in COPD patientsClin Pulm Med2015223123127
- LynchDAAustinJHHoggJCCT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner SocietyRadiology2015277119220525961632
- ColePJInflammation: a two edged-sword-the model of bronchiectasisEur J Respir Dis Suppl19861476153533593
- SethiSMurphyTFInfection in the pathogenesis and course of chronic obstructive pulmonary diseaseN Engl J Med2008359222355236519038881
- MiravitllesMSorianoJBGarcía-RíoFPrevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activitiesThorax2009641086386819553233
- MonteagudoMRodríguez-BlancoTBarrechegurenMSimonetPMiravitllesMPrevalence and incidence of bronchiectasis in Catalonia, Spain: A population-based studyRespir Med2016121263127888988
- MiravitllesMde la RozaCNaberanKAttitudes towards the diagnosis of chronic obstructive pulmonary disease in primary careArch Bronconeumol20064213816426516
- O’BrienCOGuestPJHillSLStockleyRAPhysiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary careThorax200055863564210899238
- DiazAAYoungTPMaselliDJQuantitative CT measures of bronchiectasis in smokersChest Epub20161124
- GallegoMPomaresXEspasaMPseudomonas aeruginosa isolates in severe chronic obstructive pulmonary disease: characterization and risk factorsBMC Pulm Med20141410324964956
- Martínez-GarcíaMÁSoler-CataluñaJJDonat SanzYFactors associated with bronchiectasis in patients with COPDChest201114051130113721546440
- PatelISVlahosIWilkinsonTMBronchiectasis, exacerbations indices, and inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170440040715130905
- RocheNKouassiBRabbatAMounedjiALorutCHuchonGYield of sputum microbiological examination in patients hospitalized for exacerbations of chronic obstructive pulmonary disease with purulent sputumRespiration2007741192516675894
- Garcia-VidalCAlmagroPRomaníVPseudomonas aeruginosa in patients hospitalised for COPD exacerbations: a prospective studyEur Respir J20093451072107819386694
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- ArramEOElrakhawyMMBronchiectasis in COPD patientsEgyptian Journal of Chest Diseases and Tuberculosis2012614307312
- StewardJIMaselliDJAnzuetoAClinical impact of CT radiological feature of bronchiectasis in the COPDGene cohortAm J Respir Crit Care Med2012185A3656
- Martínez-GarcíaMAde la Rosa CarrilloDSoler-CataluñaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- TulekBKivrakASOzbekSKanatFSuerdemMPhenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomographyCan Respir J2013202919623616965
- GatheralTKumarNSansomBCOPD-related bronchiectasis; independent impact on disease course and outcomesCOPD201411660561424983298
- JairamPMvan der GraafYLammersJWMaliWPde JongPAPROVIDI Study groupIncidental findings on chest CT imaging are associated with increased COPD exacerbations and mortalityThorax201570872573126024687
- MaoBLuHWLiMHThe existence of bronchiectasis predicts worse prognosis in patients with COPDScientific Reports201551096126077673
- da SilvaSMPaschoalIADe CapitaniEMMoreiraMMPalharesLCPereiraMCCOPD phenotypes on computed tomography and its correlation with selected lung function variables in severe patientsInt J Chron Obstruct Pulmon Dis20161150351327042039
- KwakHJMoonJYChoiYWHigh prevalence of bronchiectasis in adults: analysis of CT findings in a health screening programTohoku J Exp Med2010222423724221127394
- TanWCHagueCJLeipsicJFindings on thoracic computed tomography scans and respiratory outcomes in persons with and without chronic obstructive pulmonary disease: a population-based cohort studyPLoS One20161111e016674527861566
- NiYShiGYuYHaoJChenTSongHClinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systematic review and meta-analysisInt J Chron Obstruct Pulmon Dis2015101465147526251586
- DuQJinJLiuXSunYBronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysisPloS One2016113e015053226978269
- HillABThe environment and disease: association or causation?Proc R Soc Med196558529530014283879
- MarinAMonsóEGarcia-NuñezMVariability and effects of bronchial colonisation in patients with moderate COPDEur Respir J201035229530219643939
- WilkinsonTMPatelISWilksMDonaldsonGCWedzichaJAAirway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200316781090109512684248
- MiravitllesMMarínAMonsóEColour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary diseaseRespir Res2010115820470372
- PetersSCystic fibrosis: a review of pathophysiology and current treatment recommendationsS D Med201467414815124791377
- O’DonnellAEBronchiectasis in patients with COPD: a distinct COPD phenotype?Chest2013140511071108
- StockleyRABronchiectasis with chronic obstructive pulmonary disease: association or a further phenotype?Am J Respir Crit Care Med2013187878678823586376
- HurstJRElbornJSDe SoyzaABRONCH-UK ConsortiumCOPD-bronchiectasis overlap syndromeEur Respir J201545231031325653262
- BarkerAFO’DonnellAEFlumePAztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trialsLancet Respir Med20142973874925154045
- MatkovicZMiravitllesMChronic bronchial infection in COPD. Is there an infective phenotype?Respir Med20131071102223218452
- Martinez-GarciaMAMaizLDe la RosaDThe overlap with bronchiectasisAnzuetoAHeijdraYHurstJRControversies in COPDEuropean Respiratory Society2015105
- KoblizekVChlumskyJZindrVChronic obstructive pulmonary disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented careBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2013157218920123733084
- BuschRHanMKBowlerRPRisk factors for COPD exacerbations in inhaled medication users: the COPDGene study bianual longitudinal follow-up prospective cohortBMC Pulm Med2016162826861867
- GarchaDSThurstonSJPatelARChanges in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPDThorax201267121075108022863758
- PasteurMCBiltonDHillATBritish Thoracic Society Bronchiectasis non-CF Guideline GroupBritish Thoracic Society guideline for non-CF bronchiectasisThorax201065Suppl 1i1i5820627931
- VogelmeierCFCrinerGJMartínezFJGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive SummaryArch Bronconeumol201753312814928274597
- BarrechegurenMEsquinasCMiravitllesMThe asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challengesCurr Opin Pulm Med2015211747925405671
- SantosSMarinASerra-BatllesJTreatment of patients with COPD and recurrent exacerbations: the role of infection and inflammationInt J Chron Obstruct Pulmon Dis20161151552527042040
- ShawJGVaughanADentAGBiomarkers of progression of chronic obstructive pulmonary disease (COPD)J Thorac Dis20146111532154725478195
- TunneyMMEinarssonGGWeiLLung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbationAm J Respir Crit Care Med2013187101118112623348972