Abstract
Background
Although patients with COPD often have various comorbidities and symptoms, limited data are available on the contribution of these aspects to health care costs. This study analyzes the association of frequent comorbidities and common symptoms with the annual direct and indirect costs of patients with COPD.
Methods
Self-reported information on 33 potential comorbidities and symptoms (dyspnea, cough, and sputum) of 2,139 participants from the baseline examination of the German COPD cohort COSYCONET was used. Direct and indirect costs were calculated based on self-reported health care utilization, work absence, and retirement. The association of comorbidities, symptoms, and COPD stage with annual direct/indirect costs was assessed by generalized linear regression models. Additional models analyzed possible interactions between COPD stage, the number of comorbidities, and dyspnea.
Results
Unadjusted mean annual direct costs were €7,263 per patient. Other than COPD stage, a high level of dyspnea showed the strongest driving effect on direct costs (+33%). Among the comorbidities, osteoporosis (+38%), psychiatric disorders (+36%), heart disease (+25%), cancer (+24%), and sleep apnea (+21%) were associated with the largest increase in direct costs (p<0.01). A sub-additive interaction between advanced COPD stage and a high number of comorbidities reduced the independent cost-driving effects of these factors. For indirect costs, besides dyspnea (+34%), only psychiatric disorders (+32%) and age (+62% per 10 years) were identified as significant drivers of costs (p<0.04). In the subsequent interaction analysis, a high number of comorbidities was found to be a more crucial factor for increased indirect costs than single comorbidities.
Conclusion
Detailed knowledge about comorbidities in COPD is useful not only for clinical purposes but also to identify relevant cost factors and their interactions and to establish a ranking of major cost drivers. This could help in focusing therapeutic efforts on both clinically and economically important comorbidities in COPD.
Introduction
COPD is globally one of the leading causes of morbidity and mortality.Citation1 The aging global population and the ongoing exposure to risk factors is expected to further increase the prevalence of COPD.
Previous studies have revealed that COPD has a very high economic burden because of its excess health care utilization and the impact on work absence and premature retirement.Citation2–Citation7 For the countries of the European Union, the direct costs attributable to COPD were estimated as €23.3 billion in 2011, and indirect costs as €25.1 billion, although with the acknowledgement of a significant underestimation.Citation6 For Germany, the reported annual excess costs per COPD case for the year 2012 ranged between €2,595 and €8,924 for direct costs, and between €8,621 and €27,658 for indirect costs in Global initiative for Obstructive Lung Disease (GOLD) stages 1 and 4, respectively.Citation3 With its increasing prevalence, the direct and indirect costs of COPD are expected to rise substantially.Citation4,Citation8,Citation9
Besides dyspnea, cough, and sputum production as the leading symptoms,Citation1 comorbidities have been shown to increase the clinical impact of the lung disorder and have gained increased interest in recent years. A broad range of coexisting diseases occur frequently in patients with COPD, including respiratory, cardiovascular, metabolic, and mental.Citation10 Comorbid conditions have been shown to reduce patients’ health-related quality of lifeCitation11,Citation12 and alter their disease course and survival.Citation13,Citation14 Furthermore, comorbid conditions are responsible for increased health care utilization in patients with COPDCitation15–Citation17 and thus increased total health care costs.Citation3,Citation18,Citation19 The prevalence of many of these comorbidities also increases with age, and so the aging population is likely to result in further increases in these costs. However, there is high heterogeneity among studies describing the effects of comorbidities on health care utilization and costs. Some studies focused on one comorbid condition only,Citation20 while others considered a limited spectrum of comorbiditiesCitation3,Citation21 or special treatment situations only,Citation22 with the majority of studies focusing on total health care costs, rather than on COPD-related costs.
Furthermore, few studies have examined the cost-driving effect of COPD symptoms such as dyspnea.Citation23–Citation25 High symptom burden has been shown to be associated with increased health care utilization and work impairmentCitation23 as well as societal costs.Citation24,Citation25 However, these studies focused on dyspnea only or used an aggregated level of symptoms to define their burden.
Taken together, there is a lack of economic studies investigating the impact of a broad range of comorbid conditions and distinct symptoms on direct and indirect costs in a large COPD population. There are even fewer studies that put the effects of comorbid conditions on costs into the context of COPD severity. However, from a public health perspective, there is a substantial need on detailed knowledge about the cost-driving effects in patients with COPD to identify starting points for reducing disease burden, and finally, for cost containment.
Therefore, the aim of this study was to identify the role of the most prevalent comorbidities and symptoms as cost-driving factors in COPD and to consider possible interactions between the degree of airflow limitation, comorbidities, and symptoms.
Methods
Data and study design
This analysis is based on data from the baseline visit of the German COPD cohort COSYCONET (German COPD and Systemic Consequences – Comorbidities Network). In total, 2,741 patients with a clinical COPD diagnosis were included in this prospective, observational, multicenter cohort study between 2010 and 2013. Inclusion criteria were age ≥40 years and physician-diagnosed COPD. Subjects with lung cancer, or who had undergone lung volume reduction or lung transplantation, or who had experienced a moderate or severe exacerbation within the last 4 weeks, or who had physical or cognitive impairment were excluded. Participants were recruited from inpatient and outpatient health care providers and patient groups and by media campaigns and examined in 31 study centers nationwide in Germany. Detailed information on the cohort has been given elsewhere.Citation26,Citation27
COPD definition and lung function
For this analysis, COPD was defined according to GOLD as a forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) <0.7, based on post-bronchodilator spirometry.Citation1 For FEV1, percentage predicted values according to the Global Lung Function Initiative were used for spirometric severity assessment:Citation28 COPD GOLD stage 1 was defined as FEV1 ≥80% predicted, stage 2 as 50%≤ FEV1 <80%, stage 3 as 30%≤ FEV1 <50%, and stage 4 as FEV1 <30%.
Twenty patients with missing information on GOLD stages and 430 without showing an airflow limitation (FEV1/FVC ≥0.7) in spirometry were excluded, as were 152 participants with alpha-1-antitrypsin deficiency, who may differ in their comorbidity profile and bias cost estimates, in particular if the very high costs of substitution therapy are included.Citation29
Calculation of direct and indirect costs
All cost calculations were performed from a societal perspective for the price year 2012.
Information on all-cause health care utilization was assessed via standardized patient interviews and questionnaires, comprising the number of inpatient hospital days, of inpatient and outpatient days in rehabilitative care, and of physiotherapeutic treatments in the last 12 months. The number of physician visits (split into 13 specializations) was captured over the last 3 months, and was extrapolated to 12 months. Annual costs per subject were calculated by multiplying all individual utilization frequencies by standardized German unit costs for the price year 2012 as published by Bock et al.Citation30
Detailed information on unit costs can be found in Table S1.
The standardized patient interviews assessed the intake of prescribed pharmaceuticals during the previous week including their national drug code Information on Defined Daily Doses, and this information was used for the estimation of mean annual dosages,Citation31 with annual costs calculated based on pharmacy retail prices in 2012.Citation32 Vitamins, dietary supplements, non-pharmacy medicines, and over-the-counter pharmaceuticals were excluded.
Indirect costs in terms of productivity losses as a consequence of temporary inability to work or premature retirement were considered for participants under the regular retirement age of 65 years, according to the human capital approach. Mean annual German labor costs (€37,126) were used to price premature retirement.Citation33 For temporary inability to work of those who reported to take part in the labor market, the number of absence days in the last 12 months due to illness was multiplied by average costs of €177 per working day (annual labor costs of €37,126 divided by the 210 working days in 2012Citation34).
Comorbidities and symptoms
Information on a list of 33 possible comorbid conditions was assessed by asking all participants during the examination, “Has a physician ever diagnosed you with one of the following diseases?” In seven participants with missing information on single comorbidities, the answer was set to “no”.
The presence of cough and sputum production was defined by the first and second question of the COPD Assessment Test (CAT).Citation35 The response options for each symptom were dichotomized, and an answer ≥3 (on a scale of 0–5) was counted as “yes”. Dyspnea was measured by the modified Medical Research Council (mMRC) dyspnea scale. The response options for dyspnea were also dichotomized, and an answer with mMRC ≥2 (on a scale of 0–4) was used for separating less vs more breathlessness.Citation1
Covariables
Information on age, sex, smoking status, body mass index (BMI), and level of school education (basic, secondary, and higher) was assessed in standardized interviews, questionnaires, and examinations and considered as covariables. Smoking status was categorized into current, former, and never smoker. Four participants with missing information on smoking status were counted as former smokers, since this was the most frequent category.
Statistical analysis
Baseline characteristics, comorbidities, and symptoms of the total study population were summarized descriptively, and compared using analysis of variance for continuous variables and chi-square tests for categorical variables.
In a first step, generalized linear regression models included all single comorbidities with a prevalence ≥10% and the three symptom types (low vs high levels of dyspnea, cough, and sputum, respectively) as predictors of direct costs, their cost components (outpatient, hospitalization, medication, and other costs), and indirect costs. All other comorbidities were summarized and considered as an additional count (between 0 and 17 possible comorbidities with a prevalence <10%). For indirect costs, only subjects <65 years participating regularly in the labor market or reporting premature retirement were considered (n=788).
In a second step, additional regression models for direct and indirect costs were performed to study possible interactions between the three predictors COPD GOLD stage 1–4, comorbidities, and dyspnea (cough and sputum production did not show statistically significant effects in the first step). These variables were dichotomized, as follows: low extent of airflow limitation (GOLD 1/2) vs high extent (GOLD 3/4), low (≤3) vs high (>3) number of comorbidities, and low (mMRC 0–1) vs high (mMRC 2–4) level of dyspnea. A simple comorbidity count was used (between 0 and 33 possible comorbidities), dichotomized according to its median (=3) as cut-off, which is an approach that has been shown previously to be a good proxy for the burden of comorbidities.Citation36
All regression models included age group, sex, level of school education, smoking status, and BMI category as potential confounders. Since cost data typically follow a right-skewed distribution with positive values only, regression models assuming a gamma distribution and log-link were employed. An amount of €1 was allocated for all observations with zero costs for modeling. Resulting estimates can be interpreted as factors due to the log-link. Adjusted mean costs for different groups were calculated via recycled predictions with 1,000 bootstrap replications for 95% CI. The significance for all statistical tests was defined at p<0.05. Statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
The COSYCONET study complies with the Declaration of Helsinki and Good Clinical Practice Guidelines and has been approved by the ethics committee of the medical faculty of the Philipps-University Marburg, by the local ethics committees of the participating centers (a list of all participating study centers can be found at http://www.asconet.net/html/cosyconet/studzent), and by the concerned data security authority (data security agency of the federal states of Hesse, Baden-Württemberg, Lower-Saxony, and Saarland).
Written informed consent was obtained from all study participants.
Results
Characteristics of the study sample
shows the baseline characteristics of the study population, comprising 2,139 patients with COPD. The mean age was 65.4 years, with 61.1% being male. Most of the participants were in GOLD stage 2 or 3. High levels of dyspnea were reported by 48.5% of participants, with 46.7% reporting sputum production and 44.9% cough; 26.0% of participants did not have any symptoms.
Table 1 Characteristics of the study population
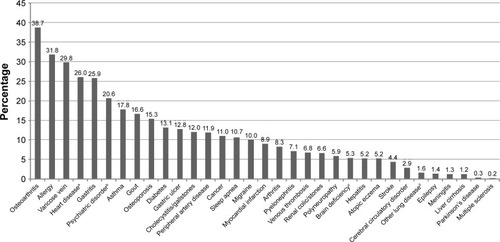
Participants reported a mean number of 3.7 comorbidities. The prevalence of all 33 comorbid conditions studied is shown in . With a prevalence of 38.7%, osteoarthritis was the most commonly reported comorbidity, followed by allergies, varicose veins, heart disease, gastritis, and psychiatric disorders.
Figure 1 Lifetime prevalence (%) of comorbid conditions.
Direct costs
Unadjusted mean annual total direct costs (all-cause) were €7,263 per patient, with 46.8% due to hospitalization, 34.1% to prescribed medication, 11.7% to physician and outpatient hospital visits, and 7.4% to rehabilitation and physiotherapy ().
Table 2 Unadjusted mean annual direct and indirect costs
The results of the regression models for total direct costs and their components are presented in . Higher COPD stage was associated with a statistically significant increase in total direct costs. Compared with COPD stage 1, stage was associated with an increase in costs of +22%, stage of +66%, and stage 4 of +102% (all p<0.01). This amplifying effect with GOLD stage was observed for all components of direct costs, except outpatient costs.
Table 3 Results of multivariate regression models for direct and indirect costs
Regarding the most prevalent comorbid conditions, osteoporosis showed the main cost-driving effect on total direct costs (+38%), followed by psychiatric disorders (+36%), heart disease (+25%), cancer (+23%), sleep apnea (+21%), gastric ulcer (+20%), cholecystitis/gallstones (+19%), migraine (+15%), and peripheral artery disease (+14%), with all p≤0.03. For the components of direct costs, psychiatric disorders had the highest cost-driving effect regarding outpatient and hospitalization costs, whereas sleep apnea showed the strongest effect on other costs and cancer showed the strongest effect on medication costs.
In terms of symptoms, a high level of dyspnea (mMRC ≥2) was associated with an increase in total direct costs of +33% (p<0.0001), with significant effects on all cost components. Cough and sputum production (CAT ≥3) were not associated with increased direct costs, either total or the cost components.
Indirect costs
The indirect costs were analyzed in a subgroup of 779 patients <65 years with complete information on retirement status or absence days. Unadjusted mean indirect costs of participants <65 years were €23,298, mainly determined by 56% of this sample being retired prematurely. Besides a high level of dyspnea (+34%), parameters associated with significantly increased indirect costs were higher age (+59%) and psychiatric disorders (+31%) ().
Interactions
shows the results of the regression models considering interactions between dichotomized COPD stage, the number of comorbidities, and dyspnea for direct and indirect costs. depicts the absolute costs of the respective groups of the models. Higher COPD stage, a high number of comorbidities, and a high level of dyspnea were associated with increases of +69%, +83%, and +43% in direct costs (all p<0.0001). There was an additional sub-additive, cost-reducing effect of having both an advanced COPD stage and a high number of comorbidities (−28%, p=0.0001), with the other interactions not reaching statistical significance.
Table 4 Interactions between COPD stage, comorbidities, and dyspnea for annual direct or indirect costs
Figure 2 Adjusted mean total direct or indirect costs (€) per year for subgroups.
Abbreviations: GOLD, Global initiative for Obstructive Lung Disease; mMRC, modified Medical Research Council.
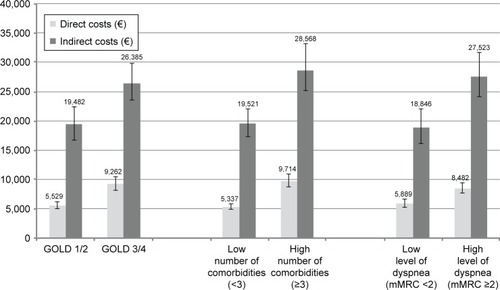
For indirect costs, cost increased with a high number of comorbidities (+46%; p=0.03). The level of dyspnea, GOLD stage, and the interaction terms did not reach statistical significance.
Adjusted mean direct and indirect costs by group (GOLD 1/2 vs GOLD 3/4, low vs high number of comorbidities, low vs high level of dyspnea) are shown in .
Discussion
This study analyzed the cost-driving effects of frequent comorbidities and symptoms, in addition to the level of airflow limitation in patients with COPD. A high level of dyspnea showed the strongest driving effect on direct health care costs, whereas cough and sputum production were not associated with any incremental direct costs. When considering the most frequently reported comorbidities, osteoporosis, psychiatric disorders, heart disease, cancer, and sleep apnea were associated with significantly increased direct health care costs. However, the effect of higher COPD stages (GOLD 3/4) was greater than the effects of the presence of any comorbid condition. A sub-additive interaction between advanced COPD stage and a high number of comorbidities reduced the independent cost-driving effects of these factors and may be an indicator for possible ceiling effects of treatment.
For indirect costs, besides a high level of dyspnea, psychiatric disorders were identified as a driver of costs, whereas other comorbid conditions did not show significant associations. However, a higher number of comorbid conditions was associated with indirect costs. No statistically significant associations between cough or sputum production and indirect costs were observed, whereas higher age was associated with increased costs.
From an economic perspective, it is important to identify possible starting points to reduce the economic burden of COPD, and therefore, it is crucial to differentiate between the costs of COPD itself and costs of concomitant circumstances. Treating comorbidities and the symptoms that in patients with COPD drive costs is a first step, and has the potential to be cost-effective or even cost-saving,Citation37,Citation38 although economic evaluations may be challenging.Citation39
The economic view on the burden of COPD does not necessarily contradict the patient’s perspective. Comparing the most relevant comorbidities from an economic and patient perspective, a clear overlap is apparent: In a previous analysis of this dataset, among the most important comorbidities with negative effects on health-related quality of life were psychiatric disorders, peripheral artery disease, sleep apnea, and heart disease, all of which were also identified as major cost drivers in this analysis.Citation40
Compared to previous studies, our results were consistent in that comorbidities in patients with COPD increase the direct costs, largely as a result of increasing health care utilization.Citation16,Citation18,Citation19,Citation41 However, there was a high variation in the effect on costs of different comorbidities. A review of the excess costs of comorbidities in COPD by Huber et al reported that pneumonia, cardiovascular disease, and diabetes were associated with the highest excess costs.Citation18 Simon-Tuval et al found myocardial infarction, congestive heart failure, mild liver disease, and diabetes as the main cost drivers in patients with COPD.Citation19 Mannino et al reported the highest effects on health care costs were for patients with COPD and comorbid cardiovascular disease, anemia, or chronic kidney disease,Citation41 whereas Schwab et al reported the highest associations with all-cause health care costs were for congestive heart failure, cerebrovascular disease, coronary artery disease, sleep apnea, and depressive disorders.Citation21 Osteoporosis, which showed the highest effect on direct costs in our analysis, has also been identified as an important contributor to cost in these previous studiesCitation21,Citation41 and has recently gained increased interest in the management of patients with COPD.Citation42,Citation43
In terms of indirect costs, few studies have considered the effects of comorbidities in patients with COPD.Citation18 It has been shown that although COPD patients have considerably higher indirect costs than lung-healthy controls, the effect of selected comorbid conditions on indirect costs does not necessarily differ between COPD patients and controls.Citation3 In their analysis of productivity losses in COPD patients, Erdal et al considered the number of comorbid conditions instead of single conditions, and reported that the number of days with lost productivity increased by five per year per additional comorbidity added,Citation44 which is in line with our finding that a high number of comorbidities shows a better association with indirect costs than single comorbid conditions.
The difficulty of these comparisons is that all studies considered different comorbidities with varying definitions, and often used other populations as reference. When considering all listed 33 comorbid conditions irrespective of their frequency in our analysis, multiple sclerosis (+425%) and Parkinson’s disease (+243%) were by far the most expensive comorbid conditions. However, these rather rare conditions are not necessarily representative for patients with COPD, and so for our analysis, we focused on common comorbidities in COPD. When considering the most prevalent comorbidities only (lifetime prevalence ≥10%, ), the effect size and statistical significance of the single conditions remained approximately the same compared to models considering all 33 comorbidities.
As to symptoms, previous studies have investigated their effect on health care utilization and costs, but studies considering cough and sputum production simultaneously or focusing on work absence or indirect costs are rare. Dyspnea, as the cardinal symptom of COPD, has been confirmed in numerous studies as a major cause of the disability associated with the disease, and as a main factor for reduced health-related quality of life or health utility of patients.Citation45 Our analysis identified dyspnea as a major driver of health care utilization, especially of inpatient services, and indirect costs, whereas cough and sputum did not have any effects on either direct or indirect costs. Even if the cut-off points for the definition of cough and sputum production were increased within a sensitivity analysis from 3 to 4 in the CAT, there were no significant associations of cough or sputum with direct or indirect costs.
A previous study involving patients in Japan with COPD reported 52% increased COPD management costs in those with mMRC ≥2, similar to the results we observed.Citation25 Furthermore, Punekar et al have described sharply increasing health care costs with increasing dyspnea levels.Citation46 However, these studies did not analyze the impact of cough or sputum on costs, although cough has been associated with worse health-related quality of life as well.Citation47 Ding et al reported associations between a higher symptom burden (as defined by an increasing CAT score) and productivity loss in a pooled sample of patients consulting for routine care in Europe, the USA, and the People’s Republic of China, but this study does not allow to disentangle the effects of the different aspects of the CAT.Citation23 Although a difference in indirect cost of approximately €8,700 per year between the groups with low vs high levels of dyspnea was observed in our study when considering potential interactions, this difference did not reach statistical significance. This could be due to a low sample size of the indirect costs analysis or high variability within this group.
Finally, we found a significant association between former smoking and direct costs, whereas current smoking was not associated with higher costs. One could speculate that arising or worsening health problems may be the reason for smoking cessation and therefore the reason for higher costs than in never smokers or even current smokers. This phenomenon has been observed previously,Citation48 and needs further investigation in longitudinal studies.
In comparison with previous studies, the strength of this study is that we included simultaneously a number of medically important comorbidities and symptoms as potential drivers of direct and indirect costs among patients with COPD, and put these aspects into context with the degree of airflow limitation. Furthermore, this study included patients with mild as well as severe or very severe airflow limitation, so that all GOLD stages were represented in an adequate frequency.
However, several limitations of our analysis have to be mentioned. First, central data are based on self-reported information by study participants, such as the presence of physician-diagnosed comorbidities (“Has a physician ever diagnosed you with …?”) and information on health care utilization and work absence due to illness. Comorbidity prevalence estimates may differ if, for example, medication data are used for the verification of comorbidities.Citation49 Furthermore, no information on the severity of comorbid conditions was available. To minimize the problem of recall bias with regard to health care utilization, we used different recall periods for various health services which is an established method of collecting data on combined primary and secondary health care usage, and is one of the few ways of collecting indirect cost data.Citation50 This approach for cost data collection has been shown to be valid.Citation51 Second, medical aids, nursing, and alternative practitioners, together with over-the-counter and non-pharmacy medicines, were excluded from our analyses. Consequently, the economic cost burden of COPD could be underestimated.
Explicitly, we did not include exacerbation history as a possible cost driver in our analysis, because both costs data and exacerbation history were assessed retrospectively for the same time period and exacerbations and their severity levels are defined by health care utilization (eg, a severe exacerbation is defined by hospitalization). Therefore, our cost calculations include to a certain amount the costs of exacerbations, and controlling for exacerbations in regression analyses would introduce circularity problems within the models and reduce their validity.
Furthermore, the design of this study cannot be used to investigate if COPD-related costs are increased by comorbid conditions and whether these conditions have the same cost-driving effects in individuals with or without COPD. When focusing on costs for medications coded with ATC “R” (respiratory system) as a proxy for COPD-related costs in an additional analysis, higher GOLD stages, asthma, sleep apnea, and dyspnea were identified as cost drivers. Other comorbid conditions did not show significant associations with this cost category, suggesting that costs of comorbidities may be comparable in individuals with or without COPD as previously suggested.Citation3 However, especially in the case of COPD which is recognized as a systemic disease with extra-pulmonary manifestations, it is challenging to disentangle disease-related costs from overall health care costs.
Nevertheless, our analysis illustrated the high prevalence of comorbidities among COPD patients, and their negative effects on the cost burden of COPD. This emphasizes the importance of a patient-centered, multidisciplinary collaboration in COPD management based on a broad range of interventions,Citation52 and of further research to establish a holistic approach to prevent, treat, and reduce coexisting comorbidities and symptoms of COPD.Citation10
Finally, our findings may be useful in decision-analytic models for cost-effectiveness analyses of COPD interventions. While older cost-effectiveness models focused on lung-function only, more contemporary models contain comorbidities and symptoms in the characterization of the disease.Citation53–Citation55 However, these models are in need of evidence on the relationship between costs and comorbid conditions or symptoms.
Conclusion
This study represents a comprehensive economic analysis of COPD in Germany, which includes patients with a high number of comorbidities and symptoms, clearly demonstrating their impact on direct and indirect costs. From a public health perspective, it is important to consider not only the management of COPD itself but also the identified primary cost drivers, and in particular, to reduce the burden due to comorbidities and dyspnea, which are also relevant from the patients’ perspective.
Acknowledgments
This work was supported by the Competence Network Asthma and COPD (ASCONET). The COSYCONET COPD cohort is funded by the German Federal Ministry of Education and Research (BMBF) with grant numbers 01GI0881 and 01GI0882 and by unrestricted grants from various pharmaceutical companies. The authors would like to thank all study centers which contributed in patient recruitment and data collection/capture as listed on http://www.asconet.net/html/cosyconet/studzent. Finally, they thank David Young of Young Medical Communications and Consulting Ltd for his critical review of the manuscript.
Supplementary material
Table S1 German unit costs
Reference
- BockJOBrettschneiderCSeidlHCalculation of standardised unit costs from a societal perspective for health economic evaluationGesundheitswesen20157715361 German25025287
Disclosure
CV reports personal fees from the pharmaceutical companies Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Janssen, Mundipharma, Novartis, Takeda, and Cipla, and grants and personal fees from Grifols outside the submitted work. The other authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (update 2017)2017 Available from: http://www.goldcopd.org/Accessed February 20, 2017
- MennPHeinrichJHuberRMKORA Study GroupDirect medical costs of COPD – an excess cost approach based on two population-based studiesRespir Med2012106454054822100535
- WackerMEJörresRASchulzHCOSYCONET-ConsortiumDirect and indirect costs of COPD and its comorbidities: results from the German COSYCONET studyRespir Med2016111394626725462
- FordESMurphyLBKhavjouOGilesWHHoltJBCroftJBTotal and state-specific medical and absenteeism costs of COPD among adults aged ≥18 years in the United States for 2010 and projections through 2020Chest20151471314525058738
- JanssonSABackmanHStenlingALindbergARönmarkELundbackBHealth economic costs of COPD in Sweden by disease severity – has it changed during a ten years period?Respir Med2013107121931193823910072
- European Respiratory SocietyThe European Lung White Book2nd revised edSheffieldEuropean Respiratory Society2013
- GershonASGuanJVictorJCGoldsteinRToTQuantifying health services use for chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187659660123328526
- McLeanSHoogendoornMHoogenveenRTProjecting the COPD population and costs in England and Scotland: 2011 to 2030Sci Rep201663189327583987
- VoglMLeidlRInforming management on the future structure of hospital care: an extrapolation of trends in demand and costs in lung diseasesEur J Health Econ201617450551726032899
- HillasGPerlikosFTsiligianniITzanakisNManaging comorbidities in COPDInt J Chron Obstruct Pulmon Dis2015109510925609943
- HuberMBWackerMEVogelmeierCFLeidlRComorbid influences on generic health-related quality of life in COPD: a systematic reviewPLoS One2015107e013267026168154
- WackerMEJörresRAKarchACOSYCONET study groupRelative impact of COPD and comorbidities on generic health-related quality of life: a pooled analysis of the COSYCONET patient cohort and control subjects from the KORA and SHIP studiesRespir Res20161718127405652
- DivoMCoteCde TorresJPBODE Collaborative GroupComorbidities and risk of mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012186215516122561964
- MillerJEdwardsLDAgustíAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsComorbidity, systemic inflammation and outcomes in the ECLIPSE cohortRespir Med201310791376138423791463
- GershonASMecredyGCGuanJVictorJCGoldsteinRToTQuantifying comorbidity in individuals with COPD: a population studyEur Respir J2015451515925142481
- JanssonSABackmanHRonmarkELundbäckBLindbergAHospitalization due to co-morbid conditions is the main cost driver among subjects with COPD-a report from the population-based OLIN COPD studyCOPD201512438138925415366
- WestneyGForemanMGXuJHenriques KingMFlenaughERustGImpact of comorbidities among Medicaid enrollees with chronic obstructive pulmonary disease, United States, 2009Prev Chronic Dis201714E3128409741
- HuberMBWackerMEVogelmeierCFLeidlRExcess costs of comorbidities in chronic obstructive pulmonary disease: a systematic reviewPLoS One2015104e012329225875204
- Simon-TuvalTScharfSMMaimonNBernhard-ScharfBJReuveniHTarasiukADeterminants of elevated healthcare utilization in patients with COPDRespir Res201112721232087
- SchwarzkopfLWackerMErtlJHapfelmeierJLarischKLeidlRImpact of chronic ischemic heart disease on the health care costs of COPD patients – an analysis of German claims dataRespir Med201611811211827578479
- SchwabPDhamaneADHopsonSDImpact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare populationInt J Chron Obstruct Pulmon Dis20171273574428260880
- DenizSŞengülAAydemirYÇeldir EmreJÖzhanMHClinical factors and comorbidities affecting the cost of hospital-treated COPDInt J Chron Obstruct Pulmon Dis2016113023303027980399
- DingBSmallMBergströmGHolmgrenUCOPD symptom burden: impact on health care resource utilization, and work and activity impairmentInt J Chron Obstruct Pulmon Dis20171267768928260874
- FooJLandisSHMaskellJContinuing to confront COPD International Patient Survey: economic impact of COPD in 12 countriesPLoS One2016114e015261827092775
- SmallMHolbrookTWoodRMullerovaHNayaIPunekarYSPrevalence and burden of dyspnoea among COPD patients in JapanInt J Clin Pract201670867668127396989
- KarchAVogelmeierCWelteTCOSYCONET Study GroupThe German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baselineRespir Med2016114273727109808
- JörresRAWelteTBalsRKochASchnoorMVogelmeierCEinfluss systemischer Manifestationen und Komorbiditäten auf den klinischen Zustand und den Verlauf bei COPD [Systemic manifestations and comorbidities in patients with chronic obstructive pulmonary disease (COPD) and their effect on clinical state and course of the disease – an overview of the cohort study COSYCONET]Dtsch Med Wochenschr201013510446449 German20198540
- QuanjerPHStanojevicSColeTJERS Global Lung Function InitiativeMulti-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equationsEur Respir J20124061324134322743675
- KarlFMHolleRBalsRCOSYCONET Study GroupCosts and health-related quality of life in Alpha-1-Antitrypsin Deficient COPD patientsRespir Res20171816028416015
- BockJOBrettschneiderCSeidlHCalculation of standardised unit costs from a societal perspective for health economic evaluationGesundheitswesen20157715361 German25025287
- WHO Collaborating Centre for Drug Statistics MethodologyGuidelines for ATC Classification and DDD Assignment 2010OsloWHOCC2009
- Wissenschaftliches Institut der AOK (WIdO) Available from: http://wido.de/arzneimittel.htmlAccessed May 20, 2011 German
- Federal Statistical OfficeStatistical Yearbook 2013 for the Federal Republic of GermanyWiesbadenFederal Statistical Office2014
- Institute for Employment Research (IAB) of the German Federal Employment Agency (BA)IAB brief reports no. 18NurembergIAB2014
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- PutchaNPuhanMADrummondMBA simplified score to quantify comorbidity in COPDPLoS One2014912e11443825514500
- BolandMRKruisALTsiachristasACost-effectiveness of integrated COPD care: the RECODE cluster randomised trialBMJ Open2015510e007284
- BolandMRTsiachristasAKruisALChavannesNHRutten-van MolkenMPThe health economic impact of disease management programs for COPD: a systematic literature review and meta-analysisBMC Pulm Med2013134023819836
- McPhailSMMultimorbidity in chronic disease: impact on health care resources and costsRisk Manag Healthc Policy2016914315627462182
- WackerMEJörresRAKarchAAssessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbiditiesBMC Pulm Med20161617027160582
- ManninoDMHiguchiKYuTCEconomic burden of COPD in the presence of comorbiditiesChest2015148113815025675282
- InoueDWatanabeROkazakiRCOPD and osteoporosis: links, risks, and treatment challengesInt J Chron Obstruct Pulmon Dis20161163764827099481
- OkazakiRWatanabeRInoueDOsteoporosis associated with chronic obstructive pulmonary diseaseJ Bone Metab201623311112027622174
- ErdalMJohannessenAAskildsenJEEaganTGulsvikAGrøonsethRProductivity losses in chronic obstructive pulmonary disease: a population-based surveyBMJ Open Respir Res201411e000049
- MiravitllesMHuertaAValleMClinical variables impacting on the estimation of utilities in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20151036737725733826
- PunekarYSWurstKShuklaAResource use and costs up to two years post diagnosis among newly diagnosed COPD patients in the UK primary care setting: a retrospective cohort studyCOPD201512326727525093809
- DesleeGBurgelPREscamillaRImpact of current cough on health-related quality of life in patients with COPDInt J Chron Obstruct Pulmon Dis2016112091209727695305
- WackerMHolleRHeinrichJThe association of smoking status with healthcare utilisation, productivity loss and resulting costs: results from the population-based KORA F4 studyBMC Health Serv Res20131327823866993
- LuckeTHerreraRWackerMCOSYCONET-ConsortiumSystematic analysis of self-reported comorbidities in large cohort studies – a novel stepwise approach by evaluation of medicationPLoS One20161110e016340827792735
- EvansCCrawfordBPatient self-reports in pharmacoeconomic studies. Their use and impact on study validityPharmacoeconomics199915324125610537432
- SeidlHMeisingerCWendeRHolleREmpirical analysis shows reduced cost data collection may be an efficient method in economic clinical trialsBMC Health Serv Res20121231822978572
- VanfleterenLESpruitMAWoutersEFFranssenFMManagement of chronic obstructive pulmonary disease beyond the lungsLancet Respir Med201641191192427264777
- BriggsABakerTRisebroughNADevelopment of the galaxy chronic obstructive pulmonary disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort modelMed Decis Making201737446948027317436
- HoogendoornMFeenstraTLAsukaiYPatient heterogeneity in health economic decision models for chronic obstructive pulmonary disease: are current models suitable to evaluate personalized medicine?Value Health201619680081027712708
- AsukaiYBaldwinMFonsecaTGrayAMungapenLPriceDImproving clinical reality in chronic obstructive pulmonary disease economic modelling: development and validation of a micro-simulation approachPharmacoeconomics201331215116123329431
