Abstract
Background
Individuals with severe alpha-1 antitrypsin deficiency (AATD) have an increased risk of developing COPD. However, outcomes during long-term oxygen therapy (LTOT) in patients with severe AATD and hypoxemia are unknown.
Patients and methods
This was a prospective, population-based, consecutive cohort study of patients on LTOT due to COPD in the period from January 1, 1987, to June 30, 2015, in the Swedish National Registry for Respiratory Failure (Swedevox). Severe AATD was identified using the Swedish AATD registry and confirmed by isoelectric focusing. Data on lung transplantation (LTx) were obtained from the two lung transplantation centers in Sweden. Mortality and causes of death were assessed based on the National Causes of Death Registry and analyzed using multivariable Cox regression.
Results
A total of 14,644 patients who started LTOT due to COPD were included in this study. No patient was lost to follow up. Patients with AATD were younger, included more males and more never smokers, and had fewer comorbidities. During a median follow-up of 1.6 years (interquartile range [IQR], 2.7) on LTOT, patients without severe AATD had a higher mortality, hazard ratio [HR] 1.53 (95% CI, 1.24–1.88), adjusting for age, sex, smoking status, body mass index, performance status, level of hypoxemia, and comorbidities. Cardiovascular deaths were increased. A higher proportion of AATD patients underwent LTx, 53 (19%) vs 118 (1%). Survival after LTx was similar for AATD and non-AATD patients and was predicted by age.
Conclusion
In oxygen-dependent COPD, patients with severe AATD have a longer survival time on LTOT, but they have a similar prognosis after lung transplantation compared with patients without AATD.
Introduction
Severe alpha-1 antitrypsin deficiency (AATD) is a common genetic condition, defined by the presence of the PiZZ phenotype that predisposes to COPD. PiZZ is present in 1%–2% of COPD patients.Citation1 The treatment of COPD patients due to severe AATD includes the usual COPD therapies, such as vaccinations, bronchodilators, rehabilitation, and surgical interventions in selected patients, and long-term oxygen therapy (LTOT) in patients with severe chronic hypoxemia.Citation2 For patients with severe AATD, alpha-1 antitrypsin augmentation therapy is not generally recommended in Sweden.
Patients with severe AATD have a shorter survival time than the general population matched for smoking habits, with increased mortality in particular due to hepatobiliary disease and early-onset emphysema, especially in smokers.Citation3 After lung transplantation (LTx), AATD patients have a longer survival time compared with COPD patients without AATD.Citation4,Citation5
Knowledge is limited on whether the prognosis after lung transplantation differs in oxygen-dependent patients with regard to their AATD status.Citation5 Ringbaek et alCitation5 reported that patients on LTOT with AATD (n=234) were younger, had fewer comorbid diagnoses, were more likely to have undergone lung transplantation, and had a longer overall survival time compared with patients without AATD.
Sweden offers the unique opportunity to study survival with LTOT in relation to severe AATD by cross-linkage between the national prospective registries of LTOT and AATD with national data on vital status with near-complete follow-up.
The aim of the current study was to evaluate whether overall survival after lung transplantation differs between the oxygen-dependent COPD patients with severe AATD compared with those without.
Patients and methods
Study design and population
This was a prospective, population-based, consecutive cohort study of patients on LTOT due to COPD between January 1, 1987, and June 30, 2015, in the Swedish National Registry for Respiratory Failure (Swedevox). Swedevox covers approximately 85% of all adults on LTOT in Sweden since January 1, 1987, as detailed elsewhere.Citation6 The Swedish indications for LTOT are in line with international guidelines.Citation7–Citation9 We excluded patients who had undergone lung transplantation before starting LTOT.
Data collection
Data were obtained from Swedevox on age, sex, smoking status (never, former, or active smoking), physician-diagnosed disease that was the main reason for initiating LTOT, body mass index (BMI), blood gases, and the World Health Organization (WHO) performance status (a 5-point scale between 0 “fully active” and 4 “completely bedridden”)Citation10 at the initiation of LTOT (baseline).
AATD was defined as the presence of the PiZZ phenotype, confirmed by isoelectric focusing at the Department of Clinical Chemistry (DCC), Malmö, which is the central laboratory for all Pi phenotyping in Sweden since 1980. The results for all individuals with the PiZZ phenotype are stored and provided to the Swedish AATD registry that was started in 1991.Citation11
Data on lung transplantations were obtained from the two centers that perform all lung transplantations in Sweden, located at the university hospitals in Lund and Gothenburg as previously described.Citation4,Citation12
Data on comorbidities and hospitalizations during the 5 years prior to the initiation of LTOT were obtained from the Swedish National Patient Register (SNPR) and the National Cancer Registry. The SNPR covers more than 99% of all hospitalizations since 1987 and about 80% of all hospital-based outpatient care since 2001 nationwide.Citation13 Diagnoses were coded according to the 9th (before 1996) and the 10th revisions of the WHO International Classification of Disease (ICD). ICD codes were grouped (ICD-9; ICD-10) as cardio-vascular disease (CVD; 390–459×; I00–I99), ischemic heart disease (410–414; I20–I25), heart failure (425, 428; I42, I50, I51.7), cerebrovascular diseases (430–438; I60–I69), venous thromboembolism (415, 451; I26, I80), digestive organ diseases (520–579×, 787; K00–K93), lung cancer (162; C34), and other cancers (140–239×; C00–D48). Vital status was obtained from the National Causes of Death Register. Registers were cross-linked using Swedish system of personal identity numbers.
Ethical considerations
The study was approved by the research ethics committee of Lund University (157/2007, 350/2008, 2014/427, and 2015/186). According to Swedish research regulations for registry-based research, individual patient consent was not required.
Statistical methods
Baseline data were tabulated using frequencies and percentages for categorical variables, mean with SD, and median with range or interquartile range (IQR) for continuous variables with normal and skewed distribution, respectively. Continuous variables with normal distribution were compared using ANOVA.
Cumulative crude survival probabilities were estimated using the Kaplan–Meier method. Differences in crude survival were calculated using the log-rank test. The 1-, 3-, 5-year survival rates were calculated using life tables. The association between AATD status and adjusted survival was analyzed using multivariable Cox regression and was expressed as hazard ratio (HR) with 95% CI. For the analysis of survival of patients on LTOT, all participants were prospectively followed from the initiation of LTOT to the first of the following events, LTOT withdrawal, lung transplantation, death, or study end (June 30, 2015). The model was adjusted for baseline age, sex, smoking status, BMI (>20 vs ≤20), WHO performance status (2–4 vs 0–1), tension of arterial oxygen (PaO2), and the presence of any of the following comorbidities during the 5 years before baseline: CVD, cancer, digestive disease, and diabetes. For survival after lung transplantation, the time period for the Cox analysis was from the date of transplantation to death or study end, and the model was adjusted for age at transplantation, sex, BMI, 1-min walk test (per 1 m), type of lung transplant (single vs bilateral), time between LTOT start and LTx and comorbidities at baseline (CVD, digestive disease, and diabetes).
Statistical significance was defined as a two-sided P-value of <0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Characteristics of participants
Up to June 30, 2015, 14,658 patients who started LTOT due to COPD were registered in Swedevox. After exclusion of patients who had undergone lung transplantation before starting LTOT (n=14; five with severe AATD), a total of 14,644 patients were included in the analysis. The median follow-up time was 1.6 (IQR, 2.7) years for the whole cohort and was significantly longer in AATD patients (2.4 years; IQR, 4.2) than in non-AATD patients (1.6 years; IQR, 2.7).
Characteristics of the study population by AATD status are provided in . Among the patients on LTOT, 1.9% had severe AATD. Compared with the COPD patients without AATD, the patients with severe AATD were younger, included a higher proportion of men and never smokers, and had a lower FEV1 (% predicted). AATD patients also had a lower PaCO2 on both air and oxygen, with similar PaO2, resulting in a higher alveolar–arterial pressure gradient of oxygen (PA–a).
Table 1 Characteristics of 14,644 patients on LTOT for COPD
Patients with severe AATD had less comorbidity at the time of starting LTOT, namely a lower prevalence of ischemic heart disease, heart failure, hypertension, cancer, depression, and diabetes mellitus (). A higher proportion of AATD patients underwent lung transplantation, 53 (19%) vs 118 (1%).
Table 2 Prevalence of comorbidities in patients on LTOT for COPD
Survival on LTOT
There were 201 (71%) deaths among the patients with AATD and 12,354 (86%) deaths among the patients without AATD during LTOT. The median survival time was 1.9 years (95% CI, 1.8−1.9) overall, and significantly longer in patients with AATD (3.5 years; 95% CI, 2.8−4.3) than in patients without AATD (1.9 years; 95% CI, 1.8−1.9; P<0.001; ). The 1-, 3-, and 5-year survival rates were 0.84 (95% CI, 0.80−0.88), 0.55 (95% CI, 0.52−0.61), and 0.36 (95% CI, 0.31−0.44) for patients with AATD compared with 0.67 (95% CI, 0.67−0.68), 0.48 (95% CI, 0.46−0.48), and 0.17 (95% CI, 0.16−0.17) for patients without AATD, respectively.
Figure 1 Survival in relation to the presence of severe AATD in patients on LTOT.
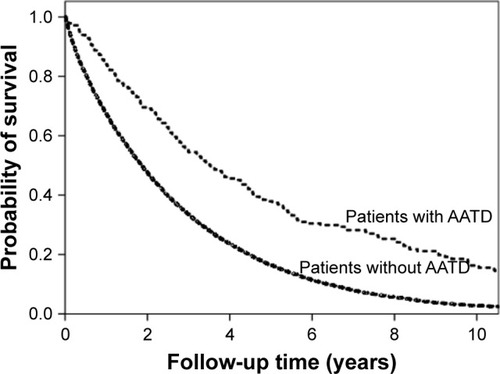
In multivariate Cox regression, patients without AATD had an increased risk of all-cause mortality, HR 1.53 (95% CI, 1.24–1.88; P<0.001), compared with patients with severe AATD (). Earlier death was also independently pre-dicted by higher age, male sex, BMI, worse WHO performance status, lower PaO2 (air), and the presence of CVD, cancer, digestive disease, or diabetes mellitus at the time of starting LTOT. Patients without AATD had more deaths from CVD (16% vs 6%; P<0.001; ).
Table 3 Multivariate Cox regression of survival in COPD patients on LTOT
Table 4 Underlying cause of death in 12,555 patients with oxygen-dependent COPD
Survival after lung transplantation
During the follow-up, 171 patients underwent LTx. Of these, 53 (31%) had severe AATD. The median time from the start of LTOT to LTx was 1.5 (IQR, 2.1) years. Of the transplanted patients, 69% were women and 96% were ex-smokers (). During the follow-up of a median of 4.4 (IQR, 6) years after LTx, 103 patients died, of whom 36 (35%) had severe AATD. The median survival time after LTx for all patients was 6.3 years (95% CI, 4.2–8.3), with no significant difference between patients with severe AATD (7.2 years; 95% CI, 3.1–11.3) and patients without AATD (6.1 years; 95% CI, 3.6–8.4; P=0.9).
Table 5 Demographic data of COPD patients on LTOT who underwent lung transplantation
The risk of death was not increased in the AATD patients compared with the non-AATD patients, HR 0.98 (95% CI, 0.60–1.60; P=0.61), after adjustment for age at transplantation, sex, BMI, 6-min walk test, single LTx vs bilateral LTx, time between LTOT start and LTx, and comorbidity (CVD, digestive disease, cancer, diabetes). The only statistically significant predictor of earlier death after LTx was higher age ().
Table 6 Multivariate Cox regression of mortality after lung transplantation in patients on LTOT
Discussion
Main findings
Of the patients with oxygen-dependent COPD, 1.9% had severe AATD. Patients with AATD were younger, had less comorbidity, and were more likely to undergo lung transplantation compared with those without AATD. Patients with severe AATD had a lower mortality rate during LTOT, especially death due to CVDs, but a similar prognosis after lung transplantation.
What this study adds?
These findings confirm those of Ringbaek et alCitation5 that patients with AATD were younger and had fewer comorbidities (CVD and diabetes mellitus), a higher rate of LTx, and a longer survival compared with other COPD patients on LTOT. In addition, in the current study, AATD patients also had lower FEV1% predicted and less diagnosed depression.Citation5 The prevalence of AATD in patients on LTOT for COPD was somewhat higher in the current study compared with that reported in the study by Ringbaek et alCitation5 (1.9% vs 1.1%), as severe AATD was identified using national data on all known PiZZ phenotypes from the laboratory performing all tests in Sweden. The prevalence is similar to the estimated prevalence of AATD across all stages of COPD.Citation1
In comparison with the non-AATD patients, we found that AATD patients had lower PaCO2 on both air and oxygen but similar PaO2, resulting in an increased PA–a oxygen gradient. This finding indicates that AATD patients have more impaired diffusion capacity than the non-AATD patients, possibly because of more severe emphysema. This complements the findings of the Long-Term Oxygen Therapy Trial (LOTT) that patients with AATD (n=11) desaturated more during a walking test, which is likely to be related to a more impaired diffusion capacity.Citation14
The current study provides novel data on survival after LTx in relation to PiZZ status as well as sex in oxygen-dependent COPD. Most studies to date have reported a 20%–40% higher mortality rate in men than in women on LTOT, but the reasons are unknown.Citation15 The sex difference in mortality in the current analysis (28% higher in men than in women) was attenuated and became nonsignificant after LTx. One explanation could be that comorbidities are contraindications for LTx, suggesting that comorbidities are an important contributing factor to the sex difference in survival on LTOT.
Mechanism
Patients with AATD, due to their higher susceptibility to develop lung impairment, are younger and have smoked less when they develop hypoxemic COPD compared with patients without AATD. Along the same line, they have fewer diagnosed and silent comorbidities, and thus more AATD patients are eligible for LTx and their survival is longer on LTOT. However, in contrast to our previously published report,Citation4 we did not find any significant difference in survival after LTx between the AATD and the non-AATD patients. This may be explained by the fact that the analysis included only patients on LTOT and that the number of patients was lower, reducing the statistical power of the analyses.
Alpha-1 antitrypsin augmentation therapy is not generally recommended in Sweden. Since 2007, the treatment is licensed in Sweden, but it is not reimbursed by the health care system.
Strengths and limitations
The strengths of the current study include the fact that it was population based, covering 85%–90% of all patients on LTOT in Sweden, with prospective follow-up of all lung transplantations and deaths nationwide. No patient was lost to follow up. The PiZZ phenotype in all AATD patients was confirmed by isoelectric focusing.
Limitations are that only smoking status and no data on cumulative smoking exposure were available for the non-AATD patients, except for those patients who had undergone lung transplantation. Pi phenotyping was available in all AATD patients but not in all non-AATD patients. However, the high prevalence of the PiZZ phenotype among the study population (1.9%) indicated that AAT has been analyzed in most patients with LTOT in Sweden.
Implications
For the clinician, AATD status may be an aid in determining a patient’s prognosis. Similar survival after LTx irrespective of the presence of AATD supports appropriate evaluation and selection of patients to be transplanted in oxygen-dependent COPD. Moreover, these data suggest that chest physician should be more observant of desaturation in patients with severe AATD. Regular follow-up of saturation and blood gases to detect the development of chronic hypoxemia is advisable. Further studies are needed on the effect of AATD on quality of life, exacerbation, and survival with more details on physiological data including diffusing capacity and exercise test.
Conclusion
In oxygen-dependent COPD, patients with severe AATD have a longer survival time but a similar prognosis after lung transplantation compared with non-AATD patients.
Acknowledgments
We thank Associate Professors Kerstin Ström and Eeva Piitulainen, founders of the Swedevox and AATD registers, for being a constant source of inspiration. We also thank all the Swedish physicians and nurses who report data to these registers. ME was supported by unrestricted grants from the Swedish Society of Medicine and the Swedish Heart-Lung Foundation, and HT was supported by unrestricted grants from the Skåne University Hospital and the Swedish Heart-Lung Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- LuisettiMSeersholmNα(1)-antitrypsin deficiency 1: epidemiology of α(1)-antitrypsin deficiencyThorax200459216416914760160
- VogelmeierCFCrinerGJMartinezFJGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive SummaryAm J Respir Crit Care Med2017195555758228128970
- TanashHEkströmMLindbergARönmarkEPiitulainenESurvival in individuals with severe alpha 1-antitrypsin deficiency (PiZZ) in comparison with a general population with known smoking habitsEur Respir J2017503 pii:1700198
- TanashHARiiseGCEkstromMPHanssonLPiitulainenESurvival benefit of lung transplantation for chronic obstructive pulmonary disease in SwedenAnn Thorac Surg20149861930193525443001
- RingbaekTJSeersholmNPerchMIversenMLangePPrognosis of patients with alpha1-antitrypsine deficiency on long-term oxygen therapyRespir Med201410881189119424906692
- GustafsonTLöfdahlKStrömKA model of quality assessment in patients on long-term oxygen therapyRespir Med2009103220921518980837
- HardingeMAnnandaleJBourneSBritish Thoracic Society guidelines for home oxygen use in adultsThorax201570suppl 1i1i4325870317
- Medical Research Council Working PartyLong term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysemaLancet1981182226816866110912
- Nocturnal Oxygen Therapy Trial GroupContinuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trialAnn Intern Med19809333913986776858
- OkenMMCreechRHTormeyDCToxicity and response criteria of the Eastern Cooperative Oncology GroupAm J Clin Oncol1982566496557165009
- PiitulainenETanashHAThe clinical profile of subjects included in the Swedish National Register on individuals with severe alpha 1-antitrypsin deficiencyCOPD201512suppl 1364125938290
- TanashHARiiseGCHanssonLNilssonPMPiitulainenESurvival benefit of lung transplantation in individuals with severe alpha1-anti-trypsin deficiency (PiZZ) and emphysemaJ Heart Lung Transplant201130121342134721821433
- LudvigssonJFAnderssonEEkbomAExternal review and validation of the Swedish national inpatient registerBMC Public Health20111145021658213
- StollerJKAboussouanLSKannerRELOTT Research GroupCharacteristics of alpha-1 antitrypsin-deficient individuals in the long-term oxygen treatment trial and comparison with other subjects with chronic obstructive pulmonary diseaseAnn Am Thorac Soc201512121796180426653189
- EkstromMPJogreusCStromKEComorbidity and sex-related differences in mortality in oxygen-dependent chronic obstructive pulmonary diseasePLoS One201274e3580622563405
