Abstract
Background
Ideally, treatment recommendations for maintenance therapy-naïve patients with COPD should be based on studies conducted specifically in this population. We have reviewed evidence from previous studies of pharmacological treatments in maintenance therapy-naïve patients with COPD and performed a new post-hoc analysis of dual bronchodilator treatment in this population, aiming to assess the effectiveness of these interventions.
Materials and methods
A literature review identified clinical trials that included analyses of patients with COPD who were maintenance therapy-naïve with long-acting β2-agonists (LABA) or long-acting muscarinic antagonists (LAMA). Additionally, a post-hoc subgroup analysis was conducted for maintenance therapy-naïve patients with COPD in two large phase III, randomized, double-blind, 24-week trials investigating the efficacy of aclidinium bromide/formoterol fumarate (AB/FF) fixed-dose combination versus monotherapy or placebo (ACLIFORM [NCT1462942] and AUGMENT [NCT1437397]).
Results
Treatment-naïve patients with COPD often represent a population of patients at the earliest stage at which most patients seek treatment. Of nine relevant studies identified, all reported positive findings for efficacy of LABA, LAMA, or LABA/LAMA treatment in maintenance therapy-naïve populations. Improvements were observed in lung function, symptoms, and health status versus monotherapy or placebo. Post-hoc analysis of ACLIFORM and AUGMENT demonstrated that AB/FF was effective in improving lung function in patients who had received no prior maintenance therapy. AB/FF showed improvements in 1 hr post-dose FEV1, trough FEV1, and patient-reported outcomes versus placebo and monotherapies. Combined with reviews of previous studies in maintenance therapy-naïve patients, these findings suggest that earlier intervention with a dual bronchodilator maintenance therapy, such as AB/FF, may provide significantly greater benefits than LAMA or LABA mono-bronchodilator therapy as a first maintenance treatment for COPD.
Conclusion
These data show that therapeutic intervention is effective in treatment-naïve patients. Intervention with dual bronchodilator therapy as a first maintenance treatment for COPD may provide greater benefits than LAMA or LABA monotherapy.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation with persistent respiratory symptoms.Citation1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report places emphasis on the assessment of symptoms and exacerbation risk for newly diagnosed patients.Citation1 These parameters enable patients to be categorized using an ABCD assessment, with specific initial pharmacological treatment recommendations defined for each group.Citation1,Citation2
The evidence base for these initial treatment recommendations comes from randomized controlled trials (RCTs) that recruited patients already taking maintenance pharmacological treatment and included patients with more advanced disease. Whether the results of these clinical trials apply to newly diagnosed patients with COPD, who are not taking any treatment, is unclear.
RCTs of long-acting bronchodilators often allow patients who are using inhaled corticosteroids (ICS) currently to continue with these drugs. The concurrent use of ICS may influence the treatment effects observed.Citation3 Furthermore, the use of maintenance long-acting bronchodilator(s) before the study may result in “step-down” effects being observed after randomization. Recommendations for the initial pharmacological management of newly diagnosed patients with COPD should ideally be based on evidence from patients who were not taking any maintenance treatment before the trial, to avoid step-down effects and/or the confounding effects of ICS use.
This paper aims to evaluate evidence from RCTs and non-interventional observational studies concerning the efficacy of pharmacological treatments in maintenance-naïve patients with COPD. We review studies that enrolled maintenance-naïve patients, and also any post-hoc analyses of this subgroup in RCTs. We also present a new pooled post-hoc subgroup analysis of data from maintenance-naïve patients with COPD in the ACLIFORM and AUGMENT studies of aclidinium bromide (AB)/formoterol fumarate (FF) (AB/FF).Citation4,Citation5 The results of these two studies have been reported previously, demonstrating the efficacy of AB/FF in improving bronchodilation and symptoms versus monotherapy or placebo and also reducing exacerbations versus placebo.Citation4–Citation6 Here we analyze the same endpoints in the treatment-naïve patient subgroup, to assess the efficacy of AB/FF induced bronchodilation in this sub-population.
Materials And Methods
Search Strategy
To establish the existing evidence base, a literature review was performed to identify clinical studies that included analyses of maintenance therapy-naïve patients with COPD. Searches in PubMed were conducted for the terms “COPD” and
treatment-naïve/treatment naïve/naïve to maintenance therapy/naïve to maintenance treatment/maintenance-naïve/maintenance naïve/long-acting β2-agonist (LABA)-naïve/LABA naïve/long-acting muscarinic antagonist (LAMA)-naïve/LAMA naïve/prior treatment/prior therapy/inexperienced/first maintenance.
Further details of the search strategy are shown in Supplementary Figure 1.
Maintenance-naïve was generally defined as naïve to the following maintenance treatments: LABA, LAMA, ICS, or systemic corticosteroids and xanthines. Some studies also excluded short-acting muscarinic antagonists, β2-agonists plus steroids, bronchodilator combinations, β2-agonists plus anticholinergic/short-acting muscarinic antagonists and leukotriene antagonists in the months preceding the study. Exclusive use of short-acting β2-agonists (SABA) was generally permitted prior to study inclusion.
Post-Hoc Analysis Of Patients In ACLIFORM And AUGMENT
We performed a post-hoc subgroup analysis of maintenance therapy-naïve patients in two large phase III, randomized, double-blind, 24-week trials investigating the efficacy of AB/FF fixed-dose combination versus either monotherapy or placebo in patients with COPD (ACLIFORM [NCT1462942] and AUGMENT [NCT1437397]). The methods used in ACLIFORM and AUGMENT have been described previously.Citation4,Citation5 Briefly, patients with moderate-to-severe stable COPD were randomized to receive AB/FF 400/12 µg, AB/FF 400/6 µg, AB 400 µg, FF 12 µg, or placebo twice daily via a multidose dry powder inhaler (GenuairTM/Pressair® [the registered trademarks of the AstraZeneca group of companies; for use within the USA as Pressair® and Genuair™ within all other licensed territories]) for 24 weeks. In this pooled post-hoc analysis, treatment-naïve patients were defined as those patients who had not received prior maintenance therapy for COPD: i.e., any LABA, LAMA, ICS, or xanthines; short-acting bronchodilators were permitted. Patients enrolled in the studies were not specifically required to have an established duration of COPD. Prior and concomitant medication data were collected and defined at screening up to approximately 2 weeks prior to randomization, and during the run-in period, resulting in the collection of medication data for at least 4 weeks before the first dose of study medication. Of the two AB/FF arms, only the currently approved 400/12 µg doseCitation7 is included here. Both the studies included in this post-hoc analysis were conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation/Good Clinical Practice Guidelines, and local regulations. The regulatory authorities approved the protocols for each country and each center had an independent ethics committee.
ACLIFORM And AUGMENT Post-Hoc Statistical Analysis
Full statistical information for ACLIFORM and AUGMENT has been described previously.Citation4,Citation5 The post-hoc analysis presented here included patients who were naïve to maintenance therapy from the pooled intent-to-treat (ITT) population (excluding those allocated to AB/FF 400/6 µg). Changes from baseline at Week 24 were analyzed for the following endpoints: pre-dose (trough) and 1 hr morning post-dose forced expiratory volume in 1 second (FEV1; mL), Transition Dyspnea Index (TDI) focal score, and St George’s Respiratory Questionnaire (SGRQ) total score. Changes from baseline in daily symptoms, as measured using the Evaluating-Respiratory Symptoms (E-RS™ [the E-RS™ is owned by Evidera. Permission has been granted for this publication. Permission to use this instrument may be obtained from Evidera ([email protected])]; formerly known as EXAcerbations of Chronic pulmonary disease Tool – Respiratory Symptoms) total score,Citation8 and morning and nighttime symptoms were analyzed over 24 weeks using the Nighttime Symptoms of COPD InstrumentCitation9,Citation10 and Early Morning Symptoms of COPD Instrument tools.Citation11 All reported data are least squares (LS) mean changes from baseline with 95% confidence intervals (CI), based on the mixed model for repeated measures: treatment effects and treatment comparisons. LS mean differences between AB/FF 400/12 µg and treatment groups are also shown (Δ). For changes from baseline in trough and morning post-dose FEV1, TDI, SGRQ, E-RS, nighttime and early morning outcomes, analyses were adjusted using the main common predictors i.e. screening response (FEV1, only), baseline scores and age as covariates, and any of the following criteria as fixed effect factors: treatment group, study, sex, smoking status, visit, prior naïve COPD patients, and interactions of treatment group-by-visit, treatment group-by-prior naïve COPD patients, and treatment group-by-visit-by-prior naïve COPD patients. Minimal clinically important difference (MCID) for each outcome: SGRQ (≥4-unit change),Citation12 trough FEV1 (change of approximately 100 mL),Citation13 TDI focal score (increase of 1 unit),Citation14 and COPD Assessment Test (CAT) score (2-point reduction),Citation15 as previously published.
Results
Treatment In Maintenance Therapy-Naïve Patients
The literature search identified nine relevant studies that showed clinical outcomes in a COPD maintenance therapy-naïve patient population or with a COPD maintenance therapy-naïve sub-population (). Of the nine studies identified, two were dual bronchodilator RCTs, four were long-acting bronchodilator monotherapy RCTs, one was a real-world study of guidelines-based treatment, and two were non-interventional observational studies. All reported positive findings for the efficacy of LABA, LAMA, or LAMA/LABA treatment in maintenance therapy-naïve populations.Citation16–Citation24 Six of the nine studies investigated the lung function effects of bronchodilators in treatment-naïve patients,Citation16,Citation18,Citation20–Citation24 while all nine studies investigated symptoms and quality of life (QoL).Citation16–Citation24
Table 1 Studies Investigating The Efficacy Of Maintenance Treatments In Treatment-Naïve Patients With COPD
Long-Acting Bronchodilator Monotherapy Studies
Four RCTs evaluated lung function, exacerbations, symptoms, and quality of life outcomes for treatment-naïve patients receiving monotherapy with either tiotropiumCitation21,Citation22,Citation24 or indacaterol.Citation23 Tiotropium or indacaterol generally showed significant improvements in a range of lung function measurements versus placebo.Citation21,Citation23,Citation24 Compared with placebo, the trough FEV1 improvements for indacaterol 150 µg and 300 µg were 170 mL and 180 mL, respectively, (P<0.001) at 6 months ().Citation23 In two RCTs of tiotropium versus placebo, the trough FEV1 improvements were 140 mL at Week 24Citation21 and 134 mL after 4 years (both P<0.001; ).Citation24
Figure 1 Treatment differences in trough FEV1 for (A) monotherapies and (B) dual therapies among the relevant publications identified in the literature search.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second.
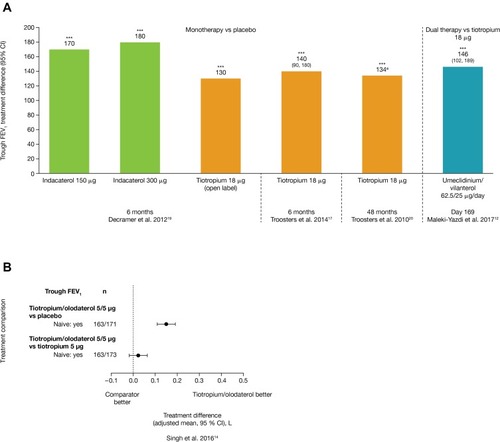
In treatment-naïve patients at 6 months, indacaterol 150 µg and 300 µg showed significant improvements (P<0.001 and P<0.05, respectively) versus placebo in symptoms and health status including SGRQ (treatment difference vs placebo for 150 µg and 300 µg of –6.1 and –2.5 units, respectively; P<0.001 and P<0.05, respectively; ), rescue use (–0.48 and –0.59 puffs/day; P<0.01), and TDI total score (1.27 and 1.04 points; P<0.001).Citation23 Tiotropium also significantly improved SGRQ versus placebo (treatment difference –4.57 units at 48 months; 95% CI –7.06 to –2.09) ().Citation24
Figure 2 Changes in SGRQ total score for (A) monotherapies and (B) dual therapies; (C) changes in TDI focal score for dual therapies, among the relevant publications identified in the literature search.
Abbreviations: CI, confidence interval; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
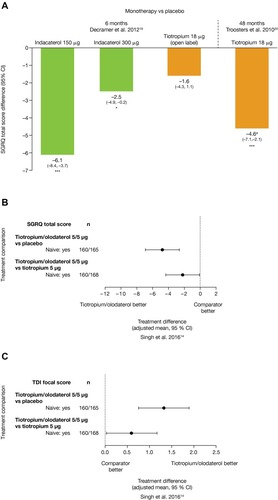
There is currently little information available on the exacerbation risk in treatment-naïve patients; however, in one of two analyses of maintenance therapy-naïve patients, tiotropium significantly increased time to first exacerbation (P<0.05), and reduced annual rates of exacerbations (P<0.05) versus salmeterol,Citation22 and the other analyses showed a numerically decreased exacerbation rate by 16% (P<0.08; non-significant) versus placebo.Citation24
One study evaluated a decline in lung function over 4 years and showed that tiotropium slowed annual decline compared with placebo (post-bronchodilator FEV1 tiotropium 42 mL/year vs placebo 53 mL/year; P<0.05).Citation24 There was also a slowed decline in the activity domain of SGRQ (difference 1.44±0.40 units; P<0.001; total score difference 4.6 units; P<0.001) with tiotropium versus placebo over 4 years.Citation24
Dual Bronchodilator Studies
Two studies presented dual bronchodilator outcomes for maintenance therapy-naïve patients as post-hoc analyses.Citation16,Citation18 Dual bronchodilation with LAMA/LABA combinations umeclidinium/vilanterolCitation16 or tiotropium/olodaterolCitation18 provided greater improvements in lung function and health status compared with monotherapy in treatment-naïve patients. Umeclidinium/vilanterol dual bronchodilation showed a significant 146 mL improvement (P<0.001) from baseline in trough FEV1 versus tiotropium monotherapy at Day 169 ().Citation16 Similarly, dual therapy with tiotropium/olodaterol showed improvements in both trough and post-dose FEV1 versus placebo and improvements for post-dose FEV1 versus tiotropium monotherapy, only. For trough FEV1, tiotropium/olodaterol versus tiotropium monotherapy showed 95% CIs crossing zero, indicating no significant difference between treatments ().Citation18
In terms of QoL, both umeclidinium/vilanterol and tiotropium/olodaterol improved total SGRQ scores from baseline over 12 or 24 weeks (≥4-unit decrease),Citation12 with both dual therapies resulting in greater improvements compared with tiotropium monotherapy (and also vs placebo for tiotropium/olodaterol).Citation16,Citation18 The effect size of improvement versus tiotropium monotherapy was approximately –2 units for both umeclidinium/vilanterolCitation16 and tiotropium/olodaterol (), less than the recognized MCID for SGRQ (≥4-unit change).Citation18
Symptom measures were reported in one study of tiotropium/olodaterol dual therapy, which showed that TDI focal score was improved versus tiotropium monotherapy with an effect size of approximately 0.5 units at 12 weeks ().Citation18 Umeclidinium/vilanterol showed significantly greater proportions (47%) of patients achieving an increase in clinically meaningful rescue-free periods (one extra rescue-free month per year or two extra rescue-free weeks out of 24) versus tiotropium monotherapy (37%; odds ratio [OR]: 1.5 [95% CI: 1.0–2.2]).Citation16
Non-Interventional Studies
There were three non-interventional, observational studies of bronchodilator therapy in treatment-naïve patients.Citation17,Citation19,Citation20 An open-label, multicenter, observational, real-world survey confirmed that initiation of Japanese guidelineCitation25-directed bronchodilator therapy in Japanese patients with untreated COPD resulted in improved lung function.Citation20 GOLD COPD stage I patients received single LAMA, GOLD stage II and III patients received LAMA plus one other of the following additional therapies: LABA, theophylline or ICS/LABA; GOLD stage IV patients received LAMA plus two of the additional therapies. There were also clinically important differences (2-point reduction)Citation15 in mean CAT scores, with improvements from 14.2 at Week 0 to 12.3 at Week 48 (P=0.022).Citation20
In a Nordic population of patients with COPD, aclidinium monotherapy in LAMA-naïve patients without concomitant maintenance therapy showed significant improvements from baseline in CAT total score (–3.8 mean change [95% CI –4.6 to –3.1]; P<0.05) and exceeded the MCID. There were also improvements from baseline in both morning and nighttime symptoms (both P<0.01).Citation17 For LAMA-naïve patients adding aclidinium to existing maintenance therapy, there were significant improvements from baseline for outcomes including CAT (–3.3; P<0.05), modified Medical Research Council Dyspnea Scale (–0.3), and morning and nighttime symptoms (all P<0.01).Citation17
An observational study in patients with COPD receiving dual therapy with tiotropium/olodaterol found that treatment-naïve patients had a higher therapeutic success rate (defined as a 10-point increase in the Physical Functioning Questionnaire between baseline and Weeks 4–6) than those with prior maintenance therapy (59.1% vs 44.5%; P<0.0001). These differences were driven by a higher response in treatment-naïve patients who were classified as GOLD B (59.8%) and C (63.0%); whereas proportions of patients achieving therapeutic success were similar for GOLD D patients, regardless of previous maintenance treatment history.Citation19
Efficacy Of AB/FF In Maintenance-Therapy-Naïve Patients: A Post-Hoc Analysis Of Patients In ACLIFORM And AUGMENT
Of 3421 patients in the pooled study population, 3394 were included in the pooled ITT population, and 1056 were naïve to maintenance therapy and included in this analysis (excluding those allocated to AB/FF 400/6 µg).
Patient demographics and baseline characteristics of treatment-naïve patients were similar across treatment groups (). Compared with treatment-exposed patients, treatment-naïve patients were younger, more likely to be current smokers, had higher baseline FEV1 and similar reversibility (). Treatment-naïve patients had moderately lower rates of prior year exacerbations, lower oxygen, and oral corticosteroid use, and lower E-RS scores at baseline. North American patients were more likely to be treatment-naïve (62.8% vs 38.9% non-naïve), whereas European patients were more likely to have received prior maintenance therapy (53.1% vs 31.2% naïve).
Table 2 Patient Demographics And Baseline Characteristics Of Patients Included In ACLIFORM And AUGMENT (Post-Hoc Analysis; ITT Population)
At Week 24, patients receiving AB/FF showed significant improvements from baseline in 1 hr post-dose FEV1 (), versus AB, FF, and placebo (LS mean difference 84 mL, 117 mL, and 284 mL, respectively; all P<0.001). AB/FF also showed significantly greater improvement from baseline in trough FEV1 versus FF (57 mL; P<0.01) and placebo (134 mL; P <0.001) (), although there was no significant difference between AB/FF and AB (14 mL; P=0.484). Patients receiving AB/FF also had significantly improved changes from baseline in TDI focal scores compared with AB (1.17; P<0.001), FF (0.92; P<0.01), and placebo (1.54; P<0.001) at Week 24 ().
Figure 3 Change from baseline in (A) 1 hr morning post-dose FEV1 and (B) trough FEV1 for treatment-naïve patients, at Week 24 of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; FEV1, forced expiratory volume in 1 sec; FF, formoterol fumarate; ITT, intent-to-treat; LS, least squares.
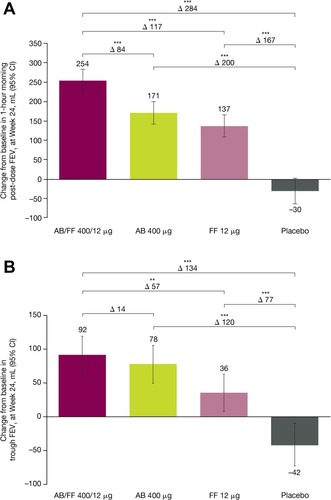
Figure 4 Patient-reported outcomes changes from baseline for treatment-naïve patients (A) TDI focal score at Week 24, (B) E-RS total score, (C) early morning COPD symptom severity and (D) nighttime COPD symptom severity over 24 weeks of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating-Respiratory Symptoms; FF, formoterol fumarate; LS, least squares; TDI, Transition Dyspnea Index.
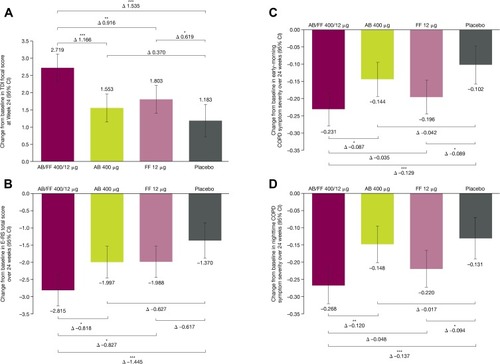
Change from baseline in E-RS total score was significantly greater with AB/FF compared with all other treatments over 24 weeks (–0.82 and –0.83 vs AB and FF, both P<0.05; –1.45 vs placebo, P<0.001; ). Overall early morning symptom severity was significantly improved from baseline with AB/FF compared with AB (–0.09; P<0.05; ) and placebo (–0.13; P<0.001); the improvement versus FF did not reach statistical significance (–0.04; P=0.317). Similarly, overall nighttime symptom severity was significantly improved from baseline with AB/FF compared with AB (–0.12; P<0.01; ), and placebo (–0.14; P<0.001); and numerically improved versus FF (–0.05; P=0.20; non-significant). It is important to note that there was a relatively large placebo response observed for TDI focal score, E-RS total score, early morning- and nighttime- symptoms.
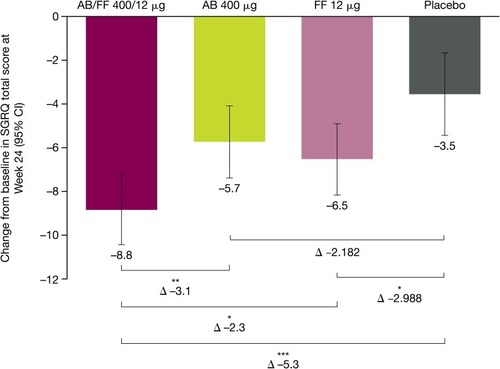
Additionally, there were significant improvements from baseline for SGRQ total score at Week 24, for AB/FF versus placebo (–5.3; P<0.001), AB (–3.1; P<0.01), and FF (–2.3; P<0.05) (). While all active treatment groups exceeded MCID versus baseline, only AB/FF (–5.3) exceeded the MCID versus placebo (≥4-unit decrease).Citation12
Figure 5 Changes from baseline for SGRQ total score for treatment-naïve patients, at Week 24 of ACLIFORM and AUGMENT (post-hoc analysis; ITT population).
Abbreviations: AB, aclidinium bromide; CI, confidence interval; FF, formoterol fumarate; ITT, intent to treat; SGRQ, St George’s Respiratory Questionnaire.
Discussion
A review of the literature revealed several RCTs that included subgroups of treatment-naïve patients. Long-acting bronchodilator monotherapies and dual bronchodilator combinations both showed evidence of significant benefits on lung function, symptoms, and QoL. There was also evidence that LAMA monotherapy appeared to slow the course of FEV1 decline in treatment-naïve patients with COPD, who were mostly GOLD stage II and III.Citation24 Furthermore, LAMA/LABA dual bronchodilator therapy provided additional benefits over monotherapy in maintenance therapy-naïve patients.Citation16,Citation18 Overall, these data combined with the new post-hoc analysis, support the case for using LAMA/LABA treatments as first-line therapy in COPD.
In general, in terms of lung function, the new post-hoc analysis of the effects of AB/FF in maintenance therapy-naïve patients demonstrated significantly greater benefits than LAMA or LABA mono-bronchodilator therapy. Compared with AB/FF, neither LAMA nor LABA monotherapy treatment was observed to provide similar levels of improvement for airflow () or symptom endpoints (). Therefore, these maintenance therapy-naïve patients in ACLIFORM and AUGMENT appeared to benefit most from initial dual bronchodilation with AB/FF, which provided a combination of anticholinergic and sympathomimetic mechanisms to improve their COPD.
Furthermore, at Week 24, the improvement with AB/FF versus placebo in trough FEV1 (134 mL) exceeded the MCID (100 mL).Citation13 AB/FF also improved patient-reported outcomes in these maintenance therapy-naïve patients to a greater extent than either of the monotherapies. At Week 24, patients receiving AB/FF had significantly improved TDI focal scores compared with monotherapies and placebo, with the improvement versus monotherapies approximately equal to the MCID (1 unit); the treatment differences versus AB and FF were 1.17 and 0.92, respectively.Citation14,Citation26 Other symptom and QoL analyses showed a similar pattern of results, supporting a greater effect of AB/FF versus monotherapies. These new data support the case that LAMA/LABA combinations can have a greater effect than long-acting bronchodilator monotherapies when used as initial treatment. GOLD recommends that initial treatment with LAMA/LABA should be reserved for individuals with a higher symptom burden.Citation2 However, there is a lack of evidence identifying the clinical characteristics that could help identify which patients are most likely to benefit from initial treatment with dual bronchodilators; this topic needs further investigation in terms of prospective studies.
A retrospective database analysis in the UK investigated real-life prescribing of first maintenance therapy in COPD from 2009–2012, and observed that the most frequent first prescription was for LAMA (40.2%), followed by ICS+LABA (29.1%), and ICS monotherapy (15.5%); only 0.4% of patients had a first prescription of LAMA+LABA.Citation27 However, this was before the introduction of LAMA/LABA combinations in a single inhaler. Additionally, 47.9% of patients initially assessed as GOLD group A and 49.0% of patients assessed as GOLD group B received an initial prescription of an ICS alone, or in combination with a bronchodilator.Citation27 This illustrates the frequent use of ICS/LABA as an initial COPD prescription. A recent observational study of COPD exacerbation risk with LAMA versus LABA/ICS found that LABA/ICS was more effective than LAMA in patients with high blood eosinophil concentrations (>4%) or counts (>300 cells per μL) and also potentially for patients with more frequent exacerbations.Citation28 LAMA and LABA/ICS showed similar effectiveness among patients with eosinophil concentrations <4%, but the elevated pneumonia risk associated with the ICS component of LABA/ICS indicates that initiation with LAMA is more appropriate for patients with low eosinophil counts; although, it should be noted this analysis did not stratify patients by COPD severity or eosinophil count.Citation28
One study of dual tiotropium and olodaterol therapy showed that effect sizes for trough FEV1 and FEV1 area under the curve from 0–3 hrs were modestly higher for maintenance therapy-treated versus maintenance therapy-naïve patients, but the magnitude of effect of SGRQ total score and TDI focal improvements were similar irrespective of treatment history.Citation18 The practical argument in favor of earlier intervention with dual bronchodilator therapy, as opposed to initiating treatment with monotherapy and later escalating to dual therapy, is that it enables an earlier reduction in the overall disease burden, and could potentially reduce the risk or rate of disease progression in some patients.Citation4,Citation16,Citation29 Furthermore, early intervention in COPD may benefit patients as they can maintain levels of activity.Citation30
A limitation of the existing evidence for efficacy of maintenance therapy in treatment-naïve patients is that patients included in the studies identified here had a wide range of disease severity from mild to very severe, suggesting that although these patients are receiving treatment for the first time, they may have been living with undiagnosed COPD for a considerable period. This precludes definitive comparison between studies. Additionally, the possibility of a publication bias exists, wherein studies showing significant results may be more likely to be published and therefore identified in our search. As with most of the other studies, our analysis was post-hoc and used a specific definition of maintenance therapy-naïve (≥4 weeks of no use of maintenance COPD medications). As such, at least some patients may have used maintenance treatments in the past, which although not received at the time of the current studies, meant there was a possibility of carry-over effects from prior treatments being observed in these studies. Additionally, our maintenance therapy-naïve patients were younger, with higher FEV1, and higher levels of current smoking than maintenance therapy-treated patients. These baseline differences might suggest the maintenance therapy-naïve group may be at an earlier, less severe stage of COPD than those already receiving maintenance therapy. There is a need for more prospective studies in patients diagnosed earlier and with milder COPD to evaluate the benefits of introducing maintenance therapy, including dual bronchodilator therapy, at an earlier stage in the development of COPD.
Conclusions
The findings presented here from retrospective analysis in treatment-naïve patients with COPD, demonstrate the effectiveness of various therapeutic interventions in this population. Of importance, intervention with dual bronchodilator therapy, such as AB/FF, as a first COPD maintenance treatment may provide greater benefits than LAMA or LABA monotherapy.
Abbreviations
AB/FF, aclidinium bromide/formoterol fumarate; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LS, least squares; MCID, minimal clinically important difference; mMRC, modified Medical Research Council; OR, odds ratio; PF-10, 10-item Physical Functioning Questionnaire; POET-COPD®, Prevention Of Exacerbations with Tiotropium in COPD; QoL, quality of life; RCT, randomized controlled trial; SABA, short-acting β2-agonist; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; WPAI, work productivity and activity impairment.
Ethics Approval And Informed Consent
Both the studies included in this post-hoc analysis were conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation/Good Clinical Practice Guidelines, and local regulations. The regulatory authorities approved the protocols for each country and each center had an independent ethics committee.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
DS is supported by the NIHR Manchester Biomedical Research Centre; and has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Apellis, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici S.p.A, Cipla, Genentech, GlaxoSmithKline, Glenmark, Johnson & Johnson, Mundipharma, Novartis, Peptinnovate Ltd., Pfizer Inc, Pulmatrix, Skyepharma, Teva, Theravance Biopharma, Menarini, and Verona Pharma. ADD has received research, consulting, and lecturing fees from Almirall, Altana, AstraZeneca, Boehringer Ingelheim (Canada) Ltd, Forest Laboratories, GlaxoSmithKline, KOS Pharmaceuticals, Merck Canada, Methapharm, Novartis Canada/USA, ONO Pharmaceutical Co., Pfizer Canada, Schering-Plough, Sepracor, and SkyePharma.
JFD has received consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, GSK, Mylan, Theravance, and Sunovion. He is a member of the Data Monitoring Committee for AstraZeneca.
EMK has participated in consulting, advisory boards, speaker panels, or received travel reimbursement from Amphastar Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Cipla, Chesi, Forest Laboratories LLC, GSK, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva Pharmaceutical Industries Ltd., and Theravance Biopharma. He has conducted multicenter clinical research trials for ~40 pharmaceutical companies. EM, FC, and DJ are employees of AstraZeneca and former employees of Almirall S.A., Barcelona, Spain. AR is a former employee of Almirall S.A., Barcelona, Spain and was an employee of AstraZeneca at the time the study was conducted. The authors report no other conflicts of interest in this work.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Nina Divorty, PhD, and Rebecca J. Douglas, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd., Glasgow, and Macclesfield, UK and was funded by AstraZeneca, Cambridge, UK in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–464). This work was funded by AstraZeneca, Cambridge, UK, with the original ACLIFORM and AUGMENT studies funded by Almirall S.A. and Forest Laboratories LLC. The sponsors did not place any restriction on authors about the statements made in the final article.
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-201728182564
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD Science Committee report 2019. Eur Respir J. 2019;53:1900164. doi:10.1183/13993003.00164-201930846476
- Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa140715425196117
- Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14(1):178. doi:10.1186/1471-2466-14-17825404569
- D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15(1):123. doi:10.1186/s12931-014-0123-025756831
- Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16:92. doi:10.1186/s12931-015-0250-226233481
- AstraZeneca PLC. Duaklir® Genuair™ Summary of Product Characteristics. 2018 Available from: http://www.medicines.org.uk/emc/medicine/29652 Accessed 622, 2018.
- Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT-Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15(1):124. doi:10.1186/s12931-014-0124-z25287629
- Hareendran A, Palsgrove AC, Mocarski M, et al. The development of a patient-reported outcome measure for assessing nighttime symptoms of chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2013;11:104. doi:10.1186/1477-7525-11-10423799883
- Mocarski M, Zaiser E, Trundell D, Make BJ, Hareendran A. Evaluation of the psychometric properties of the Nighttime Symptoms of COPD Instrument. Int J Chron Obstruct Pulmon Dis. 2015;10:475–487. doi:10.2147/COPD.S7577625834415
- Mocarski M, Hareendran A, Jen MH, Zaiser E, Make B. Evaluation of the psychometric properties of the Early Morning Symptoms of COPD Instrument (EMSCI). Value Health. 2014;17:A179.
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi:10.1081/COPD-20005051317136966
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. doi:10.1081/COPD-20005337717136971
- Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi:10.1081/COPD-20005066617136969
- Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-324621681
- Maleki-Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short-term deterioration in maintenance-naive patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three randomized trials. Adv Ther. 2017;33(12):2188–2199. doi:10.1007/s12325-016-0430-627796912
- Lange P, Godtfredsen NS, Olejnicka B, et al. Symptoms and quality of life in patients with chronic obstructive pulmonary disease treated with aclidinium in a real-life setting. Eur Clin Respir J. 2016;3:31232. doi:10.3402/ecrj.v3.3123227387608
- Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO(R) studies. Respir Res. 2016;17(1):73. doi:10.1186/s12931-016-0387-727316465
- Sauer R, Hansel M, Buhl R, Rubin RA, Frey M, Glaab T. Impact of tiotropium + olodaterol on physical functioning in COPD: results of an open-label observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:891–898. doi:10.2147/COPD.S10302327217742
- Setoguchi Y, Izumi S, Nakamura H, et al. Survey to determine the efficacy and safety of guideline-based pharmacological therapy for chronic obstructive pulmonary disease patients not previously receiving maintenance treatment. Expert Opin Pharmacother. 2015;16(15):2271–2281. doi:10.1517/14656566.2015.107467826290277
- Troosters T, Sciurba FC, Decramer M, et al. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med. 2014;24:14003. doi:10.1038/npjpcrm.2014.324841833
- Vogelmeier C, Fabbri LM, Rabe KF, et al. Effect of tiotropium vs. salmeterol on exacerbations: GOLD II and maintenance therapy naive patients. Respir Med. 2013;107(1):75–83. doi:10.1016/j.rmed.2012.09.01523102611
- Decramer M, Rossi A, Lawrence D, McBryan D. Indacaterol therapy in patients with COPD not receiving other maintenance treatment. Respir Med. 2012;106(12):1706–1714. doi:10.1016/j.rmed.2012.08.02223031496
- Troosters T, Celli B, Lystig T, et al. Tiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trial. Eur Respir J. 2010;36(1):65–73. doi:10.1183/09031936.0012780920185426
- [Guideline for diagnosis and management of COPD (chronic obstructive pulmonary disease). The Third Edition: therapy and management]. Nihon Kokyuki Gakkai Zasshi. 2009;Suppl COPD:70–144. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22712100. Accessed November 19, 2019.
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi:10.1164/rccm.201310-1863PP24383418
- Price D, Miravitlles M, Pavord I, et al. First maintenance therapy for COPD in the UK between 2009 and 2012: a retrospective database analysis. NPJ Prim Care Respir Med. 2016;26:16061. doi:10.1038/npjpcrm.2016.6127808096
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–862. doi:10.1016/S2213-2600(18)30368-030343028
- Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336–349. doi:10.1111/ijcp.2015.69.issue-325363328
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi:10.2147/COPD22371650
