Abstract
Purpose
Fatty liver disease is associated with cardiometabolic disorders and represents a potential key comorbidity in Chronic Obstructive Pulmonary Disease (COPD). Some intermediary mechanisms of fatty liver disease (including its histological component steatosis) include tissue hypoxia, low-grade inflammation and oxidative stress that are key features of COPD. Despite these shared physiological pathways, the effect of COPD on the prevalence of hepatic steatosis, and the association between hepatic steatosis and comorbidities in this population remain unclear. Liver density measured by computed tomography (CT)-scan is a non-invasive surrogate of fat infiltration, with lower liver densities reflecting more fat infiltration and a liver density of 40 Hounsfield Units (HU) corresponding to a severe 30% fat infiltration.
Patients and Methods
We took advantage of the international cohort ECLIPSE in which non-enhanced chest CT-scans were obtained in 1554 patients with COPD and 387 healthy controls to analyse the liver density at T12-L1.
Results
The distribution of liver density was similar and the prevalence of severe steatosis (density<40 HU) was not different (4.7% vs 5.2%, p=0.7) between COPD and controls. In patients with COPD, the lowest liver density quartile was associated, after age and sex adjustment, with coronary artery disease (ORa=1.59, 95% CI 1.12 to 2.24) and stroke (ORa=2.20, 95% CI 1.07 to 4.50), in comparison with the highest liver density quartile.
Conclusion
The present data indicate that a low liver density emerged as a predictor of cardiovascular comorbidities in the COPD population. However, the distribution of liver density and the prevalence of severe steatosis were similar in patients with COPD and control subjects.
Introduction
Chronic obstructive pulmonary disease (COPD) is an independent risk factor for multiple comorbidities such as diabetes, coronary artery disease and hypertension.Citation1,Citation2 These comorbidities, which are associated with obesity, are a major cause of mortality in this population, particularly in mild to moderate COPD.Citation3–Citation5 Studies report an increasing proportion of obese COPD patients, leading to major metabolic disorders.Citation6 The liver regulates multiple inflammatory pathways and free-fatty acid transportation and is particularly sensitive to ectopic fat deposition causing Fatty Liver Disease (FLD).Citation7 The increased risk of cardiovascular disease in subjects with this liver disorder is also well documented.Citation8–Citation10 Main intermediary mechanisms of FLD (and its histological component steatosis) include insulin resistance, metabolic syndromeCitation11 and some key features of COPD as reduced physical activity,Citation12 low-grade inflammation, oxidative stress and hypoxia.Citation13,Citation14 Although hepatic steatosis is a continuous process, severe fat infiltration is considered to be present when 33% or more of liver tissue on a biopsy is infiltrated by fat (stage 2–3 in the steatosis grade of the NAFLD activity score).Citation15 Liver density measured by computed tomography (CT) scan is a non-invasive surrogate of fat infiltration, with lower liver densities reflecting more fat infiltration and a liver density of 40 Hounsfield Units (HU) corresponding to a severe 30% fatty infiltration.Citation16–Citation18 Chest CTs are often available in patients with COPD and allow the assessment of the liver density in most of the cases, representing a potential «add-on» for clinicians wanting to avert comorbidities. We used the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort to document the prevalence of fatty liver infiltration in patients with COPD in comparison with healthy controls, and to explore, in the COPD population, the association with major cardiovascular comorbidities collected in ECLIPSE.
Materials and Methods
Population
The multicenter, longitudinal ECLIPSE cohort study (Clinicaltrials.gov NCT0292552) was conducted to identify surrogate markers of COPD progression.Citation19 2164 current or ex-smokers (≥10 pack-years) with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 to 4 and 582 smokers or never smokers with normal lung function who served as controls were recruited from 12 countries.Citation20,Citation21 Patients’ characteristics, blood samples and pulmonary function tests were obtained at baseline. Chest CT-scan was performed on all participants with the following protocol: 120-kV peak, 40 mA, and 1.00- or 1.25-mm slice thickness, at full inspiration. All subjects on whom a valid CT-scan slice at T12-L1 was obtained, to assess liver mean CT attenuation, were included. Comorbidities and medical history were recorded at baseline with standardized questionnaires.Citation19
Liver Density Assessment
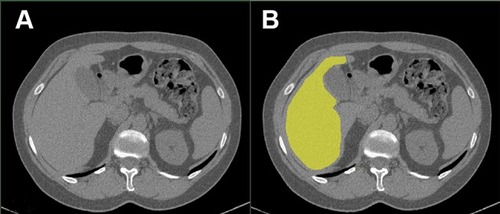
Liver density was quantified by measuring mean organ attenuation in Hounsfield unit (HU) by the same investigator using the body composition analysis platform of the Institut universitaire de cardiologie et de pneumologie de Québec and a validated image analysis software (Slice-O-Matic, Tomovision, Montréal, Québec, Canada) according to standardized techniques.Citation22,Citation23 A standardized segmentation protocol was followed by the evaluator consisting in using an automated segmentation tool to identify the liver perimeter () and then manually remove all potential artefact such as the gall blatter or the portal vein. To ensure validity, a sample of all segmented images were randomly selected and redone by the same evaluator to assess for intra-observer variation. A second imaging expert segmented the same sample to evaluate inter-observer variation, showing an excellent inter-observer validity (Inter-Class Coefficient 0.98, mean absolute difference 0.96 HU, mean relative difference 1.01%).
Statistical Analysis
We compared the prevalence of severe steatosis (liver density <40 HU) in COPD and controls using chi-square statistics. To document the association between liver density and cardiovascular comorbidities in COPD, patients with COPD were divided into quartiles of liver density. The quartiles 1 (lowest density, steatosis) and 4 (highest density, no steatosis, reference) were compared with logistic regression in order to evaluate the association (odds ratio) with coronary disease (CAD), diabetes, heart failure, hypertension and stroke. Unadjusted odds ratio (OR), age and sex adjusted OR (ORa) and age, sex and body mass index (BMI) adjusted OR (ORab) were sequentially calculated. Available inflammatory markers were compared between quartiles using ANCOVA. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA). A p-value <0.05 was considered as significant.
Results
Population and Liver Attenuation Between COPD and Controls
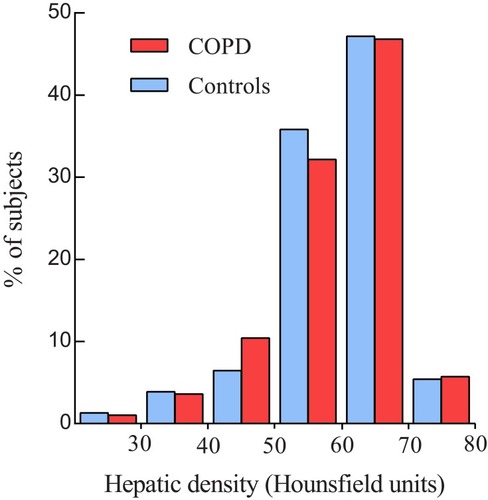
We retrieved 1941 patients (1554 COPD and 387 controls) for whom a CT slice at T12-L1 could be analysed for liver attenuation (). Patients with COPD were older (64±7 years vs 55±9 years; p<0.001) and the proportion of men was greater (61% vs 57%; p<0.001) than in controls. BMI was slightly but significantly lower in patients with COPD (26.1±5.2 kg/m2 in COPD vs 26.6±4.7 kg/m2 in controls; p= 0.046). All considered cardiovascular comorbidities were significantly more prevalent in patients with COPD. The proportion of subjects with severe liver steatosis (<40 HU) was not different between COPD and controls (4.7% vs 5.2%, respectively, p=0.7). The distribution in hepatic densities () was similar between groups (p>0.05 for all comparisons).
Table 1 Characteristics of Patients with COPD and Control Subjects
Association with Cardiovascular Comorbidities and Cytokines Levels
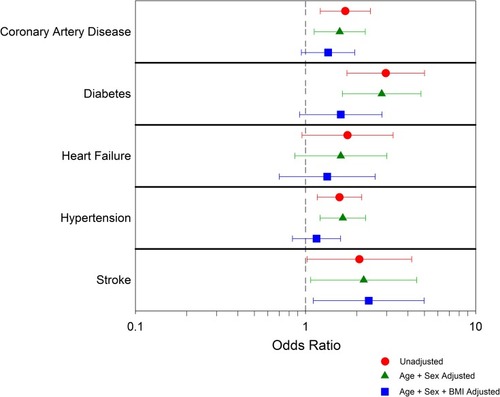
The characteristics of the COPD population by quartile of liver density are presented in . In the COPD population, compared to the reference quartile (mean attenuation 68 HU), the lowest hepatic density quartile (mean attenuation 46 HU) resulted in an unadjusted CAD OR=1.71 (95% CI 1.22 to 2.40), diabetes OR=2.96 (95% CI 1.753 to 5.007), heart failure OR=1.77 (95% CI 0.95 to 3.27), hypertension OR=1.58 (95% CI 1.17 to 2.14) and stroke OR=2.07 (95% CI 1.02 to 4.21). With age and sex adjustment, the association remains significant with CAD (ORa=1.59, 95% CI 1.12 to 2.24), diabetes OR=2.80 (95% CI 1.64 to 4.77), hypertension OR=1.66 (95% CI 1.22 to 2.25) and stroke (ORa=2.20, 95% CI 1.07 to 4.50). With age, sex and BMI adjustment, only stroke remained associated with low hepatic density (ORab=2.35, 95% CI 1.11 to 4.98) (, Supplementary Table S1). In sub-group analysis by sex, an increased risk for all cardiovascular comorbidities was observed in women (Supplementary Tables S2 and S3).
Table 2 Characteristics of COPD Patients by Liver Attenuation Quartile
Figure 3 Unadjusted and adjusted odds ratios to present cardiovascular comorbidity in the lowest liver density quartile vs the highest liver density quartile.
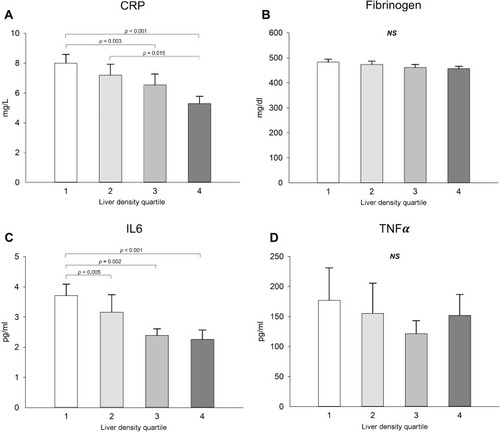
Among inflammatory markers, interleukin-6 and C Reactive Protein (CRP) were significantly higher in quartile 1 compared to quartile 4, with a clear dose–response effect (CRP and IL6 significantly decreasing with increase in hepatic density) (). Similar conclusion was reached when the analysis was stratified based on BMI (data not shown).
Figure 4 Inflammatory marker levels showing differences according to liver attenuation groups (A-D). Values are expressed with mean ± standard errors. 1 is the group with the lowest liver attenuation and 4 the group with the greatest liver attenuation. p-value for ANCOVA corrected for age, sex, Body Mass Index and smoking status.
Discussion
Despite the potential two-way relationship between COPD and liver steatosis due to chronic low-grade inflammation, intermittent hypoxia and induced adipokines,Citation24,Citation25 few data are available to draw an epidemiologic picture of liver steatosis in this population. In a previous study,Citation26 we reported that the liver fibrosis stage of non-alcoholic fatty liver disease, but not steatosis, is an independent factor of cardiovascular morbidity in COPD, in line with results in the general population.Citation27,Citation28 The present study is the first large multinational COPD cohort with controls to report liver density prevalence. The ECLIPSE data show a similar liver density distribution between COPD and controls. Our results are at variance with previous studies where the prevalence of steatosis ranging from 30% to 41% was reported in COPDCitation24,Citation25 and found to be higher than the reported 5% to 20% prevalence in the general population.Citation17,Citation29,Citation30 These discrepancies are likely related to the use of different diagnostic tools across studies, including biological scores and transient elastographyCitation31 which have the ability to detect milder fatty infiltration compared to CT scan.
Although CT-scan has its limitations in the assessment of fatty liver disease,Citation32 this modality has the advantage of being widely available in pulmonary patients and nevertheless provides a reliable assessment of severe steatosis when liver density is <40 HU.Citation32 Furthermore, our purpose was to assess the relevance of this surrogate marker for cardiovascular comorbidities in COPD, which are the main known complications of fatty liver disorders.Citation8,Citation9 CT is also a less invasive alternative to liver biopsy, the gold standard to diagnose FLD, which is a costly procedure,Citation33 prone to minor side-effects such as pain, or more severe complications, including a risk of death of 0.03%.Citation34 Moreover, intra and interobserver variabilities exist in the quantification of FLD from a biopsy.Citation35 Noninvasive tests (including biological scores and imaging) are cheaper, more easily repeatable, and lead to less interobserver variability, severe adverse events, refusal and bias than biopsy.Citation34
Using imaging data already available to the clinicians, screening for FLD could be performed in several patients with COPD. There are currently two methods to assess liver density from a CT scan. In the present investigation, we measured the mean organ attenuation with standardized segmentation which is more complex compared to the multiple region of interest (ROI) methods,Citation17 but encompasses a larger area of evaluation which may improve the assessment in case of focal fatty infiltration.Citation36,Citation37 In our experience, there is little variation between these techniques as long as they are performed on non-enhanced CT.Citation17 We thus recommend the ROI method in clinical practice, mostly because it is less time-consuming than calculating liver attenuation with standardized segmentation and available with current commercial software.
Sexual dimorphism concerns both NAFLD and cardiovascular comorbidities,Citation38,Citation39 and hormonal modulation is a key element to understand variations in risk by age and sex.Citation40,Citation41 Premenopausal women seem to be protected from developing high levels of NAFLD, while ovarian senescence appears to be associated with severe steatosis and fibrosing NASH. Due to an age >40 yo in the COPD population, we could expect more or equal risk of steatosis in women than men. In ECLIPSE, women were less frequently exhibiting low liver density (steatosis), but low liver density in women resulted in stronger association with cardiovascular comorbidities than in men.
Among its potential limitations, the ECLIPSE study did not prospectively assess alcohol consumption, which prevents us from differentiating between FLD and Nonalcoholic fatty liver disease (NAFLD) or to suggest a precise origin for steatosis. However, this limitation should not question the link that we found between liver density with cardiovascular comorbidities because hepatic steatosis leads to worse cardiovascular status, irrespective of the underlying etiology.Citation42 In the same way, we were not able to test the effect of some medications associated both with cardiovascular outcomes and fatty liver infiltration, like amiodarone.
Recent data showed that more advanced forms of NAFLD, namely fibrosis rather than steatosis are associated with cardiovascular events.Citation26–Citation28 Unfortunately, in the ECLIPSE cohort, no evaluation of liver fibrosis is available, and biological data do not include transaminases, which makes the evaluation by non-invasive NAFLD scores impossible.
The short follow-up of this cohort (3 years) also prevents us from considering the impact of hepatic density on prospective cardiovascular events, due to the limited number of such events during the study period. Finally, the representativeness of the ECLIPSE cohort in relation to the different COPD phenotypes is questionable. Previous studies have tried to identify different phenotypes of COPD patients: frequent exacerbators;Citation20,Citation43 eosinophilic patients and asthma-COPD overlap syndrome;Citation44,Citation45 and patients with severe airflow limitation at the opposite of those with milder airflow limitation but with obesity and cardiovascular comorbidities.Citation46 This last phenotype is the most likely to present both hepatic and cardiovascular comorbidities. Interestingly, in ECLIPSE, patients with GOLD 2 or 3 COPD and a body mass index >30 kg/m2 only represented 17% (266/1554) of COPD patients, which could appear low in an occidental population.Citation6,Citation47,Citation48
Conclusion
The present data indicate that a low liver density emerged as a predictor of cardiovascular comorbidities, particularly stroke, in COPD. Although we cannot conclude definitively on the clinical implications of liver steatosis in COPD, this comorbidity is likely relevant considering its association with chronic liver disease and metabolic comorbidities such as insulin resistance, ectopic fat accumulation, and lipid abnormalities.
Abbreviations
CAD, Coronary Disease; COPD, Chronic Obstructive Pulmonary Disease; CRP, C Reactive Protein; CT, Computed Tomography; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; FLD, Fatty Liver Disease; GOLD, Global Initiative in Chronic Obstructive Lung Disease; HU, Hounsfield Unit; NAFLD, Nonalcoholic Fatty Liver Disease; OR, Odds Ratio.
Ethics Approval and Informed Consent
The study is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and has been approved by the relevant ethics and review boards at the participating centres. All patients provided written informed consent. The ECLIPSE steering and scientific committees approved the protocol for this substudy.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author with the permission of the ECLIPSE Collaborative Research Group.
Author Contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosure
MM, NA and IV have no conflicts of interest to declare. DV reports research grants from Astra Zeneca outside the submitted work. JPD reports personal fees from Abbott Laboratories, AstraZeneca, Sanofi, GSK, Torrent Pharmaceuticals Ltd., Merck and Pfizer Canada Inc., outside the submitted work. HOC reports personal fees and grants from GSK during the conduct of the original ECLIPSE study and no other conflicts during the present study. JLP reports a grant from a consortium of homecare providers (ADIR assistance, Agiradom, IPS, ISIS Medical, LINDE, LVL Medical, SOS Oxygen and Vitalaire) and CPAP companies (Bréas, Philips, Resmed and Sefam), grants from Air Liquide Foundation, Astra-Zeneca, Mutualia, Philips, RESMED, Fisher and Paykel, and personal fees from JAZZ, ITAMAR, Perimetre, Philips, Fisher and Paykel, RESMED, Astra-Zeneca, SEFAM, Agiradom, ELIA and Teva outside the submitted work. FM reports grants and personal fees from Boehringer Ingelheim and GSK, grants from Nycomed and AstraZeneca, and grants and personal fees from Novartis and Grifols outside the submitted work. All fees are pooled with other revenues of the group of pulmonologists to which FM is a member and then shared among members of the group. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank Véronic Tremblay for the body composition analysis platform of the Institut Universitaire de Cardiologie et de Pneumologie de Québec for ensuring standardization of the CT image analyses. They also thank all the subjects, investigators and study site staff who participated in ECLIPSE.
References
- Lee -CT-C, Mao I-C, Lin C-H, Lin S-H, Hsieh M-C. Chronic obstructive pulmonary disease: a risk factor for type 2 diabetes: a nationwide population-based study. Eur J Clin Invest. 2013;43(11):1113–1119. doi:10.1111/eci.1214724028296
- Onishi K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol. 2017;70(2):128–134. doi:10.1016/j.jjcc.2017.03.00128325523
- Pistelli R, Lange P, Miller DL. Determinants of prognosis of COPD in the elderly: mucus hypersecretion, infections, cardiovascular comorbidity. Eur Respir J Suppl. 2003;40:10s–14s. doi:10.1183/09031936.03.0040340312762568
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. doi:10.1378/chest.128.4.264016236937
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. doi:10.1183/09031936.0013380517138679
- Wouters EFM. Obesity and metabolic abnormalities in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(Supplement_5):S389–S394. doi:10.1513/AnnalsATS.201705-371AW29161076
- Reccia I, Kumar J, Akladios C, et al. Non-alcoholic fatty liver disease: a sign of systemic disease. Metab Clin Exp. 2017;72:94–108. doi:10.1016/j.metabol.2017.04.01128641788
- Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(7):1724–1745. doi:10.3748/wjg.v20.i7.172424587651
- Liu H, Lu H-Y. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014;20(26):8407–8415. doi:10.3748/wjg.v20.i26.840725024598
- Perazzo H, Poynard T, Dufour J-F. The interactions of nonalcoholic fatty liver disease and cardiovascular diseases. Clin Liver Dis. 2014;18(1):233–248. doi:10.1016/j.cld.2013.09.01424274877
- Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46(11):1074–1087. doi:10.1111/hepr.1265626785389
- van den Borst B, Gosker HR, Schols AMWJ. Central fat and peripheral muscle: partners in crime in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(1):8–13. doi:10.1164/rccm.201208-1441OE23281350
- Minville C, Hilleret M-N, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145(3):525–533. doi:10.1378/chest.13-093824264333
- Aron-Wisnewsky J, Clement K, J-L P. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metab Clin Exp. 2016;65(8):1124–1135. doi:10.1016/j.metabol.2016.05.00427324067
- Nascimbeni F, Ballestri S, Machado MV, et al. Clinical relevance of liver histopathology and different histological classifications of NASH in adults. Expert Rev Gastroenterol Hepatol. 2018;12(4):351–367. doi:10.1080/17474124.2018.141575629224471
- Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–112. doi:10.1148/radiol.239105036116484355
- Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307–1312. doi:10.2214/AJR.06.099217449775
- Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194(3):623–628. doi:10.2214/AJR.09.259020173137
- Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.0011170718216052
- Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi:10.1186/1465-9921-11-12220831787
- Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi:10.1056/NEJMoa110548221991892
- Davidson LE, Kuk JL, Church TS, Ross R. Protocol for measurement of liver fat by computed tomography. J Appl Physiol. 2006;100(3):864–868. doi:10.1152/japplphysiol.00986.200516293702
- Martin M, Almeras N, Després J-P, et al. Ectopic fat accumulation in patients with COPD: an ECLIPSE substudy. Int J Chron Obstruct Pulmon Dis. 2017;12:451–460. doi:10.2147/COPD.S12475028203068
- Moon SW, Kim SY, Jung JY, et al. Relationship between obstructive lung disease and non-alcoholic fatty liver disease in the Korean population: korea National Health and Nutrition Examination Survey, 2007–2010. Int J Chron Obstruct Pulmon Dis. 2018;13:2603–2611. doi:10.2147/COPD.S16690230214178
- Viglino D, Jullian-Desayes I, Minoves M, et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J. 2017;49:6. doi:10.1183/13993003.01923-2016
- Viglino D, Plazanet A, Bailly S, et al. Impact of non-alcoholic fatty liver disease on long-term cardiovascular events and death in Chronic Obstructive Pulmonary Disease. Sci Rep. 2018;8(1):16559. doi:10.1038/s41598-018-34988-230410123
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.04325935633
- Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi:10.1016/j.jhep.2016.05.01327212244
- Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979–1987. doi:10.1002/hep.2359320336705
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi:10.1038/nrgastro.2013.17124042449
- Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15(5):274–282. doi:10.1038/nrgastro.2018.1029463906
- Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–485. doi:10.3748/wjg.v20.i2.47524574716
- Tapper EB, Sengupta N, Hunink MGM, Afdhal NH, Lai M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am J Gastroenterol. 2015;110(9):1298–1304. doi:10.1038/ajg.2015.24126303130
- Munteanu M, Ratziu V, Morra R, Messous D, Imbert-Bismut F, Poynard T. Noninvasive biomarkers for the screening of fibrosis, steatosis and steatohepatitis in patients with metabolic risk factors: fibroTest-FibroMax experience. J Gastrointestin Liver Dis. 2008;17(2):187–191.18568141
- Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(Suppl 4):64–70. doi:10.1111/jgh.1227124251707
- Halvorsen RA, Korobkin M, Ram PC, Thompson WM. CT appearance of focal fatty infiltration of the liver. AJR Am J Roentgenol. 1982;139(2):277–281. doi:10.2214/ajr.139.2.2776979879
- Kammen BF, Pacharn P, Thoeni RF, et al. Focal fatty infiltration of the liver: analysis of prevalence and CT findings in children and young adults. AJR Am J Roentgenol. 2001;177(5):1035–1039. doi:10.2214/ajr.177.5.177103511641164
- Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi:10.1007/s12325-017-0556-128526997
- Du T, Sun X, Yuan G, et al. Sex differences in the impact of nonalcoholic fatty liver disease on cardiovascular risk factors. Nutr Metab Cardiovasc Dis. 2017;27(1):63–69. doi:10.1016/j.numecd.2016.10.00427956025
- Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Testosterone US. Sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Hepatol. 2017;16(3):382–394. doi:10.5604/16652681.123548128425408
- Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–256. doi:10.1007/978-3-319-70178-3_1229224098
- Toshikuni N, Tsutsumi M, Arisawa T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(26):8393–8406. doi:10.3748/wjg.v20.i26.839325024597
- Donaldson GC, Seemungal TA, Bhowmik RA, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.84712324669
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC21680942
- Chou K-T, Su K-C, Huang S-F, et al. Exhaled nitric oxide predicts eosinophilic airway inflammation in COPD. Lung. 2014;192(4):499–504. doi:10.1007/s00408-014-9591-824816967
- Garcia-Aymerich J, Gómez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66(5):430–437. doi:10.1136/thx.2010.15448421177668
- Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–24. doi:10.1155/2012/73261822679617
- García-Rio F, Soriano JB, Miravitlles M, et al. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS ONE. 2014;9(8):e105220. doi:10.1371/journal.pone.010522025153331