Abstract
Rationale
Frailty in chronic obstructive pulmonary disease (COPD) patients has been associated with a higher rate of incidents, longer duration of hospitalization, poorer quality of life, and higher mortality.
Objective
To measure the prevalence of frailty among COPD patients and to evaluate associated variables.
Methods
A cross-sectional study. Subjects who visited a State Center for High-Cost Medicines to obtain free monthly COPD medicines were considered eligible. Individuals ≥40 years old who had a FEV1/FVC ratio of <0.7 post-bronchodilation were enrolled. The Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight (FRAIL) scale, Medical Research Council dyspnea scale (MRC), COPD Assessment Test (CAT), a combination of CAT/MRC [(CAT/8)+MRC], and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) severity scale were used to evaluate the enrolled subjects. Variables associated with frailty were analyzed using an ordered logistic regression and a multivariate logistic regression.
Results
The prevalence of frailty and pre-frailty among the 153 COPD subjects enrolled was 50.3% (77/153) and 35.3% (54/153), respectively. Frailty scores were correlated with CAT (correlation coefficient [cc]: 0.52, p <0.001) and MRC (cc: 0.48, p <0.001). Ordinal regression models showed that MRC and CAT were associated with fragility (p <0.0001 for both models). Higher odds of frailty were observed in GOLD groups B (p = 0.04) and D (p = 0.02). Multiple logistic regression revealed that the combination CAT/MRC≥5.5 was associated with frailty (OR 6.73; p <0.0001) and had a specificity of 80.3%, sensitivity of 62.3%, and positive and negative predictive values of 76.2% and67.8%, respectively.
Conclusion
Frailty prevalence was high and was correlated with higher MRC and CAT scores. The CAT/MRC combination [(CAT/8)+MRC] ≥5.5 was highly associated with frailty, suggesting that an additional specific evaluation for the presence of frailty is indicated.
Introduction
As a result of an aging population, frailty is a new challenge in public health.Citation1 Frailty can be defined as the loss of an individual’s ability to adapt to stress due to the decrease in or dysfunction of their physiological reserves. This state of physical vulnerability is associated with disability, and an increase in the number of falls, hospitalizations, and death.Citation2–Citation4 Frailty can result from physiological processes such as aging, or can be associated with chronic diseases such as chronic obstructive pulmonary disease (COPD).Citation4–Citation8 In fact, the prevalence of frailty among COPD patients worldwide is estimated to be between 6% and 82%.Citation9–Citation15
Frailty is a dynamic condition. Frail COPD patients are more likely to be unable to complete rehabilitation programs, and mortality amongst these patients is 80% higher than for non-frail patients.Citation12,Citation13 A study that followed up COPD patients from the National Emphysema Treatment Trial (NETT) for 24 months compared participants with two or fewer frailty parameters to those with three or more frailty parameters. The incidence rate of frailty was 6.4 per 100 person-years. Analysis revealed that frailty was associated with a higher rate and longer-duration hospitalization, and with poor quality of life.Citation10 In Brazil, the prevalence of frailty and the predictive variables associated with frailty among COPD subjects are unknown. The aim of this study was to measure the prevalence of frailty among COPD patients in Goiania city (Brazil) and to evaluate the association between predictive variables and the presence of frailty.
Methods
Design
This was an observational cross-sectional study.
Setting
The study was conducted at the Juarez Barbosa State Center for High-Cost Medicines (CEMAC), a public center responsible for providing free medicines for COPD treatment for Brazilians without socioeconomic distinction, located in Goiania city, Brazil. Data were collected from January 18th, 2018 to December 14th, 2018.
Subject Selection
All patients who reported a COPD diagnosis when visiting CEMAC to obtain free monthly COPD medicines were considered eligible. Individuals of ≥40 years of age who had a forced expiratory volume in the first second/forced vital capacity (FEV1/FVC) ratio of <0.7 post-bronchodilator documented on a pulmonary function test were enrolled. Written informed consent was obtained from all participants. Subjects were excluded if they did not complete interviews, questionnaires, or tests.
Data Collection and Diagnostic Procedures
Data were collected by one physician (ACGF) and one physical therapist (LSD) specifically trained for this purpose using a study-specific data collection instrument. The data collection instrument was pre-tested, validated, and modified during a study conducted from December 1st, 2017 to December 15th, 2017 in eight COPD patients. These subjects were not included in the study. Variables assessed by the data collection instrument included sociodemographic data, tobacco smoking, level of dyspnea, clinical impact of symptoms, medicines prescribed for COPD treatment, and total number of exacerbations in the last 12 months.
Frailty was evaluated through the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight (FRAIL) scale, a questionnaire with five self-report items for which the possible answers are “yes” or “no”, with an attribution of 1 or 0 points, respectively. The individuals were classified as non-frail (0 points), pre-frail (1 or 2 points), and frail (3 or more points).Citation16,Citation17
Comorbidities were evaluated using the Charlson Comorbidity Index (CCI).Citation18 The patient’s score is calculated as the sum of the weights of all presented comorbidities that are components of the index, and from 50 years on, an additional weight is added to each decade of life.Citation18 Dyspnea was assessed using the Medical Research Council (MRC) dyspnea scale, a scale consisting of five gradations of dyspnea, where each item corresponds to how much the dyspnea limits activities involved in daily life.Citation19 The COPD Assessment Test (CAT) was applied to evaluate the impact of COPD on quality of life.Citation20 This test consists of eight items: cough, phlegm, chest tightness, shortness of breath, activity limitations at home, confidence in leaving home, sleep, and energy. Each item is given a score from 0 to 5. By summing the score of each item, patients can be classified according to the clinical impact of the disease: mild (6 to 10 points), moderate (11 to 20 points), severe (21 to 30 points), and very severe (31 to 40 points).Citation20,Citation21
The diagnosis of COPD was confirmed by the presence of respiratory symptoms associated with a history of exposure to tobacco or biomass, and a documented pulmonary function test with an FEV1/FVC ratio of <0.7 post-bronchodilator.Citation22 The severity of the condition was assessed by considering the patient’s health status according to CAT, and the number of exacerbations and the need for hospitalization in the last 12 months, as previously described in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.Citation22
To minimize compliance bias (as the level of fragility would increase among individuals who were not on regular treatment because of low financial condition) we selected a public center providing free medication. Measurement bias was approached by using a pre-tested and validated study-specific data collection instrument applied by the same research team (ACGF and LSD), and to minimize random bias, we use consecutive recruitment.
Statistical Analysis
“Non-frail”, “pre-frail”, and “frail” were considered as outcome variables. The following predictive variables were assessed: symptoms according to CAT score, sociodemographic data, body mass index (BMI), comorbidities (CCI), risk factors (smoking), number of exacerbations, and disease severity.
To verify that predictive variables were associated with outcomes, two approaches were taken. In the first, a linear regression analysis was performed. Spearman correlation (r) was calculated to analyze the strength of associations. Then, a multiple linear regression model was created, including gender as well as variables with a p-value<0.20. In the second approach, COPD patients were classified into three categories: non-frail, pre-frail, and frail. Variables were compared using Pearson’s chi-square test or Fisher’s exact test, when applicable. The Kruskal–Wallis test with a post-hoc comparison (Dunn’s test) was used to compare the medians of quantitative variables. Then, variables with a p value<0.20 and gender were included in ordinal regression models. In these models, the dependent variable was the frailty level, stratified into three categories (non-frail[1], pre-frail [2], and frail [3]). The magnitudes of the associations were presented as regression coefficients (β) and adjusted odds ratios (ORaj) with confidence intervals (CI) of 95%. As GOLD categories were derived from CAT, two ordinal regression models were adjusted including the variables separately. The ordinal regression models were evaluated and validated to confirm the proportional odds assumption using a likelihood-ratio test and a Brant test. As MRC and CAT were predictors of frailty, we created a new variable that could express both parameters simultaneously. As the CAT can assume values up to 8 times the MRC, so that the CAT does not conceal MRC value, a new variable (CAT/MRC) was created by dividing the CAT by 8, followed by the sum of MRC: (CAT/8) + MRC. Then, we coded frailty in a binary outcome ([0] non-frail and pre-frail;[1] frail) to create a multivariate logistic regression model to assess the association between the presence of frailty and new independent variable CAT/MRC, while controlling for sex, FEV1 and inhaled medications. The cutoff value of the CAT/MRC as a frailty predictor was calculated by use of a receiver operating characteristic (ROC) curve. A p value of <0.05 was considered statistically significant. Data were analyzed using STATA software, version 15.0 (StataCorp, Texas, USA).
Sample Size
The sample size calculation was performed by estimating the proportion of the population with specified absolute precision (n = z21-α/2P(1-P)/d2). At a confidence level of 95%, with an absolute accuracy of ± 4% in the study proportion measure, and considering the prevalence of frailty in COPD patients as 6.6%, a sample of 148 subjects was estimated.Citation11
Ethical Aspects
All participants gave written informed consent in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and the study was approved by the Institutional Review Board (IRB) of the Federal University of Goias under the following number: 2,403,289. CAAE 79462917.9.0000.5078. Approval was granted on November 28th, 2017.
Results
A total of 203 individuals visited CEMAC to obtain medicines for COPD treatment during the study period.22% (45/203) were not COPD patients, but were individuals authorized to withdraw medicines on behalf of COPD patients. 2.4% (5/203) of subjects refused to participate in the study. shows the characteristics of the 153 COPD patients enrolled in this study and their frailty state.
Table 1 Characteristics of 153 COPD Patients Classified by Their Frailty State
The prevalence of frailty in the sample was 50.3% (77/153), while 35.3% (54/153) of patients were pre-frail and 14.4% (22/153) were non-frail. The education level and FEV1 (%) were significantly lower and the MRC and CAT scores were significantly higher in the frail group.
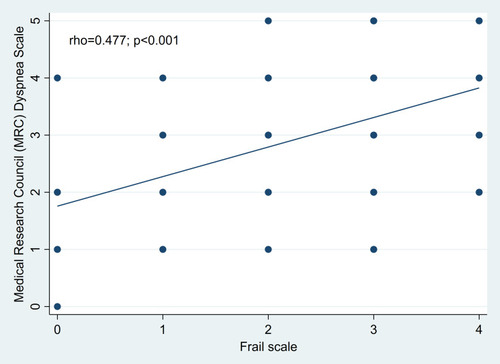
and show the correlations between frailty score and CAT score (correlation coefficient: 0.52, p <0.001) and between frailty score and MRC score (correlation coefficient: 0.48, p <0.001)
Figure 1 Correlation between FRAIL scale and COPD Assessment Test (CAT) scores in patients with COPD.
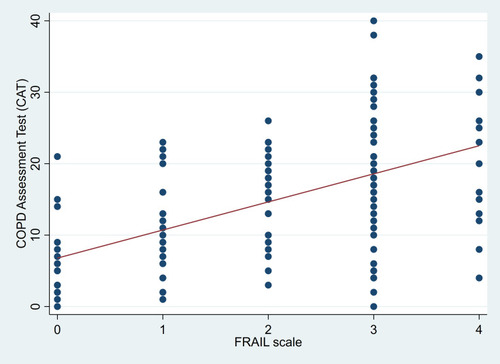
Figure 2 Correlation between FRAIL scale and Medical Research Council (MRC) Dyspnea Scale scores in patients with COPD.
The regression model presented in shows that the odds of frailty increased with increasing MRC score (Model 1: ORaj = 1.94; p <0.0001; Model 2: ORaj = 1.55; p=0.01). Considering model 1, for every one unit increase in the MRC score the odds of being pre-frail or frail, versus non-frail is multiplied 1.94 times (increases 94%), holding constant all other variables. For model 2, the increase is 1.55 times or 55%. Because of the proportional odds assumption of ordinal regression, there is a reduction of 1.94 times or 1.55 times, in the odds of being frail versus the combined categories of pre-frail and non-frail when MRC reduces one unit.GOLD B (ORaj = 2.46; p = 0.04) and D (ORaj = 3.41; p = 0.02) COPD patients had an increased chance of frailty compared to GOLD A patients. For COPD patients GOLD B the odds of being frail is 2.46 times (or 146% more) that of who were not GOLD B, holding constant all other variables. The same interpretation applies to GOLD D (3, 41 times or 241%). Also, it was found that the chance of frailty was directly related to an increase in CAT score (ORaj = 1.15; p <0.0001), that is, for every one unit increase in the CAT score the odds of being pre-frail or frail, versus non-frail is multiplied 1.15 times (increases 15%), holding constant all other variables (). Adjusting for sex, FEV1 and inhaled medications, multiple logistic regression analysis revealed that CAT/MRC was an independent predictor of frailty (ORaj = 1.86; p <0.0001) (). The area under the ROC curve of CAT/MRC combination was 0.79 (95% CI 0.72–0.86) (). Comparing the sensibility, specificity, positive and negative predictive value, and area under the ROC curve of each CAT/MRC cutoff, the best combination without a significant loss in sensibility was CAT/MRC≥5.5 (OR 6.73; 95% CI 3.26–13.9; p <0.0001). CAT/MRC≥5.5had a specificity of 80.3% (95% CI 69.5%-88.5%); sensitivity of 62.3% (95% CI 50.6%-73.1%); positive and negative predictive values of 76.2% (95% CI 66.3%-83.9%), and67.8% (95% CI 60.7%-74.1%), respectively.
Table 2 Regression Model Evaluating Predictive Variables Associated with Frailty Among the 153 COPD Patients Enrolled in the Study
Discussion
The prevalence of frailty among the 153 COPD patients evaluated was 50.3% (77/153) and the prevalence of pre-frailty was 35.3% (54/153). The level of dyspnea according to the MRC, the health status according to the CAT score, and COPD groups B or D according to the GOLD severity score were associated with frailty state. According to the literature, frailty prevalence in COPD patients varies from 6% to 82%.Citation6,Citation9–Citation15 This large variability could be explained by differences in demographics, disease severity, and comorbidities amongst study samples. Our findings are similar to the study of Park et al (2013) that found a prevalence of frailty of 57.8% in 211 COPD patients and a direct association between dyspnea and frailty.Citation23 On the other hand, a frailty prevalence of 82% was found in another study evaluating a sample of COPD patients with a mean age of 65 years,93.2% of whom were classified into groups B and D on the GOLD severity score, and 43% of whom had two or more comorbidities.Citation13 Our sample also included moderate to severe COPD patients with a similar mean age, and found a much lower frailty prevalence (50.3%). It is possible that the provision of free medicines to subjects in our sample could increase treatment adherence and have some impact on disease control and frailty prevalence. Also, the present study used the FRAIL scale to define frailty, whereas different studies have used other scales such as the Frail Non-Disabled (FIND) questionnaire, the Kihon Checklist, and the Modified Frailty Index. This also could impact on the measurement of frailty prevalence.Citation13,Citation15,Citation24
In our study, we found a significant association between frailty and CAT. Similar results were found by Maddocks and Ierodiakonou, who besides demonstrating that CAT was a predictor of frailty, additionally found that higher CAT was associated with failure to complete a rehabilitation program.Citation12,Citation13 Lahousse et al (2016) also found a higher prevalence of frailty among COPD patients with GOLD severity scores B and D.Citation14 This increased risk of frailty among GOLD severity groups B and D is probably associated with the MRC and CAT scores, since both of these parameters are used to stratify COPD patients as GOLD B or D groups, patients in group D are 8 times more likely to present frailty than the others and group B has a high prevalence of the syndrome.Citation25 We did not find a statistical difference between the frailty categories regarding inhaled corticosteroids or bronchodilators, but a predominance of double or triple therapy (LABA+LAMA or LABA+LAMA+IC) was observed in the pre-frail or frail groups. Fragility is associated with dyspnea, and as it increases, there is a need to introduce more than one bronchodilator, as described in the GOLD guidelines.Citation22 However, Pei-Ju Chen et al found that the number of medications used, was a predictor of frailty in COPD patients with and without dyspnea, irrespective of being bronchodilators.Citation26
In our sample, there was no association between BMI and FRAIL score. This finding is similar to that of a study of 121 COPD patients conducted by Limpawattana et al (2017).Citation11 In fact, although a loss of muscle mass can be found in COPD patients, BMI has not been associated with frailty, although fragility and malnutrition often coexist.Citation27,Citation28
In our sample, FEV1 was lower for frail patients. However, there was no difference in the FEV1/FVC ratio between frail and non-frail individuals (). Kusunose et al (2017), in a study of patients with mild to severe airflow limitation, also found a lower FEV1among frail subjects and no differences in FEV1/FVC or Total Lung Capacity (TLC) between the groups studied.Citation15 In fact, FEV1 describes airflow limitation better than the FEV1/FVC ratio and is, therefore, the parameter used to classify COPD severity internationally.Citation22
The present study has an important limitation. Patients were invited to take part in the study when visiting the CEMAC public center in order to obtain free COPD medicines. Since the medicines can be picked up by subjects other than the patients themselves, some COPD patients with limited mobility may not have attended CEMAC in person and thus could not be invited to participate in the study. Therefore, a selection bias is possible and the results should be not extrapolated to COPD patients who have restricted mobility.
Conclusion
Frailty has a great clinical impact, but it is not routinely investigated in COPD patients. In this sample of COPD patients, the frailty prevalence was high and was associated with higher MRC and CAT scores, parameters used routinely in the management of COPD and included in the ABCD assessment tool of the Global Initiative for Chronic Obstructive Lung Disease. As the combination of CAT/8+MRC≥5.5 was highly associated with frailty, an additional specific evaluation for the presence of frailty is indicated. If this combination is used as predictive tool for early identification of frailty, new therapeutic interventions can be made, helping doctors to better manage their patients on the risks and prognosis associated with frailty.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Ms Anna Carolina Galvão Ferreira reports a PhD scholarship from Fundação de Amparo Pesquisa do Estado de Goiás - FAPEG, personal fees from Boehringer Ingelheim, personal fees from Novartis, outside the submitted work. Prof. Dr. Marcelo Fouad Rabahi reports grants from CNPq, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank the COPD patients who participated in this study and CEMAC.
References
- DentE, MartinFC, BergmanH, WooJ, Romero-ortunoR, WalstonJD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-431609229
- FriedLP, TangenCM, WalstonJ, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A(3):M146–56. doi:10.1093/gerona/56.3.M146
- Van KanGA, RollandYM, MorleyJE, VellasB. Frailty: toward a clinical definition. JAMDA. 2008;9(2):71–72. doi:10.1016/j.jamda.2007.11.00518261696
- HoogendijkEO, AfilaloJ, EnsrudKE, KowalP, OnderG, FriedLP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi:10.1016/S0140-6736(19)31786-631609228
- VermeirenS, Vella-AzzopardiR, BeckwéeD, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. JAMDA. 2016;17(12):1163.e1e1163.e17.
- GaliziaG, CacciatoreF, TestaG, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res. 2011;23(2):118–125.21743290
- MadanSA, FidaN, BarmanP, et al. Frailty assessment in advanced heart failure. J Card Fail. 2016;22(10):840–844. doi:10.1016/j.cardfail.2016.02.00326883168
- DenfeldQE, Winters-stoneK, MuddJO, GelowJM, KurdiS, LeeCS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol. 2017;01(236):283–289. doi:10.1016/j.ijcard.2017.01.153
- MarengoniA, VetranoDL, Manes-GravinaE, BernabeiR, OnderG, PalmerK. The relationship between COPD and frailty. Chest. 2018;154(1):21–40. doi:10.1016/j.chest.2018.02.01429477493
- KennedyCC, NovotnyPJ, LeBrasseurNK, WiseRA, SciurbaFC, BenzoRP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Annalsats. 2018;16(2):1–34.
- LimpawattanaP, PutraveephongS, InthasuwanP, BoonsawatW, TheerakulpisutD, ChindaprasirtJ. Frailty syndrome in ambulatory patients with COPD. Int J COPD. 2017;12(134233):1193–1198. doi:10.2147/COPD.S134233
- MaddocksM, KonSSC, CanavanJL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. doi:10.1136/thoraxjnl-2016-20846027293209
- IerodiakonouD, KampourakiM, PoulonirakisI, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med. 2019;19(1):1–9.30606165
- LahousseL, ZiereG, VerlindenVJA, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71(5):689–695. doi:10.1093/gerona/glv15426355016
- KusunoseM, OgaT, NakamuraS, HasegawaY, NishimuraK. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res. 2017;4(1):1–7.
- MorleyJE, MalmstromTK, MillerDK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi:10.1007/s12603-012-0084-222836700
- AprahamianI, LinSM, SuemotoCK, et al. Feasibility and factor structure of the FRAIL scale in older adults. JAMDA. 2017;18(4):367.e11e367.e18.
- CharlsonME, PompeiP, AlesKL, MacKenzieCR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.3558716
- KovelisD, SegrettiNO, ProbstVS, LareauSC, BrunettoAF, PittaF. Validation of the modified pulmonary functional status and dyspnea questionnaire and the medical research council scale for use in Brazilian patients with chronic obstructive pulmonary disease. J Bras Pneumol. 2008;34(12):1008–1018. doi:10.1590/S1806-3713200800120000519180335
- JonesPW, HardingG, BerryP, WiklundI, ChenWH, Kline LeidyN. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.0010250919720809
- SilvaGPF, MoranoMTAP, VianaCMS, MagalhãesCBA, PereiraEDB. Validation of the COPD Assessment Test in Portuguese for use in Brazil. J Bras Pneumol. 2013;9(4):402–408.
- Global Initiative for Chronic Obstructive Lung Disease. Report. Global Initiative for Chronic Obstructive Lung Disease: Pocket Guide to COPD Diagnosis, Management and Prevention: A Guide for Health Care Professionals 2019; 2019.
- ParkSK, RichardsonCR, HollemanRG, LarsonJL. Physical activity in people with COPD, using the national health and nutrition evaluation survey dataset (2003-2006). Hear Lung J Acute Crit Care. 2013;42(4):235–240. doi:10.1016/j.hrtlng.2013.04.005
- ValenzaMC, Torres-SánchezI, Cabrera-MartosI, Rodríguez-TorresJ, González-JiménezE, Muñoz-CasaubonT. Physical activity as a predictor of absence of frailty in subjects with stable COPD and COPD exacerbation. Respir Care. 2016;61(2):212–219. doi:10.4187/respcare.0411826535000
- MihaltanF, AdirY, AntczakA, et al. Importance of the relationship between symptoms and self-reported physical activity level in stable COPD based on the results from the SPACE study. Respir Res. 2019;20(1):1–11. doi:10.1186/s12931-019-1053-730606211
- ChenPJ, YangKY, PerngWC, LinKC, WangKY. Effectofdyspneaonfrailtystagesandrelatedfactors in Taiwanesemenwith COPD. Int J ChronObstructPulmonDis. 2018;13:2463–2469. doi:10.2147/COPD.S172694
- MarchioroJ, GazzottiMR, MoreiraGL, et al.; PLATINO Team. Análise evolutiva antropométrica em indivíduos com DPOC na cidade de São Paulo - estudo de base populacional. J Bras Pneumol. 2019;45(6):e20170157.31365731
- BeekL, van der VaartH, WempeJB, et al. Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin Nutr. 2019;S0261-5614(19):33143–33147. doi:10.1016/j.clnu.2019.11.016

![Figure 3 ROC curve of CAT/MRC combination [(CAT/8)+MRC] to identify frailty in COPD patients.](/cms/asset/9a39ccbe-146d-4b15-b0be-09c8955a1065/dcop_a_12198225_f0003_c.jpg)