Abstract
Introduction
Dysphagia is a newly acknowledged multifactorial risk factor for the exacerbation of chronic obstructive pulmonary disease (COPD). Effective screening methods are awaited. We performed a prospective study to evaluate the impact of musculature and breathing–swallowing discoordination on the exacerbation of COPD with a novel swallowing monitor using a piezoelectric sensor.
Patients and Methods
This was the second part of a prospective study of patients with COPD from the Iizuka COPD cohort. Seventy patients with stable COPD underwent dysphagia screening, skeletal muscle mass index (SMI) and tongue pressure measurements, and swallowed 3 mL and 30 mL of water while wearing a swallowing monitor. Patients were followed for one year.
Results
During the follow-up period, 28 patients experienced exacerbations (E group), and 42 had none (non-E group). There was no significant difference in tongue pressure measurements between the two groups. The SMI in the E group was significantly lower than that in the non-E group. Among the swallowing monitor measurements, the 3 mL I-SW% (the percentage of swallows in which inspiration preceded the swallow [out of ten 3 mL swallows]) was significantly lower in the E group than in the non-E group.
Conclusion
Breathing–swallowing coordination is an independent factor related to the exacerbation of COPD. Not only the presence of discoordination but also the inability to produce an airway protection mechanism may contribute to more frequent aspiration and exacerbations.
Introduction
Chronic obstructive pulmonary disease (COPD) is an expanding issue worldwide. One of the recent topics in COPD is dysphagia. It has become clear that patients with COPD are more likely to complicate dysphagia, even at the early stages of the disease.Citation1 Dysphagia is a newly acknowledged phenotype associated with an increased risk of exacerbation.Citation1 Effective ways to screen for dysphagia in patients with COPD are being investigated.Citation2,Citation3 Many characteristics of patients make them vulnerable to dysphagia, such as smoking history, sarcopenia, dry mouth, lung hyperinflation, the high prevalence of gastroesophageal reflux disease (GERD), and reduced maximal laryngeal elevation.Citation1,Citation4 However, the uniqueness of dysphagia in this population lies in the discoordination of breathing and swallowing.Citation2,Citation5 In healthy adults, swallowing has been known to occur during exhalation. In patients with COPD, this pattern can commonly be disrupted, causing inhalation to occur before or after the swallow. Especially with larger volumes of fluids, they tend to present an inspiration before swallowing.Citation6 These changes can cause aspiration and hence an exacerbation.Citation5
A novel technique, namely, a swallowing monitor using a piezoelectric sensor (hereinafter referred to as a “swallowing monitor”), has been tested effective for studying the association between breathing–swallowing discoordination and the risk of COPD exacerbations.Citation2 However, in this study, the technique was only effective when specifically designated test foods were used. It is challenging to apply these results to daily practice. Further investigation is necessary using a common subject. The primary aim of our study was to evaluate the utility of the swallowing monitor in predicting COPD exacerbation with water. The secondary aim was to find an effective screening test for daily practice, by comparing the results of the swallowing monitor with the dysphagia screening tests. In addition to dysphagia screening tests taken in our previous study,Citation7 two more measurements were taken. The first is the skeletal muscle mass index (SMI). It is becoming evident that a low muscle mass and sarcopenia can cause exacerbations and are poor prognostic factors in patients with COPD.Citation8 The second additional technique is tongue pressure measurement. Tongue pressure measurement has become popular for assessing oral phase dysphagia and sarcopenia;Citation9 however, it has not been investigated in depth in COPD.
Patients and Methods
Patients
This study was the second part of a prospective study of patients with COPD from the Iizuka COPD cohort. Ethical approval was provided by the Iizuka Hospital Ethics Committee, as instituted by the Declaration of Helsinki (Number 17026) and written informed consent was obtained from all patients. Seventy patients with COPD were recruited from the outpatient clinic of Iizuka Hospital Department of Respiratory Medicine from March 28 to September 22, 2017. Detailed inclusion/exclusion criteria and data collection methods can be found in our previous study.Citation7 Briefly, patients diagnosed with COPD according to the GOLD 2017 criteria were included.Citation10 Those with coexisting respiratory conditions, other conditions causing dysphagia, or a poor general condition were excluded.
A COPD exacerbation was defined by the presence of new or worsening COPD symptoms (cough, sputum, wheezing, dyspnea, or chest tightness), at least one of which lasted for three days or more. An exacerbation was classified as moderate or severe if at least one of the following were required: systemic steroid administration, antibiotic therapy, or hospital admission.Citation11,Citation12 Upon inclusion, patients underwent the following dysphagia screening tests: the 10-item Eating Assessment Tool (EAT-10),Citation13 Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (FSSG),Citation14 repetitive saliva swallowing test (RSST),Citation15 water swallowing test (WST),Citation16 and simple swallow provocation test (SSPT).Citation17
In addition to the above standard dysphagia screening tests, the following measurements were taken.
Skeletal Muscle Mass Index
The skeletal muscle mass index (SMI) was measured using the InBody 770 (Biospace Co. Ltd., Seoul, Korea). In this device, skeletal muscle mass was estimated from bioimpedance analysis measurements and expressed as SMI (SMI = skeletal muscle mass/body mass × 100).
Tongue Pressure
Tongue pressure was measured using a handy probe consisting of a small balloon pressurized to 19.6 kPa with air (JM-TPM; JMS, Hiroshima, Japan). Participants compressed the balloon with their tongue to the palate at maximum effort, for seven seconds. The maximum inner pressure of the balloon was measured and recorded. Three measurements were taken, and the average value was obtained. The reliability of intraindividual measurement in healthy individuals has been confirmed, with the coefficient of variation of 5.64%.Citation18 Normal values depend on age and sex, and are still under discussion.
Swallowing Monitor
A non-invasive swallowing monitoring system using a piezoelectric sensor detecting respiratory flow, swallowing sound, and laryngeal movement was used.Citation2 The patients were put on the swallowing monitor and seated facing forward in an upright position. First, 3 mL of water was injected into the oral cavity using a syringe. The patients were told to hold this in their mouth and then swallow (as in the modified WST). This was repeated ten times. Secondly, 30 mL of water in a cup was handed to the patient. They were told to drink all the water in their usual manner (as in the WST).
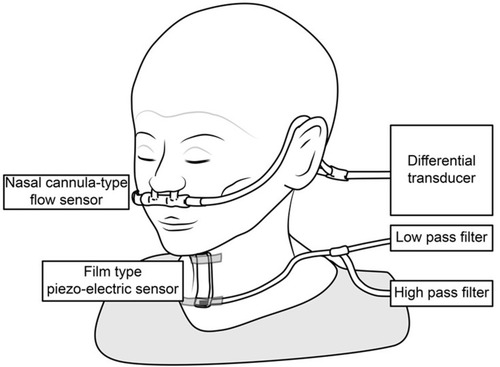
The swallowing monitor is shown in . It consists of a nasal cannula-type flow sensor, a film-type piezoelectric sensor, and signal processing units. Using a series of respiratory flow, the laryngeal sound and motion data, we evaluated (1) the duration of deglutition apnea and swallowing latency, and (2) breathing–swallowing coordination. Details on the monitor and its measurements can be found in previous reports.Citation2,Citation19
Figure 1 The swallowing monitor. The figure shows a non-invasive swallowing monitoring system using a film-type piezoelectric sensor taped to the front neck to detect swallowing sound and laryngeal movement, while a nasal canula-type flow sensor detects the respiratory flow.
The patients were followed up for one year, during which they were asked to keep a diary to record exacerbations. During the follow-up period, all medical records in our hospital and prescription records from all medical institutions were prospectively reviewed, along with the designated diaries. After one year, the patients were classified into two groups according to the presence or absence of COPD exacerbations during the follow-up period: the exacerbation group (E group) and the non-exacerbation group (non-E group). The abovementioned measurements were compared between the groups.
Statistical Analyses
Descriptive statistics for baseline data were presented as the percentage, mean, and standard deviation. Differences between the two groups were examined using Mann–Whitney’s U-test and Fischer’s exact test. P values of <0.05 were considered to indicate statistical significance. When there was a statistically significant difference between the two groups in any of the dysphagia screening results, a receiver operating characteristic (ROC) curve analysis was performed to evaluate its utility in the differentiation of the two groups. A log-rank analysis and univariate Cox regression analysis were performed to identify the best predictors of COPD exacerbations. All statistical analyses were performed using the JMP Pro software program (ver. 15; SAS Institute, Cary, NC, USA). Two-sided P values <0.05 were considered to indicate statistical significance.
Results
Details on patient background and dysphagia screening test results are explained in our previous study.Citation7 The patient background is shown in . There were 59 males (84.3%) and 11 females (15.7%). The mean age was 72.8 ± 7.5 years. During the 1-year follow-up period, 28 patients (40.0%) experienced one or more exacerbations (E group), and 42 (60.0%) experienced no exacerbations (non-E group). Patients in the E group were significantly more likely to have experienced exacerbations in the previous year than those in the non-E group. Statistically significant differences between the groups were observed in the results of the RSST, when the cut-off value was set at 2, 3, 4, or 5 swallows per 30 seconds (p < 0.01). With an RSST swallowing frequency of ≤5 times considered abnormal, the sensitivity and specificity were 42.9% and 96.4%, respectively, and the area under the ROC curve was 0.775 (95% confidence interval [CI], 0.670–0.881). Otherwise, there were no differences in patient background or dysphagia screening tests.
Table 1 Patient Characteristics
As shown in , there was no significant difference in the average of the three tongue pressure measurements between the E and non-E groups (29.5 ± 11.1 kPa vs 29.9 ± 9.4 kPa, respectively, p = 0.49). In contrast, as shown in , the SMI in the E group was significantly lower than that in the non-E group (6.8 ± 1.0 kg/㎡ and 6.1 ± 0.9 kg/㎡, respectively, p = 0.02). shows an ROC curve analysis using the skeletal muscle mass index to differentiate the two groups. When an SMI of <6.5 kg/㎡ was considered abnormal, the sensitivity and specificity were 75.0% and 57.1%, respectively, and the area under the ROC curve was 0.67 (95% confidence interval [CI], 0.536–0.796). shows the Kaplan–Meier curves with the results of the Log rank test and univariate Cox regression analysis, which were used to compare the time to the first COPD exacerbation during the follow-up period. The time to the first exacerbation in patients with an SMI of >6.5 kg/㎡ (median not available [NA]) was significantly longer than that in patients with an SMI of ≤6.5 kg/㎡ (median 325.5 days, p=0.0145). The median time to exacerbation for the group of patients with an SMI of >6.5 kg/㎡ was not reached during the follow-up, and the 1-year exacerbation-free rate was 77.4%.
Figure 2 Tongue pressure measurements in the exacerbation group and the non-exacerbation group. There was no significant difference in the average of the three tongue pressure measurements between the two groups (29.5 ± 11.1 kPa vs 29.9 ± 9.4 kPa, respectively, p = 0.49).
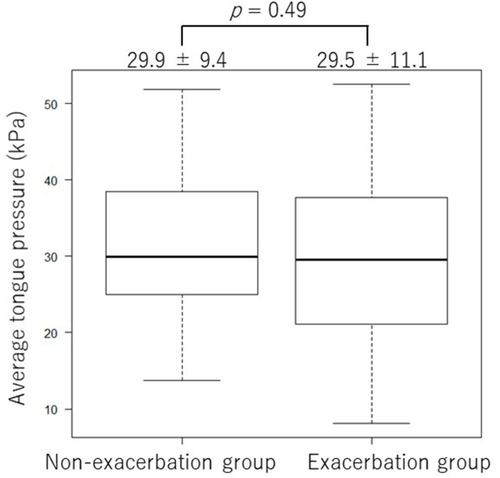
Figure 3 Skeletal muscle mass index and COPD exacerbation. (A) Skeletal muscle mass index in the Exacerbation group and the non-exacerbation group. The skeletal muscle mass index was significantly lower in the Exacerbation group than in the Non-exacerbation group (6.8 ± 1.0 kg/㎡ and 6.1 ± 0.9 kg/㎡, respectively, p = 0.02). (B) Results of the ROC curve analysis using the skeletal muscle mass index. When the cut-off value was set at 6.5 kg/㎡, the sensitivity and specificity were 75.0% and 57.1%, respectively, and the area under the ROC curve was 0.67 (95% confidence interval [CI], 0.536–0.796). (C) Kaplan–Meier curves of the time to first exacerbation (any severity) stratified by the skeletal muscle mass index cut-off value of 6.5. The time to first exacerbation was significantly longer in patients with an SMI higher than 6.5 kg/㎡ than in patients with an SMI 6.5 kg/㎡ or lower (p= 0.0145).
![Figure 3 Skeletal muscle mass index and COPD exacerbation. (A) Skeletal muscle mass index in the Exacerbation group and the non-exacerbation group. The skeletal muscle mass index was significantly lower in the Exacerbation group than in the Non-exacerbation group (6.8 ± 1.0 kg/㎡ and 6.1 ± 0.9 kg/㎡, respectively, p = 0.02). (B) Results of the ROC curve analysis using the skeletal muscle mass index. When the cut-off value was set at 6.5 kg/㎡, the sensitivity and specificity were 75.0% and 57.1%, respectively, and the area under the ROC curve was 0.67 (95% confidence interval [CI], 0.536–0.796). (C) Kaplan–Meier curves of the time to first exacerbation (any severity) stratified by the skeletal muscle mass index cut-off value of 6.5. The time to first exacerbation was significantly longer in patients with an SMI higher than 6.5 kg/㎡ than in patients with an SMI 6.5 kg/㎡ or lower (p= 0.0145).](/cms/asset/23b3c308-1a50-4bef-9a17-014085dc3f53/dcop_a_12198262_f0003_b.jpg)
Among the measurements of the swallowing monitor, the 3 mL I-SW% (the percentage of swallows in which inspiration preceded the swallow [out of ten 3 mL swallows]) was significantly lower in the E group than in the non-E group (7.2 ± 10.2 vs 17.4 ± 17.6 times, p =0.007), as shown in . shows the ROC curve analysis using I-SW% to differentiate the two groups. When the cutoff level of I-SW% was set at 0, the sensitivity and specificity were 60.7% and 71.4%, respectively, and the area under the ROC curve was 0.682 (95% confidence interval [CI], 0.562–0.802). The Kaplan–Meier curves with the Log rank test and univariate Cox regression analysis comparing the time to the first COPD exacerbation of any severity are shown in . The time to the first exacerbation was significantly longer in patients with one or more I-SW than those with no I-SW (median, not available vs 262 days, p = 0.002). Therefore, this may be a useful factor for predicting the risk of a COPD exacerbation. The median time to exacerbation for the group of patients with an I-SW% > 0 was not reached during the follow-up, and the 1-year exacerbation-free rate was 73.2%.
Figure 4 I-SW in 3 mL swallows and COPD exacerbation. (A) The incidence of I-SW in 3 mL swallows. The 3 mL I-SW% (the percentage of swallows in which inspiration preceded the swallow, out of the ten 3 mL swallows) was significantly lower in the E group than in the non-E group (7.2 ± 10.2 vs 17.4 ± 17.6 times, p =0.007). (B) Results of the ROC curve analysis using the frequency of I-SW (%) of all the 3 mL swallows. When the cut-off value was set at 0, the sensitivity and specificity were 60.7% and 71.4%, respectively, and the area under the ROC curve was 0.68 (95% confidence interval [CI], 0.562–0.802). (C) Kaplan–Meier curves of the time to first exacerbation (any severity) stratified by the presence of one or more I-SW. The time to first exacerbation was significantly longer in patients with one or more I-SW than those with no I-SW (median, not available vs 262 days, p = 0.002).
![Figure 4 I-SW in 3 mL swallows and COPD exacerbation. (A) The incidence of I-SW in 3 mL swallows. The 3 mL I-SW% (the percentage of swallows in which inspiration preceded the swallow, out of the ten 3 mL swallows) was significantly lower in the E group than in the non-E group (7.2 ± 10.2 vs 17.4 ± 17.6 times, p =0.007). (B) Results of the ROC curve analysis using the frequency of I-SW (%) of all the 3 mL swallows. When the cut-off value was set at 0, the sensitivity and specificity were 60.7% and 71.4%, respectively, and the area under the ROC curve was 0.68 (95% confidence interval [CI], 0.562–0.802). (C) Kaplan–Meier curves of the time to first exacerbation (any severity) stratified by the presence of one or more I-SW. The time to first exacerbation was significantly longer in patients with one or more I-SW than those with no I-SW (median, not available vs 262 days, p = 0.002).](/cms/asset/baac66b3-8a09-4bc0-ab13-c251ec701ac2/dcop_a_12198262_f0004_b.jpg)
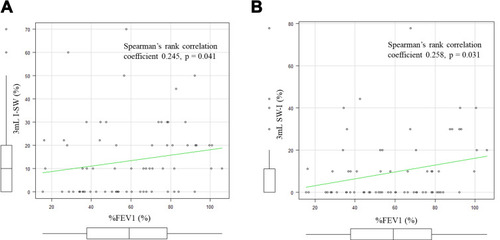
The correlation between the I-SW% and SW-I% in 3mL water and %FEV1 is shown in . As shown in , the I-SW% and %FEV1 showed a positive correlation (Spearman’s rank correlation coefficient 0.245, p = 0.041). There was also a positive correlation in the SW-I% in 3 mL water and %FEV1, as shown in (Spearman’s rank correlation coefficient 0.258, p = 0.031). However, both correlations were weak. There was no correlation between the SMI and 3 mL I-SW% (Spearman’s Rank correlation coefficient −0.0568, p = 0.641). There were no correlations between other measurements of the swallowing monitor and the results of the dysphagia screening test.
Figure 5 I-SW%, SW-I% and %FEV1. (A) The correlation between the I-SW% in 3mL water and %FEV1. The I-SW% and %FEV1 showed a positive correlation (Spearman’s rank correlation coefficient 0.245, p = 0.041). (B) The correlation between the SW-I% in 3mL water and %FEV1. The SW-I% and %FEV1 also showed a positive correlation (Spearman’s rank correlation coefficient 0.258, p = 0.031).
Discussion
We performed a prospective study to determine the utility of the swallowing monitor with water in patients with COPD. Our results show that in patients with COPD who have a higher I-SW%, the risk of an exacerbation was lower. Generally, the presence of inspiration immediately before or after a swallow is thought to be associated with a high risk of aspiration.Citation2,Citation5 Our results differed from these reports. The reason for this difference may be due to the unique swallowing changes in COPD and our study methods.
It is known that the swallowing physiology in patients with COPD differs from that in normal subjects. In patients with COPD, the maximal laryngeal elevation during swallowing is reduced, which increases the risk of penetration and aspiration.Citation4 To reduce this risk, some patients have been found to show voluntary airway-protecting behaviors during swallowing, such as prolonged airway closure and earlier laryngeal closure. Patients with COPD also have a longer pharyngeal swallowing phase.Citation20 The time between the onset of deglutition apnea to the onset of swallowing and also the duration of deglutition apnea are lengthened in swallows with the I-SW-E pattern in comparison to the E-SW-E pattern.Citation19 Therefore, the I-SW-E pattern observed in patients with COPD while swallowing large boluses is interpreted as an airway-protecting behavior to compensate for the delayed triggering of the swallowing reflex and a longer pharyngeal transit time. This is suspected to be one of the reasons why the COPD exacerbation risk was lower in those with a higher prevalence of I-SW pattern.
Regarding the methodology in the present study, we injected 3 mL of water into the patient’s oral cavity using a syringe and instructed them to hold the water in their mouth before swallowing. This may have caused patients to inhale before the procedure, as an airway protective mechanism. In other words, patients with COPD who have the capacity to compensate for their impaired breathing may have taken a breath before holding their breath to swallow. On the other hand, the patients who were not able to inhale before swallowing may have been those who were unable to compensate, thereby increasing their risk of aspiration and an exacerbation. The atypical way of swallowing water inserted by a syringe (and the fear of choking on it) may have brought forth more airway protective mechanisms in some patients. This may also help to explain why the I-SW% and SW-I% were not significantly different in the two groups when drinking 30 mL of water. In the WST, water was provided in a cup, and patients were instructed to drink it at their own pace. This may have led to less cautious behavior in the 30 mL WST than in the procedure for the 3 mL of water. This may have also been the reason why the I-SW% was higher in patients with fewer exacerbations in the previous study,Citation2 in which jelly was used instead of water. Jelly may have caused less cautious behavior than water. It may be informing to perform additional studies with emphasis on procedures and instructions that would enable a natural swallowing pattern. For instance, it may be valuable to evaluate I-SW% during a regular meal or while sleeping.
Patients with more severe COPD tended to have lower I-SW% and SW-I% values. Although statistically significant, the correlation coefficients were low, suggesting they are weak confounding factors. With the progression of COPD, lung hyperinflation and breathlessness also worsens. These factors may make it difficult for patients to inhale before swallowing for airway protection. The same can also be assumed to happen after swallowing; the more severe the COPD, the higher the intrathoracic pressure, and therefore the more difficult it is to inhale after swallowing.
The measurements of the swallowing monitor did not show any correlation with any dysphagia screening tests performed in this cohort. This suggests that breathing–swallowing discoordination is a unique and independent factor of swallowing disorder in patients with COPD.
We also studied the utility of SMI and tongue pressure. Our results show that tongue pressure measurement is not useful for differentiating patients at risk of a COPD exacerbation, while SMI may be of use. Both are useful in detecting sarcopenia. Sarcopenia is one of the leading causes of dysphagia and aspiration in the elderly population.Citation21 It is understandable that a low SMI is correlated with the risk of a COPD exacerbation. However, dysphagia in COPD is known to be multifactorial, and sarcopenia is only one of the risk factors.Citation7 Furthermore, tongue pressure is only one small part of the systemic musculature. In addition, the oral coordination needed for tongue pressure measurement was difficult to achieve in some patients. Some showed great difficulty in accomplishing the task. We suspect that these may be the reasons why the tongue pressure was not useful in detecting risky phenotypes in patients with COPD.
Our study supports the recent understanding that dysphagia in COPD is multifactorial. The inability to take airway protective measures, breathing–swallowing discoordination, lung hyperinflation, breathlessness, gastroesophageal reflux, and sarcopenia may all be causes of aspiration and COPD exacerbations. This may be the reason why, according to our previous studyCitation7 and the current study, the RSST and I-SW% in 3 mL water were useful for detecting those at risk of an exacerbation. However, in the current medical technology, it is not possible to differentiate whether a certain episode of exacerbation was due to breathing–swallowing discoordination or not. Moreover, it is not possible to differentiate whether an exacerbation (or a lower respiratory tract infection) was due to an aspiration or not. Therefore, there is clinical importance in identifying those at risk of aspiration through screening and assessment methods.
Our findings are essential towards the next step in this area of interest: intervention. Ideally, intervention that improves multiple aspects of dysphagia may be the most effective type. In the past, McKinstry et al reported improvement in dysphagic COPD patients with patient education and adequate rehabilitation.Citation22 However, its effect on exacerbations of COPD and the prognosis of COPD patients remains unclear. More studies on intervention are needed.
The present study is associated with several limitations. First, this was a relatively small study that was performed in a single center, in which there was no healthy control group. Second, instrumental swallowing assessments such as videofluoroscopy or videoendoscopy were not performed. Third, breathing–swallowing coordination was evaluated in different procedures for 3 mL and 30 mL. Three milliliters of water were inserted using a syringe, and 30 mL was given in a cup. This was done according to the modified WST and WST, respectively. The syringe administration of water may have affected the swallowing pattern. Repetitive testing of the 30mL WST may have been informative. Additionally, to evaluate breathing–swallowing coordination in a more natural setting, the use of a cup (instead of a syringe) for different volumes of water may be a topic for future studies. To evaluate swallows as naturally as possible, it may be of interest to take measurements with the swallowing monitor during meals or while sleeping. However, this is not realistic in the daily clinic setting. An alternative may be to take measurements while performing the RSST.
This is the second study to investigate the utility of the swallowing monitor in detecting patients at risk of COPD exacerbations. Further studies to unravel the pathophysiology underlying dysphagia in patients with COPD are awaited.
Conclusion
Breathing–swallowing coordination is independently related to the exacerbation of COPD. In addition to the presence of discoordination, the inability to produce an airway protection mechanism may contribute to an increased risk of aspiration and exacerbations.
Disclosure
The authors have no conflicts of interest in connection with this article.
Acknowledgments
The authors would like to express their deepest gratitude to the following members of the Iizuka Hospital Department of Respiratory Medicine for their contribution to this study: Takuto Sueyasu, Saori Nishizawa, Miyuki Munechika, Kohei Yoshimine, Yuki Ko, Mina Yasuda, Hiromi Ide, Kosuke Tsuruno, and Noriyuki Ebi. We also thank Dr. Susumu Sato for kindly contributing the artwork showing the swallowing monitor.
References
- SteidlE, RibeiroCS, GonçalvesBF, FernandesN, AntunesV, MancopesR. Relationship between dysphagia and exacerbations in chronic obstructive pulmonary disease: a literature review. Int Arch Otorhinolaryngol. 2015;19:74–79. doi:10.1055/s-0034-137643025992155
- NagamiS, OkuY, YagiN, et al. Breathing-swallowing discoordination is associated with frequent exacerbations of COPD. BMJ Open Respir Res. 2017;4:e000202. doi:10.1136/bmjresp-2017-000202
- KobayashiS, KuboH, YanaiM. Impairment of the swallowing reflex in exacerbations of COPD. Thorax. 2007;62:1017. doi:10.1136/thx.2007.084715
- TeradaK, MuroS, OharaT, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137:326–332. doi:10.1378/chest.09-048219783670
- GrossRD, AtwoodCW Jr, RossSB, OlszewskiJW, EichhornKA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:559–565. doi:10.1164/rccm.200807-1139OC19151193
- CvejicL, HardingR, ChurchwardT, et al. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2011;16:269–275. doi:10.1111/j.1440-1843.2010.01875.x21054669
- YoshimatsuY, TobinoK, SueyasuT, et al. Repetitive saliva swallowing test predicts COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2019;14:2777–2785. doi:10.2147/COPD.S22626831824143
- GuerriR, GayeteA, BalcellsE, et al. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Respir Med. 2010;104:378–388.19932014
- MaedaK, AkagiJ. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30:80–87. doi:10.1007/s00455-014-9577-y25248988
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557. doi:10.1164/rccm.201701-0218PP28128970
- KimV, AaronSD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52:1801261. doi:10.1183/13993003.01261-201830237306
- AnthonisenNR, ManfredaJ, WarrenCP, HershfieldES, HardingGK, NelsonNA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi:10.7326/0003-4819-106-2-1963492164
- HidetakaW, JunK. Translation, reliability, and validity of the Japanese version of the 10-item Eating Assessment Tool (EAT-10) for the screening of dysphagia. JJSPEN. 2014;29:871–876. [in Japanese].
- KusanoM, ShimoyamaY, SugimotoS, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–891. doi:10.1007/s00535-004-1417-715565409
- OguchiK, SaitohE, BabaM, KusudoM, TanakaT, OnogiK. The repetitive saliva swallowing test (RSST) as a screening test of functional dysphagia (2). Validity of RSST. Jpn J Rehabil Med. 2000;38:383–388. [in Japanese].
- KubotaT, MishimaH, HanadaM, NambaI, KojimaY. Dysphagia paralytica in cerebrovascular disease: screening test and its clinical application. Sogo Rihabiriteshon. 1982;10:271–276. [in Japanese].
- HayashiR, TsugaK, HosokawaR, YoshidaM, SatoY, AkagawaY. A novel handy probe for tongue pressure measurement. Int J Prosthodont. 2002;15:385–388.12170854
- YagiN, OkuY, NagamiS, et al. Inappropriate timing of swallow in the respiratory cycle causes breathing-swallowing discoordination. Front Physiol. 2018;8:676. doi:10.3389/fphys.2017.00676
- MokhlesiB, LogemannJA, RademakerAW, StanglCA, CorbridgeTC. Oropharyngeal deglutition in stable COPD. Chest. 2002;121:361–369. doi:10.1378/chest.121.2.36111834644
- CassianiRA, SantosCM, Baddini-MartinezJ, DantasRO. Oral and pharyngeal bolus transit in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:489–496. doi:10.2147/COPD.S7494525784795
- FujishimaI, Fujiu-KurachiM, AraiH, et al. Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int. 2019;19:91–97. doi:10.1111/ggi.1359130628181
- McKinstryA, TranterM, SweeneyJ. Outcomes of dysphagia intervention in a pulmonary rehabilitation program. Dysphagia. 2010;25:104–111. doi:10.1007/s00455-009-9230-319618132
