Abstract
Objective
The aim of this study was to analyse the level of serum matrix metalloproteinases (MMPs) in atopic and non-atopic COPD patients, providing guidance for clinical practice and theory for atopic COPD.
Methods
Blood samples from 50 adult male patients with COPD, including 17 atopic and 33 non-atopic patients, were submitted for detection of MMP8, MMP9, surfactant associated protein D (SPD), noradrenaline (NE), leukotriene (LT) B4, recombinant proteoglycan (PRG4), Phadiatop sIgE, and tIgE levels. Patients’ Modified Medical Research Council Dyspnea Scale (mMRC), COPD Assessment Test (CAT), pulmonary function test results, FeNO, blood cell ratio and induced sputum were collected.
Results
The level of serum tIgE in patients with atopic COPD [1876.00 kU/l (760.50, 5347.00)] was significantly higher than in patients with non-atopic COPD [377.00 kU/l (93.50, 581.50), P < 0.001]. The MMP8 levels in atopic COPD (1600 ± 1181 ng/mL) were significantly higher than in non-atopic COPD (973.3 ±921.5 ng/mL, P = 0.0494), but there was no significant difference in MMP9, SPD, NE, LTB4, and PRG4 levels between the two groups. In atopic COPD patients, the rate of leukocyte (rs = 0.63, P < 0.001) and neutrophil (rs = 0.54, P < 0.05) were positively correlated with MMP8 levels, while lymphocyte rate was negatively correlated with MMP8 (rs = −0.70, P < 0.001) and MMP9 levels (rs = −0.54, P < 0.05). Optimal scale analysis showed that NE was most closely related to the basophil rate from induced sputum and FeNO levels (Cronbach’s alpha = 85.1%). Interestingly, all atopic COPD patients with mMRC ≥2, CAT ≥ 10, and CCQ ≥16 exhibited MMP8 levels >1000 ng/mL.
Conclusion
In general, tIgE and MMP8 levels were higher in atopic COPD patients than in non-atopic patients. NE levels were closely correlated with the basophil rate of induced sputum and FeNO levels, which may play an important role in the pathogenesis and development of atopic COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease, which is characterized by persistent airflow limitation and progressive respiratory symptoms associated with airway and/or alveolar abnormalities caused by continuous exposure to noxious particles or gases.Citation1 Worldwide, COPD is predicted to cause over 6 million deaths annually, and bring heavy medical burden to patients’ families.Citation2 Our previous study showed that 32.7% of the COPD cases were atopic, and atopens are one of the most important factors causing acute attack in COPD patients.Citation3 Jin J et al found that COPD patients with increased total IgE (tIgE) levels have a longer dyspnea history and more serious symptoms than patients without this feature.Citation4 Another study in Egypt revealed that atopic COPD patients had a longer history of illness, high smoking index, and a lower FEV1.Citation5 Therefore, it is very important to study the characteristics and pathogenesis of atopic COPD.
Matrix metalloproteinases (MMPs), a major group of enzymes that regulate cell–matrix composition, are zinc-dependent endopeptidases known for their ability to cleave one or several extracellular matrix constituents, as well as non-matrix proteins.Citation6 MMPs are involved in a number of processes relevant to COPD, including growth factor, cytokine, and chemokine activity modulation; inflammatory cell migration; extracellular matrix turnover; and tissue remodeling.Citation7 A study from Poland showed that COPD patients were predisposed to produce more MMP9 and MMP9/TIMP1 complexes compared to healthy individuals.Citation8 Another study from Finland found a significant association between the levels of MMP8, neutrophil rate, and markers of their activation (lactoferrin and myeloperoxidase).Citation9 As atopy involves migration of inflammatory cells and release of inflammatory factors, MMP8, MMP9, etc. inflammatory biomarkers may play an important role in atopic COPD.Citation10 Meanwhile, previous research showed that surfactant associated protein D (SPD), noradrenaline (NE), leukotriene (LT) B4 and recombinant proteoglycan (PRG4) are also related to atopic diseases.Citation11–Citation13 Unfortunately, studies comparing their levels in atopic and non-atopic COPD patients from southern China were not available. Consequently, this study assessed the levels of serum above immune factors in atopic and non-atopic COPD patients. The aim was to analyze the clinical characteristics and pathogenesis of the disease, providing guidance for clinical practice and theory of atopic COPD.
Materials and Methods
Ethical Approval
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, with approval number: GYFYY-2016-73. Human serum samples were used in accordance with legislation in China and the wishes of donors, their legal guardians, or next of kin, where applicable, who had offered written informed consent to using the serum samples for future unspecified research purposes.
Patients
This cross-sectional study included a total of 50 adult male patients (>18 years old): 17 with atopic COPD and 33 with non-atopic COPD. Their data were obtained from the Allergy Information Repository of State Key Laboratory of Respiratory Disease (AIR-SKLRD) from January 2018 to January 2019, in south China. COPD was diagnosed by respiratory specialists using Guidelines for the Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease (ie, evidence of irreversible obstructive impairment on spirometry, as defined by a post-bronchodilator FEV1/FVC <70%).Citation14 COPD patients who have (i) contact history with one or more common inhalant allergens (such as mites, pollen, animal dander, and fungi) and (ii) Phadiatop serum IgE ≥0.35 kU/l (ThermoFisher, Uppsala, Sweden) were defined as atopic COPD. Patients undergoing immunotherapy or with parasitic infection, food allergy, cancer, or other autoimmune diseases were excluded from this study. The patients’ sex, age, questionnaire information (including Modified Medical Research Council Dyspnea Scale [mMRC] and COPD Assessment Test [CAT]), pulmonary function test results (forced vital capacity [FVC], forced expiratory volume in 1 second [FEV1]), fractional exhaled nitric oxide (FeNO), blood cell ratio, and induced sputum were collected by a skillful investigator (Chuanxu Cai) who was unaware of the Phadiatop test results. Exacerbation times were defined by patients’ history of acute attacks leading to hospitalization.
Detection of Serum IgE
Patient samples were obtained from the serum bank of the AIR-SKLRD. Around 5 mL of venous blood was taken and centrifuged at 3000 g for 10 minutes to prepare the supernatant, then stored at −80°C before detection. In this study, the Phadiatop sIgE (including fungi, pollen, insects, and dust mites) and tIgE levels of serum samples were assessed using the Phadia 1000 automatic allergy analyzer (ThermoFisher, Uppsala, Sweden) for in vitro diagnostic use. Samples with a Phadiatop sIgE concentration ≥0.35 kU/L were considered positive.
Detection of MMP8, MMP9, SPD, NE, LTB4, and PRG4
A total of 2 mL of venous blood was taken from the patients, and put into a test tube containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant. After centrifuging at 1000 × g for 10 min, the upper plasma was taken for testing. The plasma levels of MMP8, MMP9, SPD, NE, LTB4, and PRG4 were detected using the ELISA kit (DRG Instruments GmbH, Germany). Results were reported in ng/mL.
Blood Cell Ratio Measurement
Peripheral blood was taken from patients and the ratios of leukocytes to blood cells, neutrophil, eosinophils, monocyte, lymphocyte, and basophil were measured using optical microscope.
Lung Function Measurement
Lung function parameters such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximum expiratory flow (MEF 25%, 50% and 75%) were assessed using spirometers (Jaeger, Germany) according to the recommendations of the American Thoracic Society/European Respiratory Society.
Induced Sputum Measurement
Sputum was induced by ultrasonic atomization inhalation of hypertonic saline. After smear, the neutrophil, eosinophils, monocyte and lymphocyte of induced sputum were counted under an optical microscope.
Fractional Exhaled Nitric Oxide Measurement
Fractional exhaled nitric oxide (FeNO) measurements were performed in the morning prior to the lung function tests using a chemiluminescence analyzer (NIOX MINO, Aerocrine, Sweden), according to international guidelines. Results were reported in ppb.
Data Analysis
Statistical studies were conducted with SPSS 22.0 (Chicago, IL). Parametric quantitative data were presented as the mean ± standard deviation, and differences between the two groups were analyzed by T-test. Non-parametric quantitative data were presented as a median (25%, 75%), and the Mann–Whitney U-test was applied to compare two-group differences. Categorical data were reported as a percentage showing the proportion of positive results. Proportions were compared between groups with chi-square tests (χ2). Correlation analyses between non-parametric data were performed using Spearman’s tests, with the correlation coefficients presented as “rs”. The correlation between immune factors, sputum induction, and FeNO was calculated with the optimal scale analysis. P-value <0.05 was considered to be statistically significant.
Results
Baseline Characteristics
A total of 50 male patients with COPD were included. Among them, 17 were diagnosed with atopic COPD, with an age range of 45–79 years (mean 63.76 ± 8.62 years), 93.8% of the patients had smoked, and 37.5% of them had at least one exacerbation of the disease. The remaining 33 were non-atopic COPD patients, with an age range of 43–79 years (mean 63.48 ± 7.27 years), 87.1% of them had smoked, and 27.5% of the patients had at least one exacerbation of the disease. The level of serum tIgE in patients with atopic COPD [1876.00 kU/l (760.50, 5347.00)] was significantly higher than that determined in patients with non-atopic COPD [377.00 kU/l (93.50, 581.50), P < 0.001]. ()
Table 1 Baseline of Atopic and Non-Atopic COPD Patients
Comparison of MMP8, MMP9, SPD, NE, LTB4, and PRG4 Levels Between Atopic and Non-Atopic COPD Patients
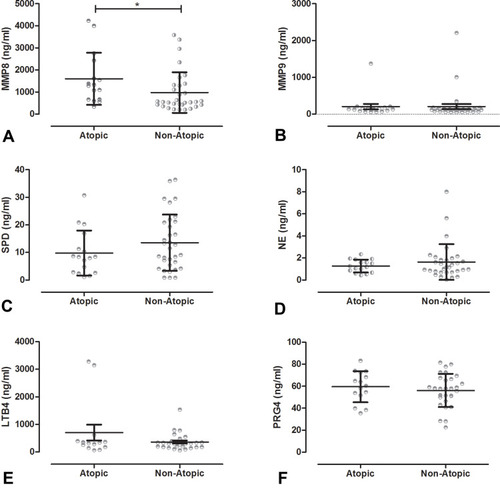
In atopic COPD, the average level of MMP8 was the highest (1600 ± 1181 ng/mL), followed by MMP9 (203.0 ± 305.6 ng/mL), and LTB4 (704.3 ± 1074 ng/mL). In non-atopic COPD, the average level of MMP8 was the highest (973.3 ±921.5 ng/mL), followed by LTB4 (361.8 ± 293.1 ng/mL), and MMP9 (205.9 ±395.6 ng/mL). The MMP8 levels in atopic COPD patients were significantly higher than those assessed in non-atopic COPD patients (z = 2.019, P = 0.0494), but there was no significant difference in the levels of MMP9, SPD, NE, LTB4, and PRG4 between the two groups. () Compared with non-atopic COPD patients (69.7%), 81.2% of the atopic COPD patients had FEV1 < 40%. Interestingly, all atopic COPD patients exhibited MMP8 levels above 1000 ng/mL. In contrast, in non-atopic COPD patients, only patients with the FEV1 below 40% had MMP8 levels above 1000 ng/mL, and there was no obvious trend in the levels of MMP9, SPD, NE, LTB4, and PRG4 ().
Table 2 Immune Factor Levels Between Different Pulmonary Function Groups in Atopic and Non-Atopic COPD Patients
Figure 1 Levels of immune factors between atopic and non-atopic COPD patients. T test: *P<0.05. (A) Levels of MMP8 in atopic COPD patients were higher than in non-atopic COPD patients. But there were no significant difference in (B) MMP9; (C) SPD; (D) NE; (E) LTB4; and (F) PRG4 between atopic COPD patients and non- atopic COPD patients.
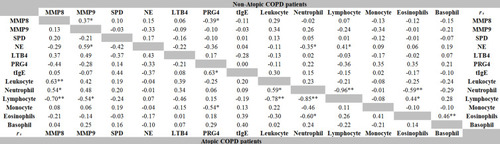
Correlations Between MMP8, MMP9, SPD, NE, LTB4, PRG4, tIgE Levels and White Blood Cell Rate in Atopic and Non-Atopic COPD Patients
In atopic COPD patients, the rate of leukocyte (rs = 0.63, P < 0.001) and neutrophil (rs = 0.54, P < 0.05) positively correlated with MMP8 levels, while the lymphocyte rate negatively correlated with the levels of MMP8 (rs = −0.70, P < 0.001) and MMP9 (rs = −0.54, P < 0.05). PRG4 levels negatively correlated with the rate of monocyte (rs = −0.54, P < 0.05), but positively correlated with tIgE levels (rs =0.63, P <0.001). In non-atopic COPD patients, NE levels negatively correlated with the rate of neutrophil (rs = −0.35, P < 0.05), but positively correlated with the lymphocyte rate (rs = 0.41, P < 0.05). Interestingly, there was a positive correlation between NE and MMP9 levels (rs = 0.59, P < 0.05) in atopic COPD, but the two were negatively correlated in non-atopic COPD (rs = −0.33, P >0.05). In addition, there was a negative correlation between the rate of MMP8 and PRG4 (Atopic COPD: rs = −0.44, P > 0.05; Non-atopic COPD: rs = −0.39, P < 0.05). ().
Comparison of Clinical Characteristics Between Atopic and Non-Atopic COPD Patients
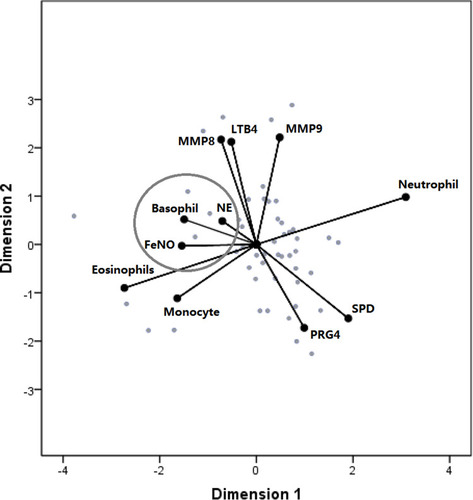
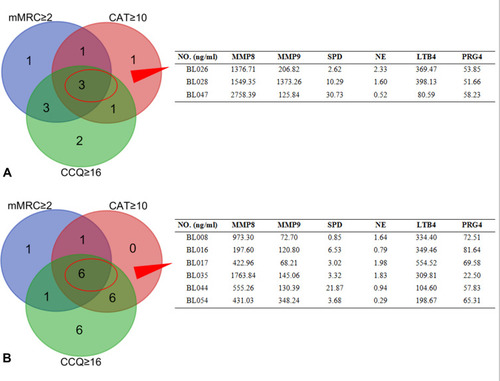
In the COPD group, 8 cases exhibited mMRC ≥ 2, 6 cases presented CAT ≥ 10, and 9 cases showed CCQ ≥ 16. Among them, 3 cases were complicated with mMRC ≥ 2, CAT ≥ 10, and CCQ ≥ 16, and their MMP8 levels were over 1000 ng/mL. In the non-atopic COPD group, 9 cases showed mMRC ≥ 2, 13 cases presented CAT ≥ 10, 19 cases exhibited CCQ ≥ 16, and 6 cases were complicated with mMRC ≥ 2, CAT ≥ 10, and CCQ ≥ 16, but only 1 case had MMP8 levels over 1000 ng/mL (). The optimal scale analysis showed that NE levels were most closely related to induced sputum’s basophil rate and FeNO levels (Cronbach’s alpha = 85.1%) ().
Figure 3 Levels of immune factors in patients with mMRC≥2, CAT≥10, and CCQ≥16. CCQ, Clinical COPD Questionnaire; CAT, COPD Assessment Test; mMRC, Modified Medical Research Council Dyspnea Scale. (A) In atopic COPD patients, there were 3 patients with mMRC≥2, CAT≥10, and CCQ≥16 in the COPD group, and all of them had MMP8 levels ≥1000 ng/mL. (B) In non-atopic COPD patients, 6 cases were complicated with mMRC ≥ 2, CAT ≥ 10, and CCQ ≥ 16, but only 1 case had MMP8 levels ≥1000 ng/mL.
Discussion
Allergens play an important role in the pathogenesis of COPD.Citation15 Our previous studies have shown that about 32.7% of COPD patients are atopic. There are various differences in the pathogenesis, symptoms, and pulmonary function of atopic vs non-atopic COPD patients. In this study, by evaluating the serum levels of MMP8, MMP9, SPD, NE, LTB4, and PRG4 in 17 cases of atopic COPD and 33 cases of non-atopic COPD, it was found that both serum tIgE and MMP8 levels were higher in patients with atopic COPD than in patients with non-atopic COPD. MMP8, which is involved in many pathological processes such as arthritis, cancer, and autoimmune disease, is a zinc- and calcium-dependent endopeptidase that can degrade all components of the extracellular matrix, and the atopy observed in COPD patients may be related to MMP8.Citation16 In addition, MMP9 is the main member of the MMPs family involved in the degradation of collagen from the vascular basement membrane, which causes vascular remodeling by changing the structure and composition of the vascular basement membrane.Citation17 SPD is mainly secreted by type II alveolar cells and regulates immunity and inflammation,Citation11 while LTB4 is a powerful neutrophil chemotactic factor which triggers the accumulation of neutrophils in the airway tissue in COPD patients.Citation12 PRG4 is an extracellular matrix protein and has been identified to act as macrophage growth-stimulating factor and joint synovial glycoprotein.Citation13 However, we found no difference in the levels of MMP9, SPD, NE, LTB4, and PRG4 between atopic and non-atopic COPD patients. But a meta-analysis showed that MMP9 rs3918242 C > T significantly correlated with increased susceptibility to COPD.Citation18
In clinical manifestations, 37.5% of patients with atopic COPD had at least one exacerbation of the disease, while only 27.5% of patients with non-atopic COPD had at least one exacerbation of the disease. This may be due to the fact that patients with atopic COPD are more susceptible to the influence of allergens in the air, which can cause acute exacerbation due to the stimulation of allergens.Citation19 In addition, there is a positive correlation between neutrophil rate and MMP8 levels in patients with atopic COPD, which may suggest that neutrophil are involved in the allergic process of airway in these patients. In the case of LPS and Th17 immune response, neutrophil from the lung can be activated, and then activate MMP8 to stimulate an inflammatory response in patients with atopic COPD.Citation20
Interestingly, there is a positive correlation between NE and MMP9 levels in atopic COPD, but the two negatively correlated in non-atopic COPD patients. NE is mainly synthesized and secreted by sympathetic postganglionic neurons and adrenergic neurons from the brain.Citation21 It has obvious vasoconstrictor effects, which can increase peripheral resistance and arterial blood pressure. A study from Poland has shown that the levels of IL-6, MMP9, and NE were higher in COPD patients than in asthmatics.Citation22
In atopic COPD, 47.1% of the patients had a poor mMRC score, compared with the non-atopic group (27.3%), suggesting that patients with atopic COPD had more severe dyspnea than non-atopic COPD patients, while there was no significant difference in the CCQ and CAT scores between atopic and non-atopic COPD patients. Surprisingly, all atopic COPD patients with mMRC ≥ 2, CAT ≥ 10, and CCQ ≥ 16 exhibited MMP8 levels over 1000 ng/mL, contrasting to non-atopic COPD patients, among which only one case presented MMP8 levels over 1000 ng/mL. There is a relationship between MMP8 levels and the mMRC score of atopic COPD patients, which might arise due to allergens in the air affecting these COPD patients; following allergen stimulation, MMP8 mediates the inflammatory response, which renders patients more prone to acute attacks and dyspnea. Harper JI et al showed that MMP8 dominated a mixture of MMPs that are detectable on the skin surface in acute atopic dermatitis.Citation23
Finally, the optimal scale analysis showed that NE was most closely related to the rate of basophils from induced sputum and FeNO levels. Airway basophils participate in local atopic response, whereas the level of FeNO can reflect the severity of airway inflammation, suggesting that there is a close relationship between atopy and airway inflammation, which corroborates with a previous American study.Citation24
However, the small sample size was the main limitation of this study, which needs further research increasing the sample size to verification. Meanwhile, this study didn’t consider the atopic patients who will develop into COPD, and only analyzed the immune factors between atopic COPD patients and non-atopic COPD patients, MMP8 were likely to play an important role in the development of atopic COPD. It needs to be supplemented by longitudinal research in the future.
Conclusion
Generally, the levels of serum tIgE and MMP8 in patients with atopic COPD were higher than those observed in patients with non-atopic COPD. The rate of neutrophil in patients with atopic COPD positively correlated with MMP8 levels, and dyspnea was more severe in patients with atopic COPD. NE levels were closely related to the rate of basophil from induced sputum and FeNO levels, which may play an important role in the pathogenesis and development of atopic COPD.
Data Sharing Statement
The data that support these findings are available on reasonable request from the corresponding author BQS. Data are not publicly available due to concerns regarding research participant privacy.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Approval was obtained from the ethic committee of The First Affiliated Hospital of Guangzhou Medical University (Reference number: GYFYY-2016-73).
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
We thank everyone involved in the collection, detection, and date management of the serum samples. We would like to thank Editage for English language editing.
References
- FaroukH, AlbasmiM, El ChilaliK, et al. Left ventricular diastolic dysfunction in patients with chronic obstructive pulmonary disease: impact of methods of assessment. Echocardiography. 2017;34(3):359–364. doi:10.1111/echo.1346928165145
- PountneyD. COPD: new inspiration needed. Nurs Older People. 2006;18(6):13–16. doi:10.7748/nop.18.6.13.s10
- HuH, HuangH, ZhengP, et al. The sensitization characteristics of adult Chinese patients diagnosed with chronic respiratory diseases. Asian Pac J Allergy Immunol. 2019;2:154.
- JinJ, LiuX, SunY. The prevalence of increased serum IgE and Aspergillus sensitization in patients with COPD and their association with symptoms and lung function. Respir Res. 2014;15(1):130. doi:10.1186/s12931-014-0130-125359094
- SamahaHMS, ElsaidAR, NasrEldinE. Total serum IgE level in COPD patients. Egypt J Chest Dis Tuberc. 2015;64(3):573–577. doi:10.1016/j.ejcdt.2015.02.005
- DasS, MandalM, ChakrabortiT, MandalA, ChakrabortiS. Structure and evolutionary aspects of matrix metalloproteinases: a brief overview. Mol Cell Biochem. 2003;253(1–2):31–40. doi:10.1023/A:102609301614814619953
- CaneJL, Mallia-MillanesB, ForresterDL, KnoxAJ, BoltonCE, JohnsonSR. Matrix metalloproteinases −8 and −9 in the airways, blood and urine during exacerbations of COPD. COPD. 2016;13(1):26–34. doi:10.3109/15412555.2015.104352226418236
- GilowskaI, KasperŁ, BogaczK, et al. Impact of matrix metalloproteinase 9 on COPD development in Polish patients: genetic polymorphism, protein level, and their relationship with lung function. Biomed Res Int. 2018;2018:6417415. doi:10.1155/2018/641741530643813
- IlumetsH, RytiläP, DemedtsI, et al. Matrix metalloproteinases −8, −9 and −12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(3):369–379.18229576
- MusiałK, ZwolińskaD. New markers of cell migration and inflammation in children with chronic kidney disease. Biomarkers. 2019;24(3):295–302. doi:10.1080/1354750X.2018.155634130516070
- KrugerS, LegensteinML, RösgenV, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology. 2017;6(5):e1310358. doi:10.1080/2162402X.2017.131035828638732
- TianW, RocksonSG, JiangX, et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med. 2017;9(389):pii: eaal3920.
- TianW, RocksonSG, JiangX, et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med. 2017;9(389):pii: eaal3920. doi:10.1126/scitranslmed.aal3920
- BousquetJ, KhaltaevN, CruzAA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2) LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160.18331513
- BożekA, RogalaB. IgE-dependent sensitization in patients with COPD. Ann Agric Environ Med. 2018;25(3):417–420. doi:10.26444/aaem/8341330260198
- NäkkiA, Rodriguez-FontenlaC, GonzalezA, et al. Association study of MMP8 gene in osteoarthritis. Connect Tissue Res. 2016;57(1):44–52. doi:10.3109/03008207.2015.109963626577236
- ZhangC, LimJ, JeonHH, et al. FOXO1 deletion in keratinocytes improves diabetic wound healing through MMP9 regulation. Sci Rep. 2017;7(1):10565. doi:10.1038/s41598-017-10999-328874756
- JiangS, YangZH, ChenYY, et al. MMP-9 genetic polymorphism may confer susceptibility to COPD. Genet Mol Res. 2016;15(2). doi:10.4238/gmr.15026272.
- HuH, LuoW, WuZ, CaiC, HuangH, SunB. A pilot study on the allergen-specific IgE to molecular components on polysensitized mite allergic asthmatic patients in Guangzhou, China. Mol Immunol. 2019;105:38–45. doi:10.1016/j.molimm.2018.11.00430481720
- De BruyneM, BogaertDJ, VenkenK, Van den BosscheL, BonroyC, RoelsL. A novel LPS-responsive beige-like anchor protein (LRBA) mutation presents with normal cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and overactive TH17 immunity. J Allergy Clin Immunol. 2018;142(6):1968–1971. doi:10.1016/j.jaci.2018.08.02630193839
- MurtazinaAR, NikishinaYO, BondarenkoNS, SapronovaAJ, UgrumovMV. Signal molecules during the organism development: central and peripheral sources of noradrenaline in rat ontogenesis. Dokl Biochem Biophys. 2016;466(1):74–76. doi:10.1134/S160767291601018X27025493
- GórskaK, Paplińska-GorycaM, Nejman-GryzP, GorycaK, Krenke R. Eosinophilic and neutrophilic airway inflammation in the phenotyping of mild-to-moderate asthma and chronic obstructive pulmonary disease. COPD. 2017;14(2):181–189. doi:10.1080/15412555.2016.126053927983888
- HarperJI, GodwinH, GreenA, et al. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br J Dermatol. 2010;162(2):397–403. doi:10.1111/j.1365-2133.2009.09467.x19804592
- SadeghiMH, WrightCE, HartS, CrooksM, MoriceAH. Does FeNO predict clinical characteristics in chronic cough? Lung. 2018;196(1):59–64. doi:10.1007/s00408-017-0074-629177539