Abstract
Background
Chronic respiratory failure may occur as a consequence of chronic obstructive pulmonary disease (COPD) and is associated with significant morbidity and mortality. Hypoxemia is determined by underlying disease characteristics and comorbidities. Severe hypoxemia is typically only found in subjects with severe airflow obstruction (FEV1<50% predicted). However, how hypoxemia relates to disease characteristics is not fully understood.
Methods
In the French Initiatives BPCO real-life cohort, arterial blood gases were routinely collected in most patients. Relationships between severe hypoxemia, defined by a Pa02<60 mmHg (8 kPa) and clinical/lung function features, comorbidities and mortality were assessed. In subjects with severe hypoxemia, clinical characteristics and comorbidities were compared between those with non-severe versus severe airflow limitation. Classification and regression trees (CART) were used to define clinically relevant subgroups (phenotypes).
Results
Arterial blood gases were available from 887 subjects, of which 146 (16%) exhibited severe hypoxemia. Compared to subjects with a PaO2≥60 mmHg, the severe hypoxemia group exhibited higher mMRC dyspnea score, lower FEV1, higher RV and RV/TLC, more impaired quality of life, lower 6-minute walking distance, less frequent history of asthma, more frequent diabetes and higher 3-year mortality rate (14% versus 8%, p=0.026). Compared to subjects with Pa02<60 mmHg and FEV1<50% (n=115, 13%), those with severe hypoxemia but FEV1≥50% predicted (n=31) were older, had higher BMI, less hyperinflation, better quality of life and a higher rate of diabetes (29% versus 13%, p=0.02). Severe hypoxemia was better related to CART-defined phenotypes than to GOLD ABCD classification.
Conclusion
In this cohort of stable COPD subjects, severe hypoxemia was associated with worse prognosis and more severe symptoms, airflow limitation and hyperinflation. Compared to subjects with severe hypoxemia and severe airflow limitation, subjects with severe hypoxemia despite non-severe airflow limitation were older, had higher BMI and more diagnosed diabetes.
Trial Registration
04–479.
Background
Chronic respiratory failure is a major complication in chronic obstructive pulmonary disease (COPD) with both a significant impact on morbidity and mortality and major therapeutic implications.Citation1,Citation2 The prevalence of hypoxemia among COPD populations is highly variable, depending on underlying disease characteristics and on comorbidities. Longitudinal data from the Swedish National Register of COPD reported a prevalence of 1.4% for resting hypoxemia.Citation3 In moderate-to-severe COPD, 7% of subjects developed resting hypoxemia after a median follow-up of 5 years.Citation4
The lung function characterization of COPD severity is based on the degree of airflow limitation as measured by forced expiratory volume in 1 s (FEV1). In parallel, clinical categories with prognostic and therapeutic relevance are defined with symptoms and exacerbations. Of note, despite its significant burden, hypoxemia is no longer included as a parameter to categorize COPD patients in the recent GOLD combined COPD categorization strategy.Citation5 A classic assumption is to consider that severe hypoxemia is only found in subjects with FEV1<50% of predicted.Citation5,Citation6 Factors associated with discrepancies between the severity of airflow limitation and hypoxemia are not completely understood.
About 40 years ago, two randomized controlled trials showed that long-term oxygen therapy (LTOT) improves survival in subjects with severe resting hypoxemia at steady state.Citation7,Citation8 Since these earlier studies, the profile of subjects with COPD has changed dramatically, while symptomatic pharmacological and non-pharmacological treatments have improved markedly. Although this has not been formally demonstrated, it seems that the disease is discovered less often at a late stage with chronic respiratory failure, and life expectancy has probably increased. In parallel, during the last two decades, major progresses have been made in the understanding of COPD heterogeneity, with the identification of clinically relevant phenotypes, endotypes and treatable traits. Importantly, our group has used classification and regression trees (CART) to develop an algorithm allocating subjects with COPD to clinical phenotypes defined by age, symptoms (dyspnea grade), lung function, nutritional status (BMI) and comorbidities. Cluster and CART analyses were performed in 3 French/Belgian cohorts and further tested inpatients from the COPD Cohorts Collaborative International Assessment (3CIA) initiative. The identified subgroups differed in terms of clinical characteristics, mortality rates and age at death (with poor prognosis groups CART 1 and 4 as compared to good prognosis group CART 5).Citation9 How such phenotypes relate to arterial blood gases remains still unknown.
Altogether, despite the profound changes mentioned above in COPD characteristics, treatments and understanding, the burden and determinants of chronic severe hypoxemia has not been reassessed in recent years. Hence, the aims of this study were (1) to assess clinical and lung function features, comorbidities and mortality associated with severe hypoxemia in a real-life COPD cohort, (2) to compare clinical and functional characteristics in subjects with non-severe (FEV1≥50% predicted) versus severe airflow limitation among those with severe hypoxemia and (3) to compare characteristics of subjects with or without severe hypoxemia, among subjects with severe airflow limitation.
Methods
Initiatives COPD Cohort
As previously described, Initiatives BPCO is a rolling real-life cohort which primarily aims to study phenotypes among subjects with COPD followed at 17 French University Hospitals.Citation10 Data collected as part of routine practice at inclusion include demographic and anthropometric characteristics, occupational exposures, smoking history, chronic bronchitis, exacerbation frequency, dyspnea assessed by mMRC dyspnea scale, health-related quality of life assessed by the Saint George Respiratory Questionnaire (SGRQ), physician diagnosed comorbidities (asthma, rhinitis, cardiovascular diseases, obesity, diabetes, mechanical limitation, psychological status), medications and post-bronchodilator spirometry (forced expiratory volume in 1 second, FEV1, forced vital capacity, FVC), plethysmography (residual volume, RV, total lung capacity, TLC)Citation11 and arterial blood gases (ABG) at rest. Patients included in the present analyses were those with available ABG at rest in stable state. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Versailles, France (trial registration #04–479), and all subjects provided informed written consent.
Arterial Blood Gases (ABG)
Arterial samples were obtained at rest on room air condition and close to sea level at each site. The following variables from ABG were collected: PaO2 and PaCO2 (mmHg) and pH. Severe hypoxemia was defined by a PaO2<60 mmHg (8 kPa). Subjects in which blood gases were performed using arterialized capillary samples were not retained for analyses.
Association Between Hypoxemia, Clinical/Lung Function Features and Phenotypes
Relationships between severe hypoxemia and clinical/lung function features, comorbidities and mortality were assessed. Severe airflow limitation was defined as post-bronchodilator FEV1 less than 50% predicted post-bronchodilator. Phenotypes analyses were based on an algorithm developed using CART in pooled French/Belgian COPD cohorts (Supplementary Figure S2C).Citation9,Citation10 Briefly, this algorithm is based on clinical and lung function variables (including cardiovascular comorbidities, diabetes and respiratory characteristics) and allows allocation of subjects into five classes corresponding to subgroups (phenotypes) identified by cluster analyses with different rates of all-cause mortality at 3 years and ages at death.
Statistical Analyses
Data are provided as median [Q1; Q3] or n (%), as appropriate. Univariate comparisons based on the presence/absence of severe resting hypoxemia and severe airflow obstruction were performed by Chi2 and t-test. Correlations were assessed using Pearson’s coefficients. Survival was analyzed using 3-year all-cause mortality.
Results
Subjects Characteristics
Among 1441 subjects in the cohort at the time of data extraction, ABG at rest in room air at stable state were available in 887 subjects. More severe airflow limitation and GOLD spirometry stages were found in COPD subjects with versus without available ABG (Supplementary Table S1). No difference was noted in terms of clinical characteristics, comorbidities, and outcomes including three-year mortality rates in patients with versus without available ABG. Characteristics of the 887 COPD subjects included in this analysis are presented in . The median values of age and percent predicted (pp) FEV1 were 64 years and 48%, respectively. The majority of the subjects (74%) were male.
Table 1 Comparison Between COPD Subjects with and without Severe Hypoxemia
Arterial Blood Gas Results
PaO2 distribution is shown in . Median PaO2, PaCO2 and pH levels were 71 [63; 79] mmHg, 40 [37; 43] mmHg and 7.42 [7.40; 7.44], respectively. A hundred and forty-six subjects (16%) exhibited severe hypoxemia defined as PaO2<60 mmHg (), and 178 subjects (20%) had hypercapnia defined as PaCO2≥45 mmHg with 6% of the subjects with a PaCO2≥52 mmHg (Supplementary Figure S1A). Fifteen subjects had acidosis, defined as pH<7.35 (Supplementary Figure S1A and B). PaO2 and PaCO2 values were weakly correlated (B).
Figure 1 Distribution of PaO2 (A) and relationships between PaO2 and PaCO2 (B). 887 COPD subjects were included. Values are in mmHg.
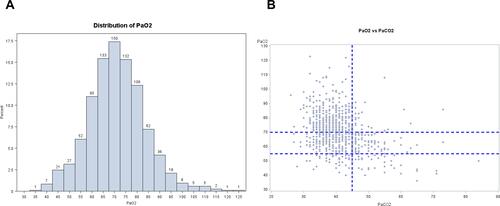
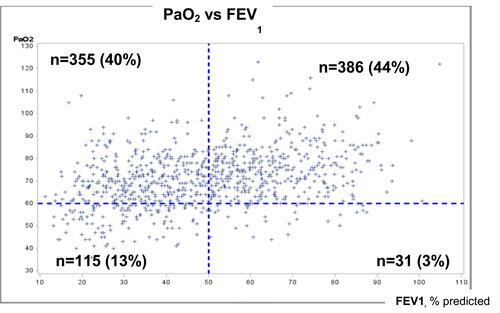
Figure 2 Relationships between PaO2 and FEV1, and distribution of COPD subjects for severe hypoxemia and severe airflow limitation. 887 COPD subjects were included. High PaO2/high FEV1 are in the right and upper part of the graph. High PaO2/low FEV1 are in the left and upper part of the graph. Low paO2/high FEV1 are in the right and lower part of the graph. Low PaO2/high FEV1 are in the left and lower part of the graph. Values are in mmHg or %.
Hypoxemia According to Airflow Obstruction and Hyperinflation
Compared to subjects with a PaO2≥60 mmHg, the severe hypoxemia group exhibited higher mMRC dyspnea score, lower FEV1, higher RV and RV/TLC, lower DLCO, more impaired quality of life, lower 6-minute walking distance, less frequent history of asthma, and higher 3-year mortality rate (14% versus 8%) (all, p<0.05, ). The relationships between PaO2 and FEV1 and the distribution of COPD subjects using thresholds of 60 mmHg PaO2 for severe hypoxemia and 50% FEV1 for severe airway limitation are presented in . Among subjects with severe hypoxemia (n=146), 31 (21.2%, 3% of the whole study population) had a FEV1≥50% predicted. Compared to subjects with severe hypoxemia and FEV1<50% (n=115), this group was older, had higher BMI, lower RV and RV/TLC, a better quality of life (SGRQ score) and a higher rate of diabetes (29% versus 13%) (all, p<0.05, ).
Table 2 Comparison Between COPD Subjects with and without Severe Hypoxemia, with or without Severe Airflow Obstruction
Among subjects with severe airflow obstruction (n=470), 355 subjects (75.5%, 40% of the whole study population) had a PaO2>60 mmHg. Compared to subjects with severe hypoxemia and FEV1<50%, this group had higher BMI, a lower rate of OSA, higher DLCO, and higher 6-min walking distance (all, p<0.05, ).
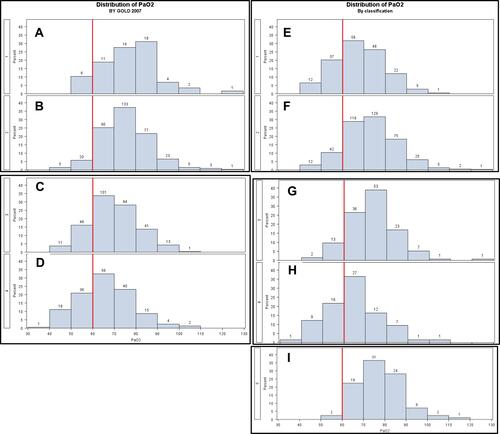
Distribution of PaO2 According to Spirometric Grade of Airflow Obstruction, GOLD Stage 2020 and CART-Defined Phenotypes
Severe hypoxemia was, respectively, present in 10%, 7%, 29% and 32% of subjects in spirometric GOLD 1, 2, 3 and 4 stages, respectively (–). GOLD ABCD classification did not allow a better discrimination of patients relative to severe hypoxemia (Supplementary Figure S2A). Interestingly, severe hypoxemia was highly prevalent in the CART-defined groups with the poorest prognosis (CART 1 and 4) with, respectively, 26.7% and 35.6% of severely hypoxemic subjects. Conversely, the CART 5 group, which is associated with a good prognosis, comprised of very few subjects (2.4%) with severe hypoxemia (–, Supplementary Figure S2B).
Discussion
Few recent studies in “modern” COPD populations have focused on the link between clinical phenotypes, outcomes and resting hypoxemia. Our results in a real-life cohort of stable COPD subjects covering the whole spectrum of light-to-very severe airflow obstruction showed that severe hypoxemia was present in 10%, 7%, 29% and 32% of subjects from GOLD 1 to 4 spirometric stages, respectively. This feature was associated with worse prognosis, more severe symptoms, airflow limitation and hyperinflation. Patients with severe hypoxemia but non-severe airflow limitation were characterized by older age, higher BMI, less hyperinflation, and more frequent diabetes. Hypoxemia was more closely linked to CART-defined phenotypes than to GOLD classifications.
The prevalence of severe hypoxemia in COPD is higher in this cohort than previously reported in other real-life COPD cohorts, where it ranged from 1.4 to 5%.Citation3,Citation4 This likely relates to a selection bias in our study including COPD subjects with available ABG follow-up in University Hospitals, and thus probably more severe than in other cohorts. Our results showing worse lung function and prognosis associated with severe hypoxemia are in line with previously published data.Citation12,Citation13 Contrary to a classical assumption, we confirm here that severe hypoxemia cannot be ruled out in case of non-severe airflow obstruction.
An interesting finding of the present study is the demonstration that the group of subjects with severe hypoxemia despite non-severe airflow limitation was older, had higher BMI and more diagnosed diabetes. The prevalence of diabetes was comparable to what has been reported in previous studies. These findings could suggest a potential interaction between diabetes and hypoxemia. Previous studies found a higher prevalence of diabetes in COPD.Citation14 Although experimental hypoxia produces insulin resistance in animal models,Citation15 evidence in humans is conflicting.Citation16 Besides, cross-sectional studies showed that adults with diabetes have lower FEV1 compared with non-diabetic subjects.Citation17 The mechanisms underlying hypoxemia in diabetes remain to be understood and may involve increased obesity, reduced physical activity, increased cigarette smoke, or disease-related modifications including inflammation and/or oxidative stress.Citation18 We can hypothesize that microangiopathy due to type 2 diabetes in the alveolar capillaries and pulmonary arterioles could also be incriminated.Citation18 Obesity may participate to explain hypoxemia in patients with non-severe airflow limitation, as suggested by the increased proportion of patients with higher BMI in discordant (non-severe airflow limitation) than in concordant (severe airflow limitation) hypoxemic patients. In obese patients, hypoxemia can relate to ventilation/perfusion mismatch in lower areas of the lungs or to alveolar hypoventilation. This latter hypothesis is unlikely since PaCO2 is not higher in the discordant than in the concordant hypoxemic group (39 [35; 42] mmHg versus 40 [37; 43] mmHg).
Finally, phenotypes analyses using the CART classification indicated that severe hypoxemia was highly prevalent in the poor prognosis groups CART 1 and 4 whereas very few severe hypoxemic subjects were found in the good prognosis group CART 5 group, highlighting that severe hypoxemia is an important feature associated with worse prognosis in COPD.Citation9 The inclusion of hypoxemia in the assessment of COPD severity may help to better categorize subjects in terms of prognosis and could eventually improve CART classification. Patients from the CART 5 group may not require ABG.
As previously reported, severe hypoxemia was associated with lower DLCO in our study than in others, where a low DLCO has been associated with specific clinical profiles including more severe dyspnea. Citation10Citation19–Citation21 However, it must be pointed out that DLCO data were often missing in our cohort, thus preventing us from deeper analyses regarding the relevance of this variable. Similarly, 6-minute walking distance was lower in severe hypoxemic subjects, but data were often missing again. Another limitation is that comorbidities are only diagnosed-comorbidities.
Conclusion
In this cohort of stable COPD subjects, severe hypoxemia was associated with worse prognosis and more severe symptoms, airflow limitation and hyperinflation. Compared to subjects with severe hypoxemia and severe airflow limitation, subjects with severe hypoxemia despite non-severe airflow limitation were older, had higher BMI and more diagnosed diabetes, underlining that the mechanisms of hypoxemia in COPD are not limited to the respiratory component of the multi-morbidity that affects these patients. CART-based classification may alleviate the need for ABGs in some patients’ categories better than GOLD classifications.
Abbreviations
ABG, arterial blood gases; BMI, body mass index; CART, classification and regression tree; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, global initiative for obstructive lung disease; HAD, hospital anxiety and depression scale; mMRC, modified Medical Research Council; OSAS, obstructive sleep apnea syndrome; RV, residual volume; SGRQ, Saint George’s respiratory questionnaire; TLC, total lung capacity.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and study protocol has been approved by the Ethics Committee of Versailles Saint Quentin University, authorization number 04-479, for protection of human beings involved in biomedical research. The study has also been approved by CCTIRS (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé), on the 6thJanuary, 2005 (04-479). All subjects provided written consent.
Consent for Publication
All subjects provided written consent. All authors provided consent to publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We thank the initiatives BPCO study group P.R. Burgel (Paris), G. Deslee (Reims), P. Surpas (Charnay), O. Le Rouzic (Lille), T. Perez (Lille), N. Roche (Paris), G. Brinchault-Rabin (Rennes), D. Caillaud (Clermont-Ferrand), P. Chanez (Marseille), I. Court-Fortune (Saint-Etienne), R. Escamilla (Toulouse), G. Jebrak (Paris), P. Nesme-Meyer (Lyon), M. Zysman (Bordeaux), A. Guillaumot (Nancy), C. Pinet (Toulon) and Brigitte Risse (ARAIRLOR, Association régionale d’aide aux insuffisants respiratoires de Lorraine, Nancy).
Disclosure
MZ reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from Chiesi, personal fees from Astra Zeneca and personal fees from GSK outside the submitted work.
PRB reports personal fees from Aptalis, personal fees from Astra-Zeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GSK, personal fees from Novartis, personal fees from Pfizer, personal fees from Vertex, personal fees from Zambon, personal fees from Insmed, outside the submitted work.
ICF reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, and GSK outside the submitted work.
GBR reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, grants and personal fees from Astra Zeneca, personal fees and non-financial support from Chiesi, outside the submitted work.
PNM reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from NOVARTIS, personal fees and non-financial support from Chiesi, outside the submitted work.
PS reports grants and personal fees from Boehringer Ingelheim outside the submitted work.
GD reports personal fees from Novartis, personal fees and grants from Astra Zeneca, personal fees from BTG/PneumRx, personal fees from Chiesi, personal fees from Boehringer Ingelheim, personal fees from GSK, outside the submitted work.
TP reports personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from GSK, personal fees from Chiesi, personal fees from Pierre Fabre, outside the submitted work.
OLR reports grants and personal fees personal fees and non-financial support from Astra Zeneca, Boehringer Ingelheim, Chiesi, Lilly and Novartis; non-financial support from Glaxo Smith Kline, Correvio, Mayoli, Mylan, MSD, PulmonX, Zambon, Novartis, MundiPharma, Pfizer, Teva, Santelys Association, Vertex and Vitalaire, all outside the submitted work.
GJ reports personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from Novartis, personal fees from Menarini, personal fees from Astra Zeneca, personal fees from Chiesi, outside the submitted work.
PC reports personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees and grants from ALK, personal fees and grants from Almirall, personal fees and grants from Boehringer-Ingelheim, personal fees from Novartis, personal fees from Teva, personal fees from Astra Zeneca, grants and personal fees from Chiesi, personal fees from Sanofi, personal fees and non-financial support from SNCF, personal fees from GlaxoSmithKline, grants and personal fees from Sanofi-Aventis, grants from Amu, outside the submitted work.
JLP reports grants from iBPCO association, during the conduct of the study.
DC has nothing to disclose.
NR reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, grant and personal fees from Pfizer, grants and personal fees from GSK, personal fees from AstraZeneca, personal fees from Chiesi, personal fees from Sanofi, personal fees from Zambon, personal fees and grants from Boehringer Ingelheim, outside the submitted work.
Additional information
Funding
References
- Albert RK, Au DH, Blackford AL, et al.; Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627.
- Bressler RB, Awe RJ. Oxygen therapy in chronic obstructive lung disease. Ann Intern Med. 1981;94:410. doi:10.7326/0003-4819-94-3-410_1
- Sundh J, Ekström M. Risk factors for developing hypoxic respiratory failure in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2095–2100. doi:10.2147/COPD.S140299
- Wells JM, Estepar RS, McDonald MN, et al. Clinical, physiologic, and radiographic factors contributing to development of hypoxemia in moderate to severe COPD: a cohort study. BMC Pulm Med. 2016;16:169. doi:10.1186/s12890-016-0331-0
- Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed April 20, 2021.
- Lim S, MacRae KD, Seed WA, Roberts CM. The value of forced expiratory volume in 1 s in screening subjects with stable COPD for PaO2 < 7.3 kPa qualifying for long-term oxygen therapy. Respir Med. 1998;92(9):1122–1126. doi:10.1016/s0954-6111(98)90405-x
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial, Ann Intern Med. 1980;93(3):391–398. PMID: 6776858. doi:10.7326/0003-4819-93-3-391
- Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686. PMID: 6110912.
- Burgel PR, Paillasseur JL, Janssens W, et al. A simple algorithm for the identification of clinical COPD phenotypes. Eur Respir J. 2017;50:1701034. doi:10.1183/13993003.01034-2017
- Burgel PR, Paillasseur JL, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36:531–539. doi:10.1183/09031936.00175109
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi:10.1183/09031936.05.00034805
- Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14:1002–1008. doi:10.1183/09031936.99.14510029
- Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52:674–679. doi:10.1136/thx.52.8.674
- Cazzola M, Bettoncelli G, Sessa E, Cricelli C, Biscione G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:112–119. doi:10.1159/000281880
- Raff H, Bruder ED, Jankowski BM. The effect of hypoxia on plasma leptin and insulin in newborn and juvenile rats. Endocrine. 1999;11:37–39. doi:10.1385/ENDO:11:1:37
- Cheng N, Cai W, Jiang M, Wu S. Effect of hypoxia on blood glucose, hormones, and insulin receptor functions in newborn calves. Pediatr Res. 1997;41:852–856. doi:10.1203/00006450-199706000-00009
- Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet Med. 2010;27:977–987. doi:10.1111/j.1464-5491.2010.03073.x
- Rogliani P, Calzetta L, Segreti A, Barrile A, Cazzola M. Diabetes mellitus among outpatients with COPD attending a university hospital. Acta Diabetol. 2014;51:933–940. doi:10.1007/s00592-014-0584-0
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi:10.1378/chest.127.5.1531
- Olsson KM, Fuge J, Meyer K, Welte T, Hoeper MM. More on idiopathic pulmonary arterial hypertension with a low diffusing capacity. EurRespirJ. 2017;50:1700354.
- Kovacs G, Agusti A, Barberà JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198:1000–1011. doi:10.1164/rccm.201801-0095PP