Abstract
Chronic obstructive pulmonary disease (COPD) is commonly associated with heart failure (HF) in clinical practice since they share the same pathogenic mechanism. Both conditions incur significant morbidity and mortality. Therefore, the prognosis of COPD and HF combined is poorer than for either disease alone. Nevertheless, usually only one of them is diagnosed. An active search for each condition using clinical examination and additional tests including plasma natriuretic peptides, lung function testing, and echocardiography should be obtained. The combination of COPD and HF presents many therapeutic challenges. The beneficial effects of selective β1-blockers should not be denied in stable patients who have HF and coexisting COPD. Additionally, statins, angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers may reduce the morbidity and mortality of COPD patients. Moreover, caution is advised with use of inhaled β2-agonists for the treatment of COPD in patients with HF. Finally, noninvasive ventilation, added to conventional therapy, improves the outcome of patients with acute respiratory failure due to hypercapnic exacerbation of COPD or HF in situations of acute pulmonary edema. The establishment of a combined and integrated approach to managing these comorbidities would seem an appropriate strategy. Additional studies providing new data on the pathogenesis and management of patients with COPD and HF are needed, with the purpose of trying to improve quality of life as well as survival of these patients.
Introduction
Chronic obstructive pulmonary disease (COPD) and heart failure (HF) frequently coexist in clinical practice.Citation1 Both conditions share some risk factors including cigarette smoking, advanced age, and systemic inflammation.Citation2 The prevalence of COPD among individuals with HF ranges from 20% to 32% of cases, and 10% of hospitalized HF patients also suffer COPD.Citation3 From the opposite side, HF is prevalent in more than 20% of patients with COPD.Citation4,Citation5 Moreover, the risk ratio of developing HF among COPD patients is 4.5 times higher than that of control individuals without the disease, after adjusting for age and other cardiovascular risk factors.Citation6 The hospital HF adjusted prevalence is three times greater among patients discharged with COPD when compared with patients without this disease.Citation2 Nevertheless, we have to take into account that most studies used inappropriate definitions for COPD and HF. They did not consider GOLD’s criteria or reproducible echocardiographic parameters.Citation7 In a recent study, the authors assessed the prevalence and prognostic implications of the coexistence of COPD and HF using objective measurements.Citation7 The prevalence of airway obstruction among chronic HF patients was 37.3% and the prevalence of ventricular dysfunction among COPD patients was 17%.Citation8 Also, the presence of ventricular dysfunction in patients with COPD tended to increase the risk of mortality during follow-up, however, the presence of airway obstruction in patients with chronic HF does not seem to influence survival.Citation8
The high prevalence of HF in COPD patients is not surprising. We have to bear in mind that these patients have a higher risk of cardiovascular morbidity and mortality which is independent from other factors, such as tobacco use.Citation9,Citation10 The forced expiratory volume in the first second (FEV1) values appear to be as good a predictor of cardiovascular mortality as cholesterol values.Citation11
For all these reasons, it is really important to recognize the coexistence of both processes early. In this article, we review the physiopathology, clinical aspects, diagnosis, and treatment of patients with both conditions, ie, COPD and HF. We therefore performed a bibliographic search in PubMed using the medical subject heading terms “COPD” and “heart failure.”
Pathophysiology
The relationship between COPD and cardiovascular events is not completely clear. Like cardiovascular diseases, there is evidence that suggests that stable COPD is associated with low grade systemic inflammation.Citation12 The risk of underlying ischemic heart disease is greater in patients with moderate or severe airflow obstruction and high circulating C-reactive protein (CRP), suggesting a certain influence of this inflammatory marker on cardiac risk. CRP was also found to be correlated with vascular structure and function in COPD patients, although not consistently.Citation13–Citation16 In addition, several markers of cardiovascular risk are associated with airflow limitation.Citation13,Citation17–Citation19
Moreover, it has also been shown that the association of obstructive sleep apnea (OSA) and COPD, which has been called overlap syndrome, is associated with vascular endothelial dysfunction, elevated inflammatory mediators, and accelerated atherosclerosis. All of these factors are associated with reduced survival. In addition, OSA is considered to lead to insulin resistance, hypertension, and cardiovascular diseases through increased sympathetic activity, inflammation, and oxidative stress. In epidemiologic studies and clinical cohorts, OSA has also been associated with an increased risk of death, mainly because of cardiovascular causes. Indeed, it has been suggested that the number of cardiovascular deaths in patients with untreated overlap syndrome is higher when compared with overlap treated patients, and also higher than those with COPD only.Citation20
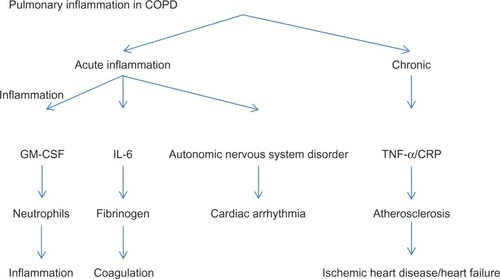
Inflammation is itself implicated in the pathogenesis of HF. Incidence of this condition was higher among Framingham subjects with elevated CRP and cytokine levels, independently of the status of established risk factors.Citation21 A hypothesis to explain the high prevalence of left ventricular systolic dysfunction in COPD patients is that systemic inflammation would accelerate coronary atherosclerosis progression, leading to the development of ischemic heart disease. The high incidence of motor alterations in the left ventricular wall and left ventricular dysfunction that we observe in COPD patients could also explain the relationship between these chronic progressive diseases ().Citation22 On the other hand, pulmonary hypertension is common in severe COPD and can lead to right HF. In turn, right HF is associated with left H F.Citation23,Citation24
Figure 1 Lung inflammation in chronic obstructive pulmonary disease may contribute to the appearance of cardiovascular events.
Skeletal muscle alterations in patients with COPD and HF include a decrease in muscle mass, size, and diameter. On the fibrillar level, atrophy of type 1 oxidative fibers occurs, and there is a relative increase in type 2a and 2b glycolytic fibers as a result of decreased oxidative enzyme activity and increased glycolytic activity, respectively.Citation25
Different techniques have demonstrated the presence of a reduced high energy phosphate concentration at rest, which is more pronounced during exercise. In addition, a faster pH decline and slower rephosphorylation after exercise are seen in patients with both COPD and HF.Citation26
Loss of muscle mass and skeletal muscle atrophy have more serious clinical and therapeutic implications in patients with COPD and HF. Muscular atrophy contributes to muscle fatigue during exercise, which causes these patients to interrupt their exercise in spite of not exhausting their cardiac and respiratory reserves.Citation27 As a result, the maximum oxygen consumption is directly related to skeletal muscle mass in both processes.Citation28 The therapeutic interventions that improve pulmonary and left ventricular function in patients with COPD and HF, respectively, do not reverse the muscular atrophy process, and do not, therefore, relieve functional intolerance. The mechanisms involved in muscular atrophy in both diseases are unknown, although they seem to be related to muscular disease, systemic inflammation, and an increase in oxidative stress, which contributes to reducing protein synthesis and accelerating protein degradation.Citation29 High circulatory values of proinflammatory cytokines have been found in patients with COPD and HF. These include 8-isoprostane, which is also found in high concentrations in the pericardial fluid of HF patients.Citation30 In summary, COPD patients have an increased risk of developing HF, since both diseases share the same pathogenic mechanism.
Diagnosis
The symptoms and physical signs of COPD and HF may coexist.Citation31 Fatigue and exertional dyspnea are frequent symptoms that are common in both conditions, and they can result in marked activity intolerance.Citation32 However, acute onset dyspnea or orthopnea, nocturnal cough, paroxysmal nocturnal dyspnea, ease of fatigue, and reduced exercise tolerance in the absence of infectious exacerbation of COPD gives rise to a diagnosis of HF. The presence of jugular venous distension, ankle edema, and hepatomegaly in COPD should be oriented toward the existence of right ventricular failure.Citation33
The electrocardiogram presents a high negative predictive value for the diagnosis of systolic ventricular function when it is normal. However, it is not a specific technique for diagnosing HF when abnormalities are present, as these abnormalities are frequent in patients with COPD and H F.Citation34
Chest radiography is not sensitive for detecting the coexistence of COPD and chronic HF, as the cardiothoracic ratio may remain normal as the heart can be long and narrow in a hyperinflated chest, and pulmonary edema can be masked by pulmonary vascular remodeling and radiolucent lung fields.Citation2,Citation33 However, it may be useful for detecting other diseases, other than COPD or HF.Citation31
In patients with COPD, plasma levels of natriuretic peptides are a fast and sensitive biomarker for diagnosing HF. Both B-type natriuretic peptide (BNP) and N-terminal fragment of B-type natriuretic peptide (NT-proBNP) are produced from a prohormone, proBNP, which is secreted by the myocytes for an increase in atrial and ventricular filling pressure.Citation35 A single BNP cutoff point for excluding or detecting HF is 100 pg/mL. Thus, HF is unlikely when BNP levels are below this value. BNP levels ranging from 100 pg/mL to 500 pg/mL in COPD patients may be due to cor pulmonale (right ventricular stretch), moderate left ventricular failure, or both. Finally, although not specifically tested in patients with a history of COPD, BNP > 500 pg/mL is suggested by several authors to indicate acute HF in COPD patients.Citation1,Citation10,Citation12,Citation33 Recently, Macchia et al demonstrated that a BNP cutoff point of 160 pg/mL in COPD patients increases, more than 10 times, the probability of finding a ventricular dysfunction with echocardiography.Citation8 For NT-proBNP, a value < 300 pg/mL excludes HF, and a value > 450 pg/mL (for patients < 50 years) or a value > 900 pg/mL (for those ≥50 years) identifies HF in patients with previous COPD presenting with acute dyspnea.Citation12 For excluding HF, the sensitivity was 94%, while for detecting H F, the specificity was 84%. In COPD patients with previous history of HF, the values of sensitivity and specificity were 97% and 47% respectively, and in those without previous COPD these values were 90% and 90% respectively.Citation36 Regardless, it should be noted that 95%–100% of patients are correctly diagnosed when natriuretic peptides are added to clinical judgment.Citation18,Citation37 The recent National Institute for Health and Care Excellence (NICE), guidelines recommend a BNP level > 400 pg/mL or a NT-proBNP level > 2000 pg/mL for HF diagnosis.Citation38 If doubts exist, cardiac imaging techniques could be applied.
Using an echocardiogram in COPD patients can detect left ventricular dysfunction (diastolic or systolic) which is associated with the presence of cardiovascular disease in a high percentage of cases.Citation39 In addition, the echocardiographic evaluation of the right ventricle, which determines the systolic pulmonary artery and interventricular septal pressures, is essential to assess the presence of cor pulmonale in COPD patients and to establish their short-term prognosis.Citation40 NICE guidelines recommend an echocardiogram be performed within two weeks in patients with BNP values > 400 pg/mL or NT-proBNP > 2000 pg/mL, or within 6 weeks in those patients with BNP values between 100 pg/mL and 400 pg/mL or NT-proBNP between 400 pg/mL and 2000 pg/mL.Citation38 If an echocardiogram is normal, HF would be excluded. On the other hand, HF diagnosis should be kept in mind in COPD patients with left ventricular ejection fraction > 40% and abnormal left ventricular mass, or enlarged left atrium by echocardiography.Citation1
Although echocardiography is the reference test for diagnosing HF, it could be limiting in patients with obesity or those with COPD who have a poor echocardiographic window due to pulmonary hyperinflation.Citation41 In these cases, evaluating the right ventricle by magnetic resonance imaging (MRI) can be more objective. Apart from providing accurate and reproducible measurements of left ventricular volumes and left ventricular ejection fraction that are not affected by lung hyperinflation, this technique is also valuable in the evaluation of right ventricular volume and function. However, this complementary test has some disadvantages such as the acquisition time, limited availability, and higher costs than echocardiography, which limits its usefulness in clinical practice. Thus, the MRI should be reserved to evaluate left ventricular function in HF patients with technically limited echocardiogram images.Citation2
In the resting pulmonary function tests we can see that the obstructed airflow, the destruction of pulmonary tissue in emphysema, and the respiratory muscle weakness in COPD patients, provoke an obstructive type respiratory defect. In turn, HF is accompanied by the development of a restrictive disorder, which is partly due to heart enlargement and pulmonary venous congestion that occurs in this disease. Thus, patients with COPD and HF have combined obstruction and restriction in pulmonary function tests.Citation42 However, lung function can improve after treatment of HF. Therefore, in patients with this condition, it is recommended to repeat the spirometry after treatment to make a final diagnosis of COPD.Citation32
During exercise, the dynamic hyperinflation caused by the increased residual function capacity, tachypnea caused by COPD, and the increased dead space and CO2 consumption resulting from HF, produce an abnormal ventilation/perfusion relationship.Citation25 In addition, a drop in carbon monoxide diffusing capacity occurs, caused by COPD and HF, limiting the exertion capacity.
Arterial blood gas detects alterations in the gas exchange that occur in COPD and HF patients. During exertion, COPD causes a decrease in O2 arterial pressure (PaO2) and an increase in CO2 arterial pressure (PaCO2), while the HF worsens the gas exchange. This favors hypoxemia and increases PaCO2.Citation43
Patients with COPD and HF have significantly impaired cardiopulmonary exercise testing responses. These tests reveal a significantly poorer response in subjects with both diseases by several variables, including peak oxygen consumption, minute ventilation/carbon dioxide production slope, and heart rate recovery at 1 minute. This finding may impact the clinical interpretation of cardiopulmonary exercise testing data in patients with COPD and HF.Citation44
In summary, plasma levels of natriuretic peptides can be useful in patients with COPD. These parameters perform better for excluding, rather than for detecting, HF. In case of doubt, cardiac imaging techniques should be used. To investigate simultaneous COPD in HF patients, several pulmonary function tests should be performed.
Therapy
There are few prospective studies and randomized clinical trials regarding combined treatment for patients with COPD and HF. Most of these results come from retrospective studies, clinical trial subgroup analyses, or meta-analyses with outcomes other than evaluation of treatment for both conditions (combined therapy). We have to consider the fact that the treatment for COPD may have an influence on the clinical course of HF and vice versa.Citation42 Now, we offer a brief review of the therapeutic aspects of both.
Pharmacological treatment for HF that influences COPD patient prognosis
In COPD patients, HF should be treated according to usual HF guidelines as there is no evidence that HF should be treated differently in the presence of this respiratory disease.Citation7 Although β-blocker therapy improves symptoms and survival among patients with chronic HF, it is frequently withheld in patients with COPD due to concerns of the diminution of the β2-agonist bronchodilator effect and the worsening of bronchospasm.Citation45 Although a Cochrane meta-analysis concluded that β1-selective β blockade was safe, only 35% of patients with chronic HF and COPD receive β2-blocker therapy.Citation45 Recently, the NICE and the European Society of Cardiology (ESC) guidelines stated that COPD is not a contraindication for the use of β-blockers.Citation38,Citation46 Cardioselectivity is a priority, metoprolol, bisoprolol, and nebivolo are the best candidates.Citation47 A study has shown that treatment with bisoprolol in patients with COPD and HF decreased FEV1 but without deleterious effects on symptoms and quality of life, and that a selective β1-blocker is preferable to a nonselective β-blocker in these patients.Citation34 In a studyCitation48 of patients with moderate to severe airflow obstruction and HF, treatment with bisoprolol and carvedilol was well tolerated and beneficial effects on lung function were seen. However, bisoprolol was superior to carvediol on respiratory parameters.Citation48 The benefits of selective β1-blocker treatment in HF clearly outweigh any potential risk associated with treatment in COPD patients, even in those with severe obstruction. Low dose initially and gradual up-titration is recommended. Mild deterioration in pulmonary function or presence of mild respiratory symptoms should not prompt discontinuation.Citation46 Physicians may be even more reluctant to continue β-blocker therapy during an acute exacerbation of COPD when a patient’s respiratory status is most tenuous, although it has been shown that among patients with HF, continuing β1-selective β-blockers during hospitalization for COPD appears to be safe.Citation45
Treatment with statins, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin-receptor blockers (ARBs) can reduce the morbidity and mortality of COPD patients.Citation49,Citation50 Some retrospective studies, such as the one by Mancini et alCitation49 have shown a reduced risk of hospitalization in COPD patients treated with statins who also received treatment with ACE inhibitors and/or ARBs. In fact, in patients with absolute contraindications for β-blockers, the association of ACE inhibitors and ARBs could be a treatment option for increasing survival, as shown in a meta-analysis that has evaluated this topic.Citation49 Additionally, Mortensen et al have also reported that the use of statins and ACE inhibitors prior to hospital admission is associated with a decreased mortality in subjects hospitalized for COPD exacerbation.Citation50 Recently, in a prospective study by Bartziokas et al, the use of statins was associated with a low risk of COPD exacerbation and more severe COPD.Citation51 Nonetheless, results from these studies should be cautiously interpreted. We think that new controlled studies to establish their real usefulness are needed. In the meantime, the use of these medications should only be recommended when COPD patients have an associated condition with clear indication.Citation52
The use of diuretics is another important aspect that must be evaluated in these patients, given that high doses of loop diuretics can produce metabolic alkalosis, with the presence of hypoventilation as a compensatory mechanism, which in turn could worsen hypercapnia.Citation53,Citation54 In addition, although large prospective studies are not available, the use of high doses of diuretics in HF patients is associated with a high risk of kidney dysfunction, morbidity, and mortality.Citation55
A recent studyCitation56 that assessed the pharmacologic treatment of patients with COPD and HF showed that only 22% of patients with both conditions were prescribed β-blockers, as opposed to 81% of those without COPD. This contrasted markedly with the prescription of ACE inhibitors and ARBs, where no significant difference was noted between these two groups. Most COPD patients were prescribed aldosterone antagonists while a few were prescribed loop diuretics.Citation56
Pharmacological treatment of COPD in patients with HF
COPD should be treated according to clinical guidelines in patients with HF as there is no direct evidence that this respiratory disease should be treated differently in the presence of HF.Citation7 This statement is based on findings from large long-term studies in patients with HF and comorbid COPD.Citation57–Citation59
Inhaled β2-agonists are part of the mainstay of the COPD pharmacological treatment. However, they can induce adverse cardiac effects in COPD patients with pre-existing cardiovascular disease, especially in those with coexistent HF. An observational study found an increased risk of death and hospital admission among patients with HF treated with inhaled β-agonists,Citation60 possibly indicating a need for close follow-up of patients with severe HF who are on this treatment for COPD. Oral β2-agonists should be avoided, and both the dose and frequency of nebulized therapy should be minimized. Patients with HF and concomitant COPD who require a regular long-acting inhaled bronchodilator, should start treatment with a long-acting antimuscarinic rather than long-acting β2-agonists.Citation47 However, in a recent study where patients with COPD and HF were assessed, β2-agonists were the most frequent therapy for COPD, followed by inhaled corticosteroids and anti-muscarinic drugs.Citation56
Furthermore, the use of corticosteroids can increase the risk of hydrosaline retention in HF patients. There are studies that show that COPD patients who received high doses of corticosteroids (doses of prednisone above 20 mg/day) had a higher risk of presenting with an episode of decompensated HF than those who received these drugs in lower doses.Citation61 Inhaled corticosteroids have a lower risk for side effects than oral administration, they can be administered if indicated according to clinical guidelines.
Finally, methylxanthines are in decreasing use, do not seem to be a good alternative treatment for patients with COPD and HF. They carry an increased risk for arrhythmias.Citation42
Noninvasive ventilation in patients with COPD and HF
Noninvasive ventilation (NIV), added to conventional therapy, improves the outcome of patients with acute respiratory failure due to hypercapnic exacerbation of COPD or heart failure in situations of acute pulmonary edema. NIV improves gas exchange and symptoms in patients with COPD, reducing the need for endotracheal intubation, hospital mortality, and hospital stay when compared with conventional oxygen therapy. NIV may also avoid reintubation and may decrease the length of invasive mechanical ventilation. In acute cardiogenic pulmonary edema, NIV accelerates the remission of symptoms and the normalization of blood gas parameters, reduces the need for endotracheal intubation, and is associated with a trend toward lower mortality. The ventilation modality used in acute pulmonary edema does not affect patient prognosis.Citation62
In patients with cor pulmonale secondary to a chronic pulmonary disease like COPD, the use of Biphasic Positive Airway Pressure (BiPAP), can improve the right ventricular function and decrease plasma levels of natriuretic peptides.Citation63 Furthermore, recent studies have concluded that administering this treatment method can play an important role for HF patients with associated muscle fatigue and hypercapnia.Citation64,Citation65
In summary, the combination of COPD and HF presents many therapeutic challenges. The beneficial effects of cardioselective β1-blockers should not be denied in stable patients who have HF and coexistent COPD. Statins, ACE inhibitors, and ARBs may reduce the morbidity and mortality of COPD patients. Moreover, caution is advised with use of inhaled β2-agonists for the treatment of COPD in patients with HF. NIV, when added to conventional therapy, improves the outcome of patients with acute respiratory failure due to hypercapnic exacerbation of COPD or heart failure in situations of acute pulmonary edema. The establishment of a combined and integrated approach to managing these comorbidities would seem an appropriate strategy.
Conclusion
COPD is commonly associated with HF in clinical practice. Both conditions incur significant morbidity and mortality. Clinicians attending these patients should perform an integrated approach to objectively identify both diseases at an early stage, and to optimize control of respiratory and cardiovascular conditions. This will result in a reduction in the morbidity and mortality of these patients. Additional studies providing new data on the pathogenesis and management of patients with COPD and HF are needed, with the purpose of trying to improve quality of life as well as survival of these patients.
Disclosure
The authors declare no conflicts of interest in this work.
References
- Le JemtelTHPadelettiMJelicSDiagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failureJ Am Coll Cardiol200749217118017222727
- HawkinsNMPetrieMCJhundPSChalmersGWDunnFGMcMurrayJVHeart failure and chronic obstructive pulmonary disease: diagnosis pitfalls and epidemiologyEur J Heart Fail200911213013919168510
- NiHNaumanDHershbergerREManaged care and outcomes of hospitalization among elderly patients with congestive heart failureArch Intern Med199815811123112369625402
- LainscakMHodoscekLMDüngenHDThe burden of chronic obstructive pulmonary disease in patients hospitalized with heart failureWien Klin Wochenschr20091219–1030931319562292
- UkenaCMahfoudFKindermannMThe cardiopulmonary continuum systemic inflammation as ‘common soil’ of heart and lung diseaseInt J Cardiol2010145217217620570377
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for Diagnosis, Management, and Prevention of COPD Updated Feb 2013. Available from: http://www.goldcopd.org/Guidelines/guidelines-resources.htmlAccessed April 26, 2013
- MacchiaARodríguez MoncalvoJJUnrecognised ventricular dysfunction in COPDEur Respir J2012391515821700606
- De BloisJSimardSAtarDAgewallSNorwegian Heart Failure RegistryCOPD predicts mortality in HF: the Norwegian Heart Failure RegistryJ Card Fail201016322522920206897
- van der MolenTCo-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequencesPrim Care Respir J201019432633420842323
- HoleDJWattGCDavey-SmithGHartCLGillisCRHawthorneVMImpaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population studyBMJ199631370597117158819439
- HanninkJDCVan HelvoortHACDekhuijzenPNRHeijdraYFHeart failure and COPD: partners in crime?Respirology201015689590120546188
- IwamotoHYokoyamaAKitaharaYAirflow limitation in smokers is associated with subclinical atherosclerosisAm J Respir Crit Care Med20091791354018931335
- EickhoffPValipourAKissDDeterminants of systemic vascular function in patients with stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008178121211121818836149
- MillsNLMillerJJAnandAIncreased arterial stiffnes in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular riskThorax200863430631118024535
- McAllisterDAMaclayJDMillsNLArterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007176121208121417885263
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- SabitRBoltonCEEdwardsPHArterial stiffness and osteoporosis in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007175121259126517363772
- ZureikMBenetosANeukirchCReduced pulmonary function is associated with central arterial stiffness in menAm J Respir Crit Care Med2001164122181218511751184
- MarinJMSorianoJBCarrizoSJBoldovaACelliBROutcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndromeAm J Respir Crit Care Med2010182332533120378728
- VasanRSSullivanLMRoubenoffRDinarelloCAHarrisTBenjaminEJInflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart StudyCirculation2003107111486149112654604
- SteelePEllisJHvan DykeDSuttonFCreaghEDaviesHLeft ventricular ejection fraction in severe chronic obstructive airways diseaseAm J Med197559121281138548
- BoussugesAPinetCMolenatFLeft atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler studyAm J Respir Crit Care Med20001622 Pt 167067510934104
- LouieEKRichSLevitskySBrundageBHDoppler echocardiographic demonstration of the differential effects of right ventricular pressure and volume overload on left ventricular geometry and fillingJ Am Coll Cardiol199219184901729350
- GoskerHRLencerNHFranssenFMvan der VusseGJWoutersEFScholsAMStriking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPDChest200312351416142412740256
- GoskerHRWoutersEFvan der VusseGJScholsAMSkeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectivesAm J Clin Nutr20007151033104710799364
- JondeauGKatzSDZohmanLActive skeletal muscle mass and cardiopulmonary reserve. Failure to attain peak aerobic capacity during maximal bicycle exercise in patients with severe congestive heart failureCirculation1992865135113561423946
- HarringtonDAnkerSDChuaTPSkeletal muscle function and its relation to exercise tolerance in chronic heart failureJ Am Coll Cardiol1997307175817649385904
- JackmanRWKandarianSCThe molecular basis of skeletal muscle atrophyAm J Physiol Cell Physiol20042874C834C84315355854
- GanWQManSFSenthilselvanASinDDAssociation between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysisThorax200459757458015223864
- StevensonLPerloffJThe limited reliability of physical signs for estimating hemodynamics in chronic heart failureJAMA198926168848882913385
- ZengQJiangSUpdate in diagnosis and therapy of coexistent chronic obstructive pulmonary disease and chronic heart failureJ Thorac Dis20124331031522754671
- ChhabraSKGuptaMCoexistent chronic obstructive pulmonary diseaseheart failure: mechanisms, diagnostic and therapeutic dilemmasIndian J Chest Dis Allied Sci201052422523821302600
- DavieAPMcMurrayJJValue of ECGs in identifying heart failure due to left ventricular systolic dysfunctionBMJ199631370523003018704562
- BoomamaFvan der MeirackerAHPlasma A- and B-type natriuretic peptides: physiology, methodology and clinical useCardiovasc Res200151344244911476734
- TungRHCamargoCAJrKrauserDAmino-terminal pro-brain natriuretic peptide for the diagnosis of acute heart failure in patients with previous obstructive airway diseaseAnn Emerg Med2006481667416781921
- McCulloughPAHollanderJENowakRMUncovering heart failure in patients with a history of pulmonary disease: rationale for the early use of B-type natriuretic peptide in the emergency departmentAcad Emerg Med200310319820412615582
- Al-MohammadAMantJThe diagnosis and management of chronic heart failure: review following the publication of the NICE guidelinesHeart201197541141621296784
- RenderMLWeinsteinASBlausteinASLeft ventricular dysfunction in deteriorating patients with chronic obstructive pulmonary diseaseChest199510711621687813269
- BiernackiWFlenleyDCMuirALMacNeeWPulmonary hypertension and right ventricular function in patients with COPDChest1988946116911753191757
- DevereauxRBLiebsonPRHoranMJRecommendations concerning use of echocardiography in hypertension and general population researchHypertension198792 Pt 2II97II1042948915
- Villar AlvarezFMéndez BailónMde Miguel DíezJChronic obstructive pulmonary disease and heart failureArch Bronconeumol200945838739319595494
- NavarroEsteva JAlonsoBInsuficiencia cardiaca y enfermedad pulmonar obstructiva crónica. [Heart failure and chronic obstructive pulmonary disease]Arch Med20051 Available from: http://www.archivosdemedicina.comAccessed February 28, 2013 Spanish
- GuazziMMyersJVicenziMCardiopulmonary exercise testing characteristics in heart failure patients with and without concomitant chronic obstructive pulmonary diseaseAm Heart J2010160590090521095278
- StefanMSRothbergMBPriyaAPekowPSAuDHLindenauerPKAssociation between β-blocker therapy and outcomes in patients hospitalized with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertensionThorax2012671197798422941975
- DicksteinKCohen-SolalAFilippatosGGuidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM)Eur Heart J200829192388244218799522
- HawkinsNMPetrieMCMacDonaldMRHeart failure and chronic obstructive pulmonary disease. The quandary of beta-blockers and beta-agonistsJ Am Coll Cardiol201157212127213821596228
- LainscakMPodbregarMKovacicDRomanJvon HaehlingSDifferences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trialRespir Med2011105Suppl 1S44S4922015086
- ManciniGBReduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary diseaseJ Am Coll Cardiol200647122554256016781387
- MortensenEMCopelandLAPughMJImpact of statins and ACE inhibitors on mortality after COPD exacerbationsRespir Res2009104519493329
- BartziokasKPapaioannouAIMinasMKostikasKBanyaWDaniilZDStatins and outcome after hospitalization for COPD exacerbation: a prospective studyPulm Pharmacol Ther201124562563121729761
- DeMiguelDíezJMoránCaicedo LRodríguezRodríguez PJuarezMoralesMCPuenteMaestuLAlvarez-SalaWalther LLa enfermedad pulmonar obstructiva crónica como factor de riesgo cardiovascular [COPD as a cardiovascular risk]Aten Primaria Epub352012 Spanish
- AronowWSTreatment of heart failure in older persons. Dilemmas with coexisting conditions: diabetes mellitus, chronic obstructive pulmonary disease, and arthritisCongest Heart Fail20039314214712826772
- RuttenFHCramerMJLammersJWGrobbeeDEHoesAWHeart failure and chronic obstructive pulmonary disease: an ignored combination?Eur J Heart Fail20068770671116531114
- EshaghianMRelation of loop diuretic dose to mortality in advanced heart failureAm J Cardiol200697121759176416765130
- O’KellyNRobertsonWSmithJDexterJCarroll-HawkinsCGhoshSShort-term outcomes in heart failure patients with chronic obstructive pulmonary disease in the communityWorld J Cardiol201243667122451854
- CalverleyPMAndersonJACelliBTORCH investigatorsSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- TashkinDPCelliBSennSUPLIFT Study InvestigatorsA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- CalverleyPMAndersonJACelliBTORCH InvestigatorsCardiovascular events in patients with COPD: TORCH study resultsThorax201065871972520685748
- AuDHUdrisEMFanVSCurtisJRMcDonellMBFihnSDRisk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunctionChest200312361964196912796175
- SouvereinDCUse of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control studyHeart200490885986515253953
- RialpCervera GDelCastillo Blanco APérezAizcorreta OParraMorais Lpor el GT-IRA de la SEMICYUCNoninvasive mechanical ventilation in chronic obstructive pulmonary disease and in acute cardiogenic pulmonary edemaMed Intensiva Epub11152012 Spanish
- ThorensJBRitzMReynardCHaemodynamic and endocrinological effects of noninvasive mechanical ventilation in respiratory failureEur Respir J19971011255325599426094
- MasipJBetbeséAJPáezJVecillaFCañizaresRPadróJNon-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trialLancet200035692482126213211191538
- ResterholtzTKempfJBertonCNoninvasive pressure support ventilation (NIPSV) with face mask in patients with acute cardiogenic pulmonary edema (ACPE)Intensive Care Med1999251212810051074