Abstract
Purpose
There is an unmet clinical need for an accurate and objective diagnostic tool for early detection of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). DETECT (NCT03556475) was a multicenter, observational, cross-sectional study aiming to develop and validate multivariable prediction models for AECOPD occurrence and severity in patients with chronic obstructive pulmonary disease (COPD) in China.
Patients and Methods
Patients aged ≥40 years with moderate/severe COPD, AECOPD, or no COPD were consecutively enrolled between April 22, 2020, and January 18, 2021, across seven study sites in China. Multivariable prediction models were constructed to identify AECOPD occurrence (primary outcome) and AECOPD severity (secondary outcome). Candidate variables were selected using a stepwise procedure, and the bootstrap method was used for internal model validation.
Results
Among 299 patients enrolled, 246 were included in the final analysis, of whom 30.1%, 40.7%, and 29.3% had COPD, AECOPD, or no COPD, respectively. Mean age was 64.1 years. Variables significantly associated with AECOPD occurrence (P<0.05) and severity (P<0.05) in the final models included COPD disease-related characteristics, as well as signs and symptoms. Based on cut-off values of 0.374 and 0.405 for primary and secondary models, respectively, the performance of the primary model constructed to identify AECOPD occurrence (AUC: 0.86; sensitivity: 0.84; specificity: 0.77), and of the secondary model for AECOPD severity (AUC: 0.81; sensitivity: 0.90; specificity: 0.73) indicated high diagnostic accuracy and clinical applicability.
Conclusion
By leveraging easy-to-collect patient and disease data, we developed identification tools that can be used for timely detection of AECOPD and its severity. These tools may help physicians diagnose AECOPD in a timely manner, before further disease progression and possible hospitalizations.
Plain Language Summary
Chronic obstructive pulmonary disease causes breathing-related problems and airflow blockage. As the disease progresses, patients often experience worsening of symptoms in a process called exacerbation. Exacerbation can lead to poorer quality of life and even hospitalization. Physicians can better manage the disease if exacerbations are detected early. However, it is difficult for physicians to always detect exacerbations early because hospitals and medical centres sometimes lack accurate and objective measurement tools.
In this study, Yin et al developed two measurement tools to help physicians detect and manage exacerbations in their patients with COPD. The first tool indicates whether a patient is experiencing an exacerbation, using information on disease-related history, and current signs and symptoms. The second tool indicates whether the patient has a severe exacerbation, using information on previous exacerbations, as well as current disease-related symptoms.
Physicians can better manage chronic obstructive pulmonary disease by using these measurement tools to detect exacerbations early on, which can help to prevent substantial worsening of the disease.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by persistent airflow limitation and chronic respiratory symptoms, is one of the leading causes of morbidity and mortality globally.Citation1 In China, COPD affects close to 100 million individuals (13.6% of adults aged ≥40 years),Citation2 and was identified as one of the top three causes of disability-adjusted life-years in 2017.Citation3 COPD exacerbations are part of the natural course of the disease and are defined as an acute worsening of respiratory symptoms that result in a need for additional therapy.Citation4 A meta-analysis of seven randomized controlled trials comprising 18,746 patients reported nearly one-quarter of patients with COPD experienced ≥1 severe exacerbation in the previous year;Citation5 in China, patients with COPD experience 0.5–3.5 episodes of an acute exacerbation of COPD (AECOPD) annually.Citation6 Acute exacerbations may enhance disease progression and are major drivers of economic burden. Among patients in China who were hospitalized for AECOPD, long-term mortality was 37.2%.Citation7 The mean per capita expenditure for AECOPD increased from 3,472 USD to 4,538 USD between 2009 and 2017.Citation8 Given that there is a limited window of time between exacerbation onset and possible hospitalization, early detection and intervention for AECOPD are crucial to improve disease prognosis.Citation9
There are several challenges in detecting AECOPD, particularly in primary healthcare settings in China, leading to frequent misdiagnoses and missed diagnoses. These include: 1) there exists a lack of objective measurements, and diagnosis relies largely on clinical symptoms and the types of healthcare resources used;Citation10 2) spirometry is currently the most reproducible and objective method of measuring airflow limitation,Citation1 but many community hospitals in China lack access to spirometry equipment;Citation11 3) comorbidities are common in patients with COPD,Citation6 which may enhance the challenge of differential diagnosis; 4) there is a low level of COPD-specific knowledge among physicians in primary hospitals;Citation12 and 5) patients may lack COPD awareness and a formal COPD diagnosis.Citation2
There is a clear unmet clinical need for an accurate and objective diagnostic tool for early detection of AECOPD. Most existing models are predictive of future exacerbations, but do not detect current exacerbations.Citation13 One diagnostic tool that captures exacerbation frequency, severity and duration using a patient-reported daily symptom diary is the exacerbations of chronic pulmonary disease tool (EXACT); however, this tool fails to detect up to two-thirds of clinically important outpatient COPD exacerbations.Citation14 To address this gap, the DETECT study (NCT03556475) aimed to develop and validate multivariable diagnostic models to identify AECOPD in patients diagnosed with COPD and non-COPD patients who present with respiratory symptoms (primary objective), and severe AECOPD in patients diagnosed with AECOPD (secondary objective) in community hospitals across China. Development of an AECOPD identification tool using independent variables that are easy to obtain in the clinic setting will help to address the challenges currently faced in China’s primary healthcare settings, as well as the urgent global need for early AECOPD detection and improved disease intervention.Citation1
As a prerequisite to the study, a literature search (to December 31, 2016) was conducted through PubMed, Embase, Ovid, Science Direct and Web of Science. Search terms included (“COPD” or “chronic obstructive pulmonary diseases” or “AECOPD”) AND (“diagnosis” or “diagnostic” or “screen”), and the search field was limited to the article title. Of 523 studies screened, 108 full-text study publications were accessed and reviewed for potential candidate variables to use in development of the multivariable prediction models. These variables were reviewed and verified by respiratory experts in China before application to the present study.
Methods
Study Design and Population
DETECT was an observational, multicenter, cross-sectional study conducted in seven tertiary hospitals with respiratory experts across six regions of China (Eastern, Southern, Northern, Central, Western and Northeastern regions). Patients were consecutively enrolled between April 22, 2020 and January 18, 2021. Key eligibility criteria were: 1) ≥40 years of age; 2) diagnosed with moderate/severe stable COPD (with a diagnosis of COPD, predicted ratio of forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC]< 0.7, and FEV1< 0.8 predicted), AECOPD (with a diagnosis of COPD, predicted ratio of FEV1/FVC< 0.7, and consulted the outpatient clinic or emergency department due to acute worsening of respiratory symptoms with an AECOPD diagnosed by more than two clinical experts), or no COPD (with chronic respiratory symptoms including cough, sputum, wheeze, and dyspnea, and ≥2 high-risk factors including symptoms, exposure, health history, and recent history of respiratory event) per Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 criteria.Citation15 Full inclusion and exclusion criteria can be found in Table S1. COPD exacerbation was defined as worsening of ≥2 major symptoms or 1 major and ≥1 minor symptom for ≥2 consecutive days (or 1 day for very rapid symptom development); major symptoms include dyspnea, increase in sputum volume, and increase in sputum color/purulence, and minor symptoms include sore throat, nasal discharge and/or congestion, fever without other cause, increased cough, and increased wheeze. Mild exacerbations were defined as having symptoms that did not require hospitalization, systemic steroids, or antibiotics for ≥3 days; moderate exacerbations were defined as having symptoms that required systemic corticosteroids and/or antibiotics for ≥3 days but did not require hospitalization or >24 hours in the emergency department; severe exacerbations were defined as having symptoms that required hospitalization (including >24 hours in the emergency department).
Considering a 20% margin for incomplete data and estimated two-sided 95% confidence interval (CI) widths of 0.191 and 0.155 that were based on sensitivity and specificity of 0.70 for internal validation, we aimed to enroll 300 patients, including 90 non-COPD patients with high-risk factors, 90 moderate-to-severe COPD patients, and 120 AECOPD patients (60 mild/moderate, and 60 severe). At a face-to-face visit, information on patient demographics, signs and symptoms, disease and medication history, and health status were collected. Laboratory and spirometry tests were not mandatory, but data were collected if available. The data obtained by respiratory physicians were collected through case report forms, medical records, and patient-reported outcome (PRO) questionnaires. The study was designed and conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practices, Good Pharmacoepidemiology Practices, and the study was approved by the Ethics Committee of The First Hospital of China Medical University (Reference number [2018] 2018–153-2). Written informed consent was obtained from patients prior to study initiation. The study findings were reported in accordance with the guidelines for Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD).
Outcomes and Variables
For the primary objective, the outcome was the occurrence of AECOPD (ie occurrence versus no occurrence). For the secondary objective, the outcome was AECOPD severity (ie severe versus mild/moderate AECOPD). Potential candidate variables identified from the literature search that had >10% missing data were not considered (excluding variables such as drug use, comorbidities, and event-type variables, where missing data were interpreted, for example, as no drug use or no event). The full lists of candidate variables are shown in Tables S2 and S3.
Statistical Analyses
The full analysis set (FAS) comprised patients who fulfilled the inclusion/exclusion criteria with complete information for all candidate variables. Multivariable prediction models were used to construct the identification tools for AECOPD occurrence and AECOPD severity. For the development of the prediction model for AECOPD occurrence (primary outcome), all patients in the FAS (which comprised patients with stable COPD, AECOPD, or no COPD) were included in the analysis. For the development of the prediction model for AECOPD severity (secondary outcome), only patients with AECOPD were included. For each study outcome, three training models were built as the source of apparent performances. Performance measures used were the Akaike information criterion (AIC), Bayesian information criterion (BIC), adjusted R2 and area under the receiver operating characteristic curve (AUC); a smaller AIC or BIC score, and a higher adjusted R2 or AUC were desirable. A stepwise procedure was used to select candidate variables with significance levels <0.15 to enter the model, and <0.20 to stay in the model for multivariate analysis. Stepwise logistic regression is commonly used for variable selectionCitation16 and this method was prespecified during the study design. Age and sex were forced to stay in the model for the primary outcome; age, sex, and most recent exacerbation in the past 12 months were forced to stay in the model for the secondary outcome. The bootstrap method, which is recommended for optimism and overfitting correction,Citation17 was subsequently used for internal model validation, and this procedure was repeated until sensitivity and specificity >0.70 was reached. Cut-off values, that is, the value that maximizes the Youden index (sensitivity + specificity – 1), were calculated to support the clinical utility of both AECOPD occurrence and AECOPD severity prediction models. Individual scores were derived using the logistic regression equations of the most optimal models. The study followed relevant statistical guidance.Citation18 All statistical analyses were carried out on non-missing data using SAS version 9.4. Missing data were handled using a complete-case approach and data were not imputed unless otherwise specified. For categorical variables, unknown and missing data were presented as a separate category, with the denominator excluding unknown or missing values as appropriate.
Results
Patient Demographics and Baseline Characteristics
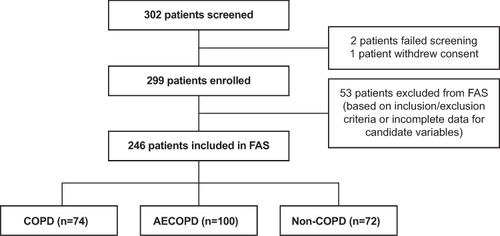
Of the 302 patients screened, two did not meet inclusion/exclusion criteria and one patient withdrew consent. A total of 299 patients were enrolled in the study, and 246 patients were included in the FAS (). Baseline characteristics of patients in the FAS are presented in . Briefly, 74 (30.1%) patients had COPD, 100 (40.7%) patients had AECOPD, and 72 (29.3%) had no COPD. Among those who had AECOPD, 48 (48.0%) were mild/moderate while 52 (52.0%) were severe (Table S4). Mean (standard deviation [SD]) age was 64.1 (9.6) years and most patients were male (n=211, 85.8%); 126 (51.2%) had exacerbations in the past 12 months, and 12 (4.9%), 33 (13.4%), and 91 (37.0%) patients had exacerbations resulting in ≥1 emergency room treatment, hospitalization, and outpatient treatment, respectively.
Table 1 Patient Demographics and Baseline Characteristics (FAS)
Patient Core Symptoms
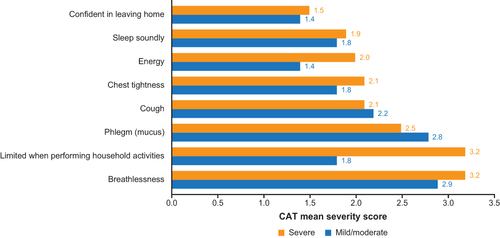
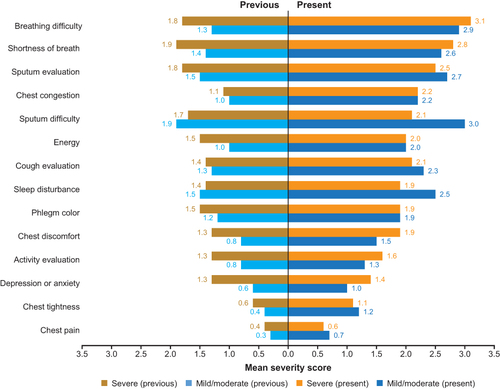
Among patients in the FAS, those with COPD or AECOPD had the highest severity scores (1.8–3.0) for breathing difficulty, shortness of breath, sputum evaluation and difficulty, and cough evaluation (). COPD assessment test (CAT) symptoms with the highest severity scores (2.0–3.0) were breathlessness, phlegm, and being limited when performing household activities (). For non-COPD patients, symptoms with highest severity scores (1.9–2.1) were related to cough and sputum ( and ). Symptoms with the greatest difference in severity scores between patients with AECOPD and patients with COPD were breathing difficulty (3.0 versus 1.8), chest congestion (2.2 versus 1.2), and chest tightness (2.0 versus 1.1) ( and ). Symptom severity scores were largely similar between patients with severe versus mild/moderate AECOPD ( and ) although being limited when performing household activities had a much higher CAT score in patients with severe versus mild/moderate AECOPD (3.2 versus 1.8) ().
Figure 2 Severity scores for core symptoms (patients in FAS). We assigned the following values for phlegm color: 1=clear phlegm, 3=yellowish orange phlegm, 5=dark green phlegm.
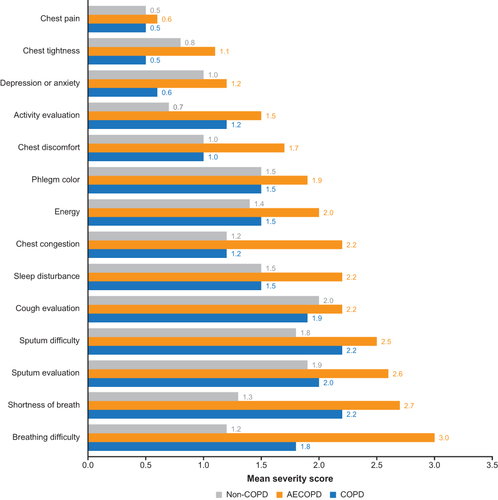
Figure 3 Severity scores for CAT symptoms (patients in FAS).
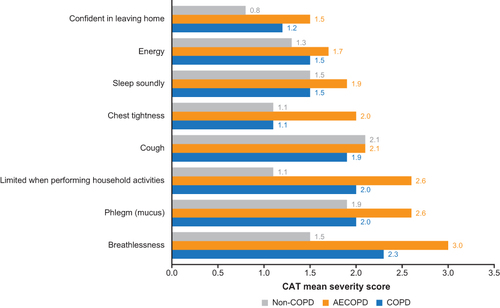
Figure 4 Severity scores for core symptoms in AECOPD patients in FAS. We assigned the following values for phlegm color: 1=clear phlegm, 3=yellowish orange phlegm, 5=dark green phlegm.
Primary Outcome Analysis
Performance results of the training models and that after internal validation are summarized in for the primary outcome. Model 2 was considered the most ideal model for identifying patients with AECOPD (sensitivity 0.84 [95% CI: 0.75–0.90], specificity 0.77 [95% CI: 0.71–0.85], AIC 245.82, BIC 277.37, adjusted R2 0.47, AUC 0.86) (), and had an intercept of –6.4549 (). The following variables were significantly associated with an increase in AECOPD occurrence (P<0.05) and entered the final model: GOLD classification, most recent exacerbation in the past 12 months, number of exacerbations that resulted in hospitalization in the past 12 months, phlegm color, respiratory rate, and chest tightness. Age and sex were not significantly associated with the occurrence of AECOPD (P>0.05) (). The optimal cut-off value corresponding to the Youden index was 0.374 (derived using the logistic regression equation of model 2), indicating that patients were categorized as test positive (ie potentially having AECOPD) when ≥0.374, or as test negative when
<0.374 (Supplement 1).
Table 2 Model Performance Results for the Primary Outcome Analysis
Table 3 Occurrence of Exacerbation Multivariable Logistic Regression Model (FAS=246)
Secondary Outcome Analysis
Performance results of the training models and that after internal validation are summarized in for the secondary outcome. Model 3 was considered the most ideal model for identifying severe AECOPD (sensitivity 0.90 [95% CI: 0.83–0.98], specificity 0.73 [95% CI: 0.60–0.86], AIC 136.93, BIC 157.77, adjusted R2 0.22, AUC 0.81) (), and had an intercept of –0.6810 (). The following variables were significantly associated with AECOPD severity (P<0.05) and entered the final model: exacerbation in the past 12 months, number of exacerbations that resulted in hospitalization, limited when performing household activities, and time of onset of symptoms. Age and sex were not significantly associated with AECOPD severity (P>0.05) (). The optimal cut-off value corresponding to the Youden index was 0.405 (derived using the logistic regression equation of model 3), indicating that patients were categorized as test positive (potentially having severe AECOPD) when ≥0.405, or as test negative when
<0.405 (Supplement 2).
Table 4 Model Performance Results for the Secondary Outcome Analysis
Table 5 Severity of AECOPD Multivariate Logistic Regression Model (AECOPD Patients in FAS=100)
COPD Knowledge
Total COPD knowledge questionnaire (COPD-Q) scores (mean±SD) in the COPD (6.1±2.0) and AECOPD (6.3±2.1) groups were higher than that in the non-COPD group (4.2±2.6). The questions with the lowest proportion of correct answers were: “COPD medicines keep the disease from getting worse” (FAS: 13.0%; AECOPD patients: 12.0%), and “Stopping smoking will keep COPD from getting worse” (FAS: 9.8%; AECOPD patients: 10.0%). The questions with the highest proportion of correct answers were: “People with COPD may feel short of breath” (FAS: 78.0%; AECOPD patients: 90.0%), and “Cigarette smoking or second-hand smoke causes most COPD” (FAS: 71.1%; AECOPD patients: 79.0%) (Table S5).
Discussion
To the best of our knowledge, this is the first study comprising a broad spectrum of respiratory patients to develop a diagnostic model for identifying patients with AECOPD, using key patient characteristics and disease-related factors that were easy to obtain and applicable to daily clinical practice. The model demonstrated high clinical applicability, and may help clinicians (especially those in primary care) enhance their awareness and ability to diagnose patients with AECOPD, guide disease management, and reduce inappropriate prescribing and patient monitoring.
Many models have predicted future exacerbations, but none have detected current exacerbations. Herer et al reported prediction models for AECOPD using multidimensional indices spanning patient characteristics, symptoms, use of COPD maintenance medications, and number of exacerbations in the previous year, and reported sensitivity of 21.9–84.4, specificity of 51.6–87.1, and AUC of 0.50–0.78.Citation19 Others have used a machine-learning algorithm to predict the risk of severe exacerbation, with internal validation yielding an AUC of 0.82.Citation20 In a systematic review of 27 predictive models from 25 studies, it was found that only two out of 25 studies validated their models, and only three out of 27 models used high-quality statistical approaches for model development and evaluation.Citation13 In contrast, instead of predicting risk, our models were constructed to detect AECOPD occurrence and severity. Several previous studies have proposed models to detect AECOPD, using COPD-Q,Citation21 patient-reported daily symptomsCitation14,Citation22 or patient features;Citation23 however, all of these may be subjective and may limit model utility. For example, patient-reported symptoms require frequent monitoring and may result in poor patient compliance.Citation14 The sensitivity (62.5–99.3%), specificity (36.0–100.0%), and AUC values (0.75–0.91) of these models varied widely.Citation21–23 In contrast, our models included data for both patient symptoms and disease-related characteristics, which can be easily collected by respiratory physicians, further enhancing their clinical utility. In our study, the optimal models selected for diagnosis of AECOPD and its severity demonstrated high sensitivity (0.84 and 0.90, respectively) and specificity (0.77 and 0.73, respectively), with high AUC values (0.86 and 0.81, respectively), and low AIC (245.82 and 277.37, respectively) and BIC (136.93 and 157.77, respectively) values, thus indicating strong diagnostic accuracy. Collectively, these features reflect high clinical applicability for our models.
In the primary model, variables that were significant risk factors for AECOPD occurrence were COPD disease-related characteristics, and signs and symptoms. COPD disease-related characteristics (GOLD classification, exacerbation in the past 12 months, and number of exacerbations resulting in hospitalization) were important discriminating factors in the detection of AECOPD. This is consistent with findings from the ECLIPSE study, where deterioration of lung function and a history of exacerbations were associated with the risk of subsequent exacerbations.Citation24,Citation25 Other variables associated with AECOPD occurrence were chest tightness and respiratory rate, which are inherent pathophysiological manifestations of AECOPD.Citation1,Citation26,Citation27 Phlegm color was also associated with AECOPD occurrence; bacterial infections are common in AECOPD and are indicated by deepening sputum color.Citation28 Age is a common risk factor for AECOPD,Citation29 and previous studies have demonstrated that there is an increased risk of AECOPD in females.Citation24,Citation30 As such, these variables were included in our model to increase sensitivity and specificity. However, age and gender, while showing correlation, were not significantly associated with occurrence of AECOPD in our model.
In the secondary model, the number of exacerbations leading to hospitalization was significantly associated with severity of AECOPD. This corroborates previous findings, which showed that a history of prior severe exacerbations resulting in hospitalization was positively associated with new severe exacerbations and mortality.Citation31,Citation32 Interestingly, we found that exacerbation in the past 12 months was strongly associated with mild/moderate AECOPD, indicating that past exacerbation may be a protective factor. This may be due to a higher proportion of patients with mild/moderate compared with severe AECOPD in our study that sought outpatient treatment (79.2% versus 36.5%), although these values may be impacted by memory bias or the small sample size. History of outpatient treatment may have a positive impact on patient behavior and lead to standardization of medication, device use, and increased disease awareness, which in turn may improve disease management.Citation33 In addition, patients who are more engaged with COPD management may be more likely to seek professional healthcare advice when they perceive a worsening of their respiratory symptoms,Citation9 as well as adhere to treatment,Citation34 which in turn is associated with lower rates of severe exacerbations.Citation35 Other important risk factors for severe AECOPD were being limited when performing household activities, and having morning symptoms. This is in line with previous studies, in which increased exacerbation symptoms (breathlessness, cough and sputum, and chest symptoms) were associated with reduced daily physical activity,Citation36 and the rate of severe exacerbation was higher in patients with severe morning symptoms.Citation37 Similar to the primary outcome model, age and gender were not associated with severe AECOPD.
The diagnostic tool for identifying patients with AECOPD may help clinicians, particularly those in primary care settings, to enhance their awareness of AECOPD risk factors and establish a diagnosis of AECOPD. The tool may be used as a supplement to established rating scales or existing predictive models, and may also improve patients’ self-management behavior. In this study, we found that all patients had poor COPD knowledge, especially pertaining to the progressive nature of COPD. Low levels of disease knowledge may impact how patients with COPD interpret the complex changes in their COPD symptoms,Citation9,Citation38 and have been shown to be significantly associated with poorer self-management behavior.Citation39 Use of this diagnostic tool may help patients improve their knowledge of COPD and reporting behavior, thereby reducing future exacerbations, hospitalizations, and healthcare costs, and ultimately improve quality of life.Citation9
This study has several limitations. Firstly, the model developed in this study was validated internally, but not externally. Future prospective studies need to be conducted to validate and improve the present diagnostic model. Nonetheless, the variables identified here were consistent with those associated with AECOPD in previous studies.Citation24,Citation25,Citation31,Citation32 Secondly, potential candidate variables for model development were not selected based on structured criteria, which may have introduced bias. Nonetheless, these variables were identified by expert opinion and through a review of literature, as recommended by others.Citation40,Citation41 Additionally, variables related to lung function and laboratory examinations, which may further improve the accuracy of the model, were not included in this study due to substantial missing data. These variables are not readily available in clinical practice, particularly in primary care settings, and are therefore of limited use in a diagnostic model. Finally, the small sample size in this study may have introduced bias, and this should be considered when applying these models in clinical practice.
Conclusion
By accessing readily available clinical patient and disease data, we developed diagnostic tools for identifying AECOPD and severe AECOPD. In addition, this study highlighted the need to improve patient education on COPD disease characteristics and AECOPD symptoms for more timely disease management. In primary healthcare settings, these diagnostic tools can aid physicians to detect AECOPD before further disease progression, and potential hospitalizations. Future studies should externally validate these models and directly compare their performance to other diagnostic tools.
Abbreviations
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; AIC, Akaike information criterion; AUC, area under the receiver operating characteristic curve; BIC, Bayesian information criterion; BMI, body mass index; CAT, chronic obstructive pulmonary disease assessment test; COPD, chronic obstructive pulmonary disease; COPD-Q, chronic obstructive pulmonary disease knowledge questionnaire; CI, confidence interval; EXACT, exacerbations of chronic pulmonary disease tool; FAS, full analysis set; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council; OR, odds ratio; PEF, peak expiratory flow; PRO, patient-reported outcome; SD, standard deviation; TRIPOD, Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis; USD, United States Dollar.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of The First Hospital of China Medical University (Reference number [2018] 2018-153-2). Written informed consent was obtained from patients prior to study initiation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and/or interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Editorial assistance was provided by Liting Hang BSc (Hons), PhD, and Alice Carruthers BSc (Hons), PhD of Nucleus Global Shanghai, China.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2022 report); 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf. Accessed February 14, 2022.
- Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
- Guo J, Chen Y, Zhang W, Tong S, Dong J. Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: a meta-analysis. Int J Surg. 2020;78:28–35. doi:10.1016/j.ijsu.2020.04.010
- Cai BQ, Cai SX, Chen RC, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People’s Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395. doi:10.2147/COPD.S58454
- Zhang Y, Lin YX. Risk factors analysis for one-year and long-term mortality in patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease [in Chinese]. Chin J Tuberc Respir Dis. 2019;42(12):895–900.
- Liang L, Shang Y, Xie W, Shi J, Tong Z, Jalali MS. Trends in hospitalization expenditures for acute exacerbations of COPD in Beijing from 2009 to 2017. Int J Chron Obstruct Pulmon Dis. 2020;15:1165–1175. doi:10.2147/COPD.S243595
- Wilkinson TMA, Donaldson GC, Hurst JR, Seemungal TAR, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298–1303. doi:10.1164/rccm.200310-1443OC
- Montes de Oca M, Laucho-Contreras ME. Is it time to change the definition of acute exacerbation of chronic obstructive pulmonary disease? What do we need to add? Med Sci. 2018;6(2):50. doi: 10.3390/medsci6020050
- Shen N, He B. Is the new GOLD classification applicable in China? Lancet Glob Health. 2013;1(5):e247–e248. doi:10.1016/S2214-109X(13)70064-0
- Zhou XM, Wu C, Zhao L, et al. A cross-sectional survey of the knowledge on chronic obstructive pulmonary disease in physicians of tertiary hospitals in Northern China [in Chinese]. Chin J Internal Med. 2016;55(9):717–720.
- Guerra B, Gaveikaite V, Bianchi C, Puhan MA. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26(143):160061. doi:10.1183/16000617.0061-2016
- Mackay AJ, Donaldson GC, Patel ARC, Singh R, Kowlessar B, Wedzicha JA. Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT). Eur Respir J. 2014;43(3):735–744. doi:10.1183/09031936.00110913
- Global Initiative for Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention; a guide for health care professionals; 2017. Available from: http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed February 14, 2022.
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:10.1186/1751-0473-3-17
- Iba K, Shinozaki T, Maruo K, Noma H. Re-evaluation of the comparative effectiveness of bootstrap-based optimism correction methods in the development of multivariable clinical prediction models. BMC Med Res Methodol. 2021;21(1):9. doi:10.1186/s12874-020-01201-w
- Harrell JFE. Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York: Springer; 2015.
- Herer B, Chinet T. Acute exacerbation of COPD during pulmonary rehabilitation: outcomes and risk prediction. Int J Chron Obstruct Pulmon Dis. 2018;13(13):1767–1774. doi:10.2147/COPD.S163472
- Tavakoli H, Chen W, Sin DD, FitzGerald JM, Sadatsafavi M. Predicting severe chronic obstructive pulmonary disease exacerbations. Developing a population surveillance approach with administrative data. Ann Am Thorac Soc. 2020;17(9):1069–1076. doi:10.1513/AnnalsATS.202001-070OC
- Trappenburg JCA, Touwen I, de Weert-van Oene GH, et al. Detecting exacerbations using the clinical COPD questionnaire. Health Qual Life Outcomes. 2010;8:102. doi:10.1186/1477-7525-8-102
- Jones PW, Wang C, Chen P, et al. The development of a COPD exacerbation recognition tool (CERT) to help patients recognize when to seek medical advice. Int J Chron Obstruct Pulmon Dis. 2022;17:213–222. doi:10.2147/COPD.S337644
- Claxton S, Porter P, Brisbane J, et al. Identifying acute exacerbations of chronic obstructive pulmonary disease using patient-reported symptoms and cough feature analysis. NPJ Digit Med. 2021;4(1):107. doi:10.1038/s41746-021-00472-x
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
- Meeraus WH, Mullerova H, El Baou C, Fahey M, Hessel EM, Fahy WA. Predicting re-exacerbation timing and understanding prolonged exacerbations: an analysis of patients with COPD in the ECLIPSE cohort. Int J Chron Obstruct Pulmon Dis. 2021;16:225–244. doi:10.2147/COPD.S279315
- Gil HI, Zo S, Jones PW, et al. Clinical characteristics of COPD patients according to COPD assessment test (CAT) score level: cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2021;16:1509–1517. doi:10.2147/COPD.S297089
- Shah SA, Velardo C, Farmer A, Tarassenko L. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res. 2017;19(3):e69. doi:10.2196/jmir.7207
- Allegra L, Blasi F, Diano P, et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med. 2005;99(6):742–747. doi:10.1016/j.rmed.2004.10.020
- Stone RA, Lowe D, Potter JM, Buckingham RJ, Roberts CM, Pursey NJ. Managing patients with COPD exacerbation: does age matter? Age Ageing. 2012;41(4):461–468. doi:10.1093/ageing/afs039
- Kilic H, Kokturk N, Sari G, Cakır M. Do females behave differently in COPD exacerbation? Int J Chron Obstruct Pulmon Dis. 2015;10:823–830. doi:10.2147/COPD.S78952
- Hunter LC, Lee RJ, Butcher I, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open. 2016;6(1):e009121. doi:10.1136/bmjopen-2015-009121
- Santibáñez M, Garrastazu R, Ruiz-Nuñez M, et al. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLoS One. 2016;11(6):e0158727. doi:10.1371/journal.pone.0158727
- Park HJ, Byun MK, Kim T, et al. Frequent outpatient visits prevent exacerbation of chronic obstructive pulmonary disease. Sci Rep. 2020;10(1):6049. doi:10.1038/s41598-020-63064-x
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198–3204. doi:10.1378/chest.128.5.3198
- Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi:10.1136/thx.2009.113662
- Crook S, Büsching G, Keusch S, et al. The association between daily exacerbation symptoms and physical activity in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:2199–2206. doi:10.2147/COPD.S156986
- Sun T, Li X, Cheng W, et al. The relationship between morning symptoms and the risk of future exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1899–1907. doi:10.2147/COPD.S255030
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J. 2002;20(4):799–805. doi:10.1183/09031936.02.03242002
- Yang H, Wang H, Du L, Wang Y, Wang X, Zhang R. Disease knowledge and self-management behavior of COPD patients in China. Medicine. 2019;98(8):e14460. doi:10.1097/MD.0000000000014460
- Grant SW, Collins GS, Nashef SAM. Statistical primer: developing and validating a risk prediction model. Eur J Cardiothorac Surg. 2018;54(2):203–208. doi:10.1093/ejcts/ezy180
- Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health. 2020;8(1):e000262. doi:10.1136/fmch-2019-000262