Abstract
Purpose
To assess the relationship between suboptimal Peak Inspiratory Flow (sPIF), inhalation technique errors, and non-adherence, with Healthcare Resource Utilisation (HCRU) in Chronic Obstructive Pulmonary Disease (COPD) patients receiving maintenance therapy via a Dry Powder Inhaler (DPI).
Patients and methods
The cross-sectional, multi-country PIFotal study included 1434 COPD patients (≥40 years) using a DPI for maintenance therapy. PIF was measured with the In-Check DIAL G16, and sPIF was defined as a typical PIF lower than required for the device. Inhalation technique was assessed by standardised evaluation of video recordings and grouped into 10 steps. Patients completed the “Test of Adherence to Inhalers” questionnaire. HCRU was operationalised as COPD-related costs for primary healthcare, secondary healthcare, medication, and total COPD-related costs in a 1-year period.
Results
Participants with sPIF had higher medication costs compared with those with optimal PIF (cost ratio [CR]: 1.07, 95% CI [1.01, 1.14]). Multiple inhalation technique errors were associated with increased HCRU. Specifically, “insufficient inspiratory effort” with higher secondary healthcare costs (CR: 2.20, 95% CI [1.37, 3.54]) and higher total COPD-related costs (CR: 1.16, 95% CI 1.03–1.31). “no breath-hold following the inhalation manoeuvre (<6 s)” with higher medication costs (CR: 1.08, 95% CI [1.02, 1.15]) and total COPD-related costs (CR 1.17, 95% CI [1.07, 1.28]), and “not breathing out calmly after inhalation” with higher medication costs (CR: 1.19, 95% CI [1.04, 1.37]). Non-adherence was not significantly associated with HCRU.
Conclusion
sPIF and inhalation technique errors were associated with higher COPD-related healthcare utilisation and costs in COPD patients on DPI maintenance therapy.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic and progressive lung disease, impacting the lives of approximately 384 million patients worldwide.Citation1,Citation2 In addition to the large burden of disease associated with COPD, it also poses a large economic burden on the healthcare system and society.Citation3 While inhaled therapy allows high-dose local drug administration and reduces unnecessary systemic exposure, incorrect use of inhalers is a substantial problem among patients with COPD.Citation4 Notably, incorrect inhaler use negatively affects the medication dose that is delivered into the airways, and as such, bronchoconstriction and airway inflammation are insufficiently reduced.Citation5
Effective inhaler use depends on a complex constellation of factors including proper inhaler preparation, a correct inhalation manoeuvre, and medication adherence. A correct inhalation manoeuvre includes the ability to achieve sufficient Peak Inspiratory Flow (PIF) which is of particular importance in patients who are using Dry Powder Inhalers (DPIs). In contrast to pressurised metered-dose inhalers (pMDI) and soft mist inhalers (SMI), DPIs require the patient to generate a minimum inspiratory flow to release the drug from its carrier in the inhaler. The optimal PIF a patient would ideally generate to release the drug from its carrier differs per DPI and has been determined previously.Citation6–10 A PIF lower than the optimal PIF threshold, and therefore considered suboptimal (sPIF), has recently been associated with poorer health status in COPD patients.Citation11 Among other patient- and disease characteristics, deteriorated muscle function and COPD disease severity can drive sPIF.Citation12 In the PIFotal study, 29% of all participants did not generate an optimal PIF for their DPI during a typical inhalation manoeuvre.Citation11 From this population, 54% showed that they were capable of generating an optimal PIF for their device, but failed to do so during a typical inhalation manoeuvre.Citation11 This may indicate that sPIF is a potentially treatable trait in COPD management. While the clinical impact of sPIF during inhaler use has been studied, its potential economic relevance is currently unknown.Citation13
A recent systematic literature review yielded few papers describing the economic burden of inhalation technique errors in patients with COPD,Citation14 with most studies focussing on a specific DPI.Citation15–18 Although previous (modelling) studiesCitation19–21 described the economic impact of COPD patients making inhaler errors in general, there is a need for more detailed economic analyses investigating the association of individual inhalation errors and healthcare resource utilisation (HCRU) and associated costs.
Another relevant factor hampering optimal disease management in COPD is non-adherence to medication. It is known from real-world studies that a substantial proportion of the COPD population is non-adherent to their prescribed medication regimen.Citation22–27 COPD patients with higher adherence to prescribed regimens tend to have lower total healthcare costs compared with adherent patients.Citation28,Citation29 Although the impact of medication non-adherence on health status has been investigated extensively, there is a limited body of literature regarding economic evaluations with real-world data.Citation30
This multi-country PIFotal sub-study aims to explore the association of sPIF, inhalation technique errors, and non-adherence with HCRU and associated costs in COPD patients using DPI maintenance therapy.
Methods
Study Design
The PIFotal COPD study was a cross-sectional, observational, real-world study in five European countries (Greece, the Netherlands, Poland, Portugal, and Spain) and Australia (NCT04532853).Citation11,Citation31 Patients were recruited and included in the study between October 2020 and May 2021. Local medical ethics committees reviewed and approved the study protocol, and all participants provided written informed consent. A flow chart of study procedures is provided elsewhere.Citation11
Study Population
A minimalist approach to the inclusion/exclusion criteria was used in order to ensure a real-world representation of patients as much as possible. Patients with a clinical diagnosis of COPD, aged 40 years or older, who were treated with a DPI as maintenance therapy for their COPD in the previous 3 months, were eligible for participation. Patients were excluded from participation if they were unable to give informed consent, were participating in other trials with COPD medication, if they had had COPD exacerbation in the 6 weeks prior to participation or if they had a life-threatening disease with life expectancy of <6 months.
Peak Inspiratory Flow
PIF (L/min) was assessed using the In-Check DIAL G16 (Clement Clarke, UK).Citation32 The In-Check DIAL G16 can be set to mimic the resistance of the patient’s inhaler. If a patient used multiple inhalers, the assessment priority determined which inhaler would be evaluated in the study (Table S1).
With the In-Check DIAL G16, PIF was assessed in three ways: 1) day-to-day typical PIF at the resistance of patient’s inhaler, 2) maximum PIF at the resistance of patient’s own inhaler 3) maximum PIF at low internal resistance.Citation32 For the typical PIF measurement, patients were asked to inhale with the In-Check DIAL G16 as they normally would with their DPI.Citation11 For both maximal PIF measurements, patients were instructed to breathe out completely to empty the lungs and then inhale as forcefully and fast as possible. Maximal measurements were performed twice. The maximum of the two attempts were included in the data analysis. A participant was categorized as having an optimal PIF when their typical PIF was equal or higher than the PIF that is required for their specific DPI (Table S1). Participants who exhibited a typical PIF lower than that required for their device were categorized as having sPIF.
Pragmatically, patients were further categorized based on their PIF into the following clinically relevant subgroups:
“Can and will do”: Patients with optimal PIF
“Can, but will not do”: Patients with a typical PIF below the optimal PIF for their device, but able to perform a maximum PIF that is equal to or higher than the optimal PIF group.
“Cannot do”: Patients with both their typical and their maximum PIF below the optimal PIF for their device.
These PIF subgroups were formed as part of the PIFotal study,Citation11 and as clinical recommendations are expected to differ between these subgroups we aimed to assess whether HCRU and associated costs differed between the subgroups. More specifically, patients who cannot produce a sufficiently high PIF, even with maximal effort (“cannot do” group), will most likely not benefit from interventions targeting inhalation technique improvement, while it may be a viable option for the patients in the “can, but will not do” subgroup. Additionally, for patients in the “cannot do” group, healthcare professionals could consider selecting a different DPI with lower resistance or inhalers with zero internal resistance such as pMDIs or an SMI.
Inhalation Technique Errors
Inhalation technique was observed and documented by video recording and rated offline by two independent observers. Checklists on inhaler-specific and inhaler-independent commonly made errors were used, based on recommendations of the Lung Alliance Netherlands (www.inhalatorgebruik.nl) or Aerosol Drug Management Improvement Team (www.inhalers4u.org). Inhalation technique was evaluated by grouping errors into 10 categories (). Differences between the two independent observers were resolved by discussion. In case no consensus was reached, a third independent expert was consulted to reconcile the disagreement.
Table 1 Overview of Inhaler Step Categories
Medication Adherence
Adherence was calculated based on the 12-item Test of Adherence to Inhalers (TAI-12).Citation33 Items 1 to 10 could be scored 1–5 points each. Item 11 (patient’s knowledge on medication regimen) and item 12 (device inhalation technique) were scored with “incorrect” or “correct” (1 or 2 points, respectively). Due to the more accurate way of testing the inhalation technique in the study, we replaced item 12 of the TAI-12 with an objective assessment of the inhalation technique video, as the 12th item concerns physician-observed critical inhalation technique errors. A participant was considered non-adherent when one or more of the following three criteria were met: 1. TAI-10 sum of scores for item 1–10 <50, or 2. TAI item 11 was scored by the physician as “incorrect”, or 3. when any of the following inhalation technique errors were observed (replacement of item 12): “failure to seal teeth and lips around mouthpiece”, “insufficient inspiratory effort” or “patient did not breathe out calmly after inhalation” (relevant errors based on the association with health status as described in a previous study).Citation11
Healthcare Resource Utilisation
HCRU was defined as the 1) COPD-related primary healthcare costs, 2) COPD-related secondary healthcare costs, 3) COPD-related medication costs, and 4) total COPD-related healthcare costs (sum of 1 −3), categorised in Table S3. HCRU in the previous 6 months was calculated from either the medical records or self-reported by the participant during the visit (retrieved from medical records for primary healthcare data in 34.1%; secondary healthcare data 32.4%; and the number of hospital admissions in 30.0%, the majority of HCRU data was self-reported). Although HCRU was measured in a 6-month period, HCRU results were doubled to provide annual estimates. To calculate the primary and secondary care costs from the HCRU data, we obtained the unit costs from the Dutch National Healthcare Institute “Zorginstituut Nederland”. This institute uses a list of all available healthcare costs in the Netherlands. As the last version of this list dates from 2014, costs in this study analysis were corrected for inflation with a cumulative factor of 6.5% to represent the cost year 2020 (Table S3). The average yearly COPD-related medication costs were calculated based on the daily prescription of DPIs and pMDIs at the day of the study visit, based on the lowest price marketed in the Netherlands which differed per inhaler and per dose. For pMDIs and Short-Acting Beta-Agonists, when daily use was reported as “as required”, average use for the specific inhaler in this study was assumed. Whenever a device was not available on the Dutch market, prices in the UK Dictionary of Medicines and Devices (DM+D) database were used. If these were also unavailable, prices from online pharmacies were used (Tables S3 and S4). Analyses of costs were expressed both as cost ratio and absolute cost changes in euro (€). Cost ratios (CR) were estimated by calculating the ratios of the mean costs for the considered subgroup of patients to those for the reference group.
Statistical Analyses
Generalised linear mixed-effects models were used, allowing a random intercept for clusters at the level of general practitioners (GPs), to determine the associations between sPIF, inhalation technique errors, non-adherence, and HCRU cost components. Models were created to estimate the cost ratios (CR), with 95% Confidence Intervals (CI), for the predictors of the COPD-related total, primary healthcare, secondary healthcare, and medication costs separately. As there were almost no missing HCRU data, with the exception disease severity (GOLD status) as a potential confounder, we performed complete case analyses instead of using imputed data. All candidate confounders (eg, COPD medication regimen and comorbidities, see Table S5) were added to the models one by one, sorted by bias potential (the change in the coefficient of the main predictor due to its inclusion in the model) in descending order. Whenever the bias potential was ≥5%, the candidate confounder was retained in the model. An overview of confounders included in the models can be found in Table S6.
Additionally, the associations of the number of inhalation technique errors with the HCRU components were assessed. To this end, an error count was used as a categorical predictor. Models were also created to estimate the cost ratio with the PIF subgroups “can and will do”, “can, but will not do”, “cannot do” as categorical predictors on the HCRU components, with the “can and will do” as the reference group.
Two separate sensitivity analyses were performed. First, all analyses were repeated excluding non-adherent patients, as adherence was previously associated with economic outcomes in COPD.Citation30 Second, all analyses were repeated using an adjusted definition for non-adherence: TAI-10 sum of scores for item 1–10 <50, which is regarded as the cut-off value for the TAICitation33 (in place of the pre-specified non-adherence measure comprising TAI-10, TAI-11, and three critical inhalation technique errors).
Sample Size Calculation
A sample size calculation was performed prior to study execution for the main study objectives, and not specifically for the analysis concerning HCRU.Citation31 All statistical analyses were performed using Stata version 17.
Results
Study Population
A total of 1434 patients with COPD from Australia, Greece, the Netherlands, Poland, Portugal, and Spain were included in the study (Table S5). Of these patients, 50.1% were female, and the mean (SD) age was 69.2 (9.3) years. COPD severity was available for 801 patients and classified as GOLD stage I in 23.6%, II in 54.9%, III in 17.4%, and IV in 4.1%. Both patients with an optimal and sPIF most frequently used a Long-Acting Beta Agonist/Inhaled Corticosteroid (LABA/ICS) in DPI (40%), followed by a Long-Acting Muscarinic Antagonist (LAMA; 33%) and LAMA/LABA (25%) in a DPI. An overview of all the used medications for COPD is provided in Table S7.
Of the 1389 patients with PIF data, 71% (n = 987) revealed an optimal PIF for their DPI (“can and will do”), and 29% (n = 402) had an sPIF. 16% of the patients were categorised as “can, but will not do” (n = 219), and 13% (n = 183) as “cannot do”. Patient characteristics are shown in .
Table 2 Patient Characteristics and HCRU in the 6-Months Prior to Study Visit, Stratified by Peak Inspiratory Flow Sufficiency (Optimal/Suboptimal)
An overview of all HCRU is presented in . As data for ability to work were available for 18.2% of the total population, of which 5 participants (0.3% of the total population) reported lost working hours in the 7 days prior to study visit, this indirect cost was excluded from the analyses.
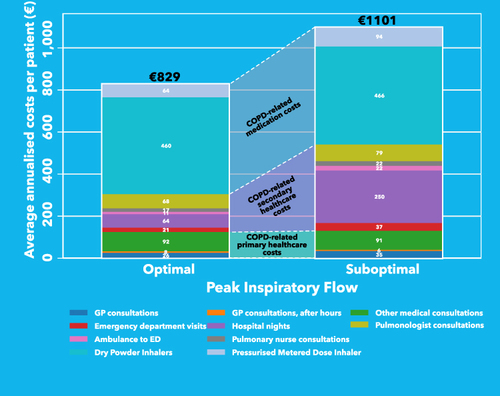
Patients with an optimal PIF had an average of €829 total COPD-related costs per year, while patients with sPIF had an average of €1101 total COPD-related costs per year. This gap of €272 was largely caused by higher costs for secondary healthcare for patients with sPIF, specifically by more costs from hospital nights (, Table S8).
Figure 1 Composition of HCRU and total COPD-related yearly healthcare costs per patient. Please note that the composition of HCRU in this figure consist of raw data and is not adjusted for potential confounders. ICU nights were not reported.
Associations of Peak Inspiratory Flow, Adherence, Inhalation Technique Errors with Healthcare Resource Utilisation
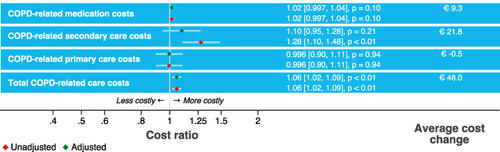
Although all HCRU costs were higher in patients with sPIF (), the adjusted regression models showed that only medication costs were associated with PIF (CR: 1.07, 95% CI [1.01, 1.14], p = 0.03). Patients with sPIF had on average 7% higher medication costs compared to those with optimal PIF, corresponding to €38 yearly higher medication costs. The difference was largely caused by higher yearly costs for pMDI prescription in patients with sPIF (on average €63.7 in patients with optimal PIF vs €94.4 in patients with sPIF). sPIF was associated with 53% higher secondary healthcare costs compared to patients with optimal PIF (CR: 1.53, 95% CI [0.86, 2.75], p = 0.15).
From the ten individual inhalation errors (), errors made in the inhalation steps “Breathe in”, “Hold breath” and “Breathe out calmly after inhalation” were associated with higher HCRU costs (). Specifically, an error in the inhalation step “Breathe in” was associated with higher secondary healthcare costs (CR: 2.20, 95% CI [1.37, 3.54], p < 0.01) and higher total COPD-related healthcare costs (CR: 1.16, 95% CI [1.03, 1.31], p = 0.01), an error in the step “Hold breath” with higher medication costs (CR: 1.08, 95% CI [1.02, 1.15], p = 0.01) and higher total healthcare costs (CR: 1.17, 95% CI [1.07, 1.28], p < 0.01), and an error in the step “Breathe out calmly after inhalation” with higher medication costs (CR: 1.19, 95% CI [1.04, 1.37], p = 0.01).
Figure 2 Associations of Peak Inspiratory Flow, adherence, inhalation technique errors, and cost ratios for HCRU.
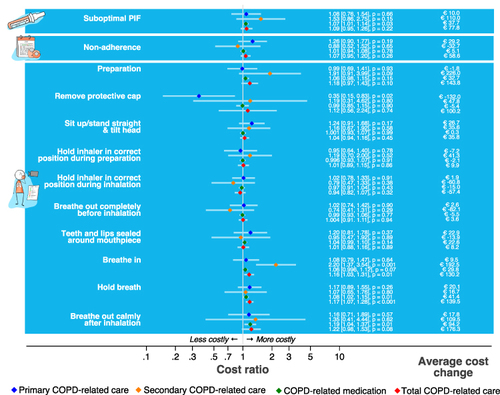
Patients who incorrectly removed the cap of the inhaler had on average lower primary healthcare costs (CR: 0.35, 95% CI [0.15, 0.83], p = 0.02), but this association disappeared when including only adherent patients in the sensitivity analysis (CR: 2.39, 95% CI [0.13, 45.61], p = 0.56).
Non-adherence was not significantly associated with any of the HCRU cost components (). The results from the sensitivity analysis, using a different definition for non-adherence (TAI-10 score <50), did not relevantly change the results.
An increase in cumulative errors in all ten inhalation steps was significantly associated with higher total COPD-related healthcare costs (CR: 1.06, 95% CI [1.02, 1.09], p < 0.01) (), meaning that each additional error was associated with a 6% increase in costs. In the adjusted models, no significant associations were observed between the number of inhalation technique errors and specific cost components ().
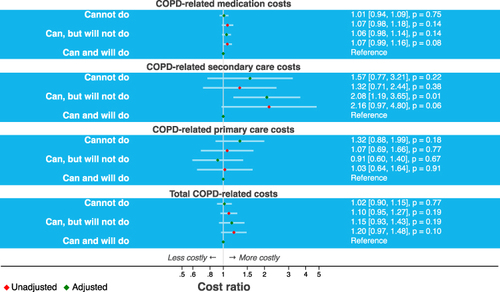
Patients from the PIF subgroup “can, but will not do” (n = 219, 16%) had significantly higher secondary healthcare costs compared to patients from the PIF subgroup “can and will do” (n = 987, 71%) (CR: 2.08, 95% CI [1.19, 3.65], p = 0.01, ).
Discussion
Main Findings
sPIF was significantly associated with higher medication costs and markers of disease severity, such as specialist care and hospital admissions. Moreover, there was a clear trend suggesting that sPIF was related to higher COPD-related healthcare costs. Consistent with the findings for PIF, errors associated with the inhalation steps, “Breathe in”, “Hold breath” and “Breathe out calmly after inhalation” were significantly associated with higher COPD-related healthcare costs. Additionally, a higher cumulative number of inhalation errors was significantly associated with higher total COPD-related healthcare costs. Non-adherence was not significantly associated with any of the healthcare components.
Interpretation and Comparison with Previous Studies
sPIF has been frequently described in other studies among COPD patients and has been implied as a key factor influencing health status and health economic outcomes.Citation11,Citation34–37 To our knowledge, we are the first to demonstrate that, while adjusting for potential confounders, sPIF is associated with higher COPD-related medication costs, as patients with sPIF were more likely to have been prescribed with pMDIs in addition to their DPI maintenance therapy. This finding further emphasizes the importance of interventions targeted towards tackling sPIF and inhalation technique improvement for those patients who are not able to produce sufficient PIF for their DPI. Future studies should investigate the magnitude of such individualised cost-saving interventions for COPD patients on DPI maintenance therapy.
The economic relevance of inhalation technique errors found in this study is in line with an Italian cohort study,Citation19 which studied the association between critical inhaler errors and yearly HCRU costs in COPD patients who switched their device in the last year (without receiving adequate training). Patients making critical inhaler errors had, on average, an 18.8% higher yearly difference (€234) in HCRU costs compared to patients without inhalation errors.Citation19 The costs were mainly driven by hospital admissions and emergency room visits. It is therefore crucial that patients receive adequate inhalation technique training, especially when healthcare professionals are considering changing the inhaler device (eg, from DPI to SMI/pMDI).Citation38
A previous systematic review reported that non-adherence to COPD medication is associated with worsened economic outcomes.Citation30 Although non-adherence in our study was high, we found no association between non-adherence and HCRU. We previously showed that non-adherence was not associated with health status (assessed with the Clinical COPD Questionnaire and the COPD Assessment Test) or exacerbation frequency,Citation11 and hereby likely does not affect HCRU and associated costs. However, the cross-sectional rather than longitudinal assessment of adherence, coupled with the reliance (in part) on patient recollection rather than actual measurements (eg, via a dose counter on the inhaler), may have contributed to the inconsistency between studies.Citation30
We observed that sPIF is likely associated with higher COPD-related healthcare costs, especially secondary healthcare and medication costs. Higher medication costs could be due to sPIF causing suboptimal drug delivery to the lungs, with subsequent undertreatment of patients, leading to more frequent inhaler use. Although patients with sPIF did not have significantly higher total COPD-related HCRU, they did tend to generate higher costs for secondary healthcare, particularly for COPD-related hospital admissions. A plausible explanation for this gap in healthcare costs between patients with optimal vs suboptimal PIF is that decreased drug delivery due to suboptimal clinically relevant PIF would lead to suboptimal disease control. Of note, access to secondary healthcare is dependent on several factors (eg, willingness of GPs to refer a patient to a specialist; the affordability of accessing secondary care; the perception of how unwell the patient actually is) that we could not take into account. These results may therefore be an underestimation of the actual association between sPIF and secondary healthcare costs. In a recent study based on the same study population, we demonstrated that sPIF is associated with poorer health status and a trend towards more severe exacerbations.Citation11 The finding of a trend towards more hospital admissions in the sPIF group in this study is in line with these earlier findings.
Interestingly, when dividing patients based on PIF subgroups; COPD-related secondary healthcare costs, medication costs, and total COPD-related costs were on average higher in the “Can, but will not do” group than in the “Cannot do” group. However, the increase in COPD-related primary healthcare costs was more pronounced in the “Cannot do” group than in the “Can, but will not do” group (). A possible explanation for these results might be that the “Can, but will not do” group is unstable in terms of PIF, meaning sometimes they do achieve optimal PIF and therefore the healthcare costs are in between the costs for the “Cannot do” group and “Can, and will do” group. The findings reveal that it is valuable to assess whether PIF is sufficient at every opportunity in clinical practice and to choose the most appropriate inhaler type for the patient based on internal device resistance.Citation12
Strengths and Limitations
This study has strengths and several limitations worth noting. The major strength of this study is the multi-country, real-world approach, providing evidence to healthcare professionals to manage COPD and reduce associated costs.
The missing disease severity data resulted in an inability to eliminate associated potential confounders, as disease severity has been shown to impact HCRU.Citation32,Citation39,Citation40 However, in patients for whom disease severity was known, there was no evidence for a negative association between disease severity and sPIF (). Additionally, we did account for the errors “Hold breath” and “Breathe out calmly after inhalation”, which could be considered indirect measures of disease severity as an incorrect exhalation (such as a cough reflex) reflects a greater degree of airflow obstruction, disabling a patient to exhale calmly after an extended breath-hold of 6 seconds or longer.
We cannot exclude residual confounding, although the analyses were adjusted for a comprehensive set of potential confounders based on the literature and clinical expertise, such as diabetes mellitus and cardiovascular- and lung comorbidities (see Table S6).
Our analysis regarding indirect costs (eg, self-reported work absence and productivity loss) was limited by a low sample of participants that were in active employment. Of over 1400 study participants, 261 were actively employed. Lost working hours in the 7 days prior to the study visit were only reported by five patients and were therefore excluded from the models considering total costs over the 1-year follow-up period. A previous study found that 40% of patients with moderate or severe COPD retired prematurely due to their symptoms and on average retired at the age of 54.Citation41 Considering that the mean age of our study population was 69.2 years, it seems understandable why the number of actively employed patients was so low. Another limitation of this study is that for the association between the number of inhalation errors and HCRU, we assumed that every inhalation error was equally important. This might not be the case in reality, where one error may be associated with a larger difference in total healthcare costs than the other, and we should thus interpret these findings with caution. Additionally, for the participating sites on the Northern Hemisphere, most visits were executed during the winter months. Participating patients were required to supply information about the past 6 months, which makes the final data not perfectly representable over a period of a year, while HCRU might be substantially different from patients during the summer months. Finally, it is worthwhile to mention the conditions in which the study was performed, ie, the COVID-19 pandemic and its accompanying restrictions. Therefore, the participating patients likely had less social contact as compared to periods before the pandemic, which might make exacerbation rates not representative.
Conclusions
This study demonstrated that sPIF and the inhalation technique errors “Breathe in”, “Hold breath”, and “Breathe out calmly after inhalation” were associated with higher COPD-related healthcare utilisation and costs. These findings underline that PIF and inhalation technique are factors to consider when initiating and evaluating DPI therapy. Not only may this aid in improving health status of patients with COPD using a DPI as maintenance therapy but also in reducing hospital admissions, medication use and costs, and ultimately reducing total COPD-related healthcare costs globally.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author J.K.
Ethics Approval
The PIFotal COPD study complies with the Declaration of Helsinki and its protocol has received approvals from the following institutional ethics committees/institutional review boards: Australia: Human Research Ethics Committee (HREC 3) University of Sydney; Greece: Research Ethics Committee University of Crete; Poland: Komisja Bioetyczna przy Beskidziej Izble Lekarskiej – Bielsko Biala; Komisji Bioetycznej przy Śląskiej Izbie Lekarskiej; Silesian Medical Society (Śląska Izba Lekarska); Bioethics Committee at Lower Silesian Medical Association; Bioethics Committee at the Medical University of Bialystok; Portugal: North Health Regional Administration (ARS Norte); Matosinhos Local Health Unit (ULS Matosinhos); Guimarães Hospital; Center Health Regional Administration (ARS Centro); Regional Health Administration of Lisbon and Tagus Valley (ARS LVT); Spain: Comité de Ética de la Investigación (CEI) Islas Baleares; CEI Hospital Universitario de Gran Canaria; The Netherlands: Medisch Ethische Toetsingscommissie (METC) Assen exempted this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
ML, JvC, LD, IvdH, YJ, BM, KS, NS, were employed by the General Practitioners Research Institute (GPRI) at the time of the study. In the past three years (2019–2021), GPRI conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Teva).
JvB has received grants and/or consultancy fees from Aardex, AstraZeneca, Chiesi, European Commission (COST Action CA19132 “ENABLE,” supported by the COST [European Cooperation in Science and Technology]), GSK, Lung Alliance Netherlands, Novartis, Nutricia, Pfizer, Teva and Trudell Medical, all unrelated to this study and paid to his employer (University Medical Center Groningen).
SB-A has received grants from TEVA, and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Sanofi and Mylan.
JCdS reports or personal fees from AstraZeneca, Bial, Boehringer Ingelheim, GSK, Medinfar, Mundipharma and Sanofi.
BC received an honorarium from GSK and Sanofi.
RD has received grants and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Chiesi, Focus Care, and Glenmark.
RG has received personal fees from AstraZeneca, GSK and Chiesi.
MGP receives grants from AstraZeneca, GSK and Boehringer Ingelheim.
AG and LH-G are employees of Boehringer Ingelheim.
FL received grants and personal fees from GSK, personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini International, Novartis, Orion, and Trudell International, outside the submitted work.
TM has no competing interests to declare.
JM received grants from Boehringer Ingelheim, during the conduct of the study, and grants from AstraZeneca, Chiesi, Novartis, and GSK, outside the submitted work.
DP reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Theravance and Zentiva (Sanofi Generics); grants from the British Lung Foundation, Respiratory Effectiveness Group, UK National Health Service, and AKL Research and Development Ltd; personal fees from Cipla, GlaxoSmithKline, Kyorin, Merck, Mundipharma, Airway Vista Secretariat, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, and WebMD Global LLC; non-financial support from Efficacy and Mechanism Evaluation programme and Health Technology Assessment; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and 5% shareholding in Timestamp, which develops adherence monitoring technology.
MR-R receives grants and personal fees from AstraZeneca and GSK; and personal fees from Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Pfizer, TEVA and BIAL.
IT reports grants and personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Chiesi and Elpen.
OU reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Edmond Pharma, Chiesi and GSK; grants from Edmond Pharma; and personal fees from Napp, Mundipharma, Sandoz, Takeda, Cipla, COVIS, Novartis, Mereobiopharma, Orion, and Menarini.
JK reports grants, personal fees and non-financial support from AstraZeneca, GSK and Boehringer Ingelheim; grants and personal fees from Chiesi Pharmaceuticals and TEVA; grants from Mundipharma; personal fees from MSD and COVIS Pharma; and also holds 72.5% of shares in the General Practitioners Research Institute.
The authors report no other conflicts of interest in this work.
Acknowledgments
The members of the PIFotal study group would like to acknowledge Dr. Judith Cohen and Dr. Wilma Zijlema for their assistance with the review, drafting, and editing of the paper, and Dr. Hans Wouters for his contribution to the project administration in the initial phase of the project.
They would also like to thank all contributing researchers: Maria João Barbosa, Ana Margarida Cruz, Liliana Silva, Duarte Araújo, Eurico Silva, Daniel Castro, João Ramires, Ana Fernandes, Catarina Carvalho, Raquel Castro, Jerzy Zientek, Ewa Pasko, Witold Drzastwa, Tomasz Kachel, Kornelia Ciekalska, Krzysztof Wytrychowski, Bernard Panaszek, Krzysztof Kowal, Ebian Brill, Willemien Feenstra, Geert Struik, Hans Schuurman, Mariette van Oostrum, Hennie Holwerda Meekma, Boudewijn Dierick, George Amofa, Esther Kuipers, Lennard Ringnalda, Boris Tyndall, Mark Drenth, Peter Mast, Hilbert Talsma, Raoul Wolfs, Cobie Hoogeboom, Hanneke van Andel, Paul Stoutenberg, Nancy van de Laak, Tessa Hillaert, Laura Holtzer, Natascha Fehrmann, Anniek Makkinga – Maassen van den Brink, Annemarie Hilbink, Erik Feenstra, Murat Tek, Sabrina Burer, Jan van Ginkel, Rinze Boersma, Alyssa Bonger, Miguel Roman Rodriguez, Marina García Pardo, Alejandra Valero Suau, Laura López Velasco, Cecilia Amato, Francisco Palmer Simó, Alberto Abenza, Rosa Llull Vila, Bartolomé Llompart Van Belzen, Silvia Jimeno Martínez, Francesc Moranta Ribas, Margarita Perelló Oliver, Yolanda Gómez López, Patricia Ibañez Gómez, María Nieves Mendieta Lagos, Laura Bueno López, Virginia María Mirabal Sánchez, Ana Delia Rodríguez Delgado, Nils Fischer, Alicia González Sansó, Nayra Ramírez Mendoza, Valeria Gazzaneo, Paula Merced Guillama Rodríguez, Virginia Naranjo Guerrero, Jose Angel Suarez Caballero, Isidoro Souto Bethencourt, Juan R. Dominguez Beatell, Elena Vanesa Rojas Manrique, Maria Jose Sanz Orejas, Cary Perez Lorenzo, Jesús Antonio Pérez Jiménez, Silvia Lara Afonso Trujillo, Bartolomé Dominguez Del Río Boada, Stavroula Papageorgakopoulou, Eleytheria Vakouti, Claire Gkatzoudi, Thodoris Krasanakis, Dimitris Kounalakis, Izoldi Bouloukaki, Nikolaos Tsakountakis, Emmanouela Chronaki, Katherine Mary Borg and Kamila Abutalieva for their time and efforts to perform the study measurements and complete patient inclusion, even in the challenging times of the pandemic.
Finally, they would like to thank the participants who generously gave their time to participate in the study.
Additional information
Funding
References
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412. doi:10.1183/09031936.06.00025805
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: www.goldcopd.org. Accessed September 13, 2022.
- Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J COPD. 2020;15:439–460. doi:10.2147/COPD.S234942
- Bosnic-Anticevich SZ. Inhaler device handling: have we really started to address the problem? Eur Respir J. 2017;49(2):1700120. doi:10.1183/13993003.00120-2017
- Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi:10.1016/J.AMJMED.2008.09.042
- Ghosh S, Pleasants RA, Ohar JA, Donohue JF, Drummond MB. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J COPD. 2019;14:585–595. doi:10.2147/COPD.S195438
- van der Palen J. Peak inspiratory flow through Diskus and Turbuhaler, measured by means of a peak inspiratory flow meter (In-Check DIAL ®). Respir Med. 2003;97(3):285–289. doi:10.1053/rmed.2003.1289
- Clark AR, Weers JG, Dhand R. The confusing world of dry powder inhalers: it is all about inspiratory pressures, not inspiratory flow rates. J Aerosol Med Pulm Drug Deliv. 2020;33(1):1–11. doi:10.1089/jamp.2019.1556
- Virchow JC, Weuthen T, Harmer QJ, Jones S. Identifying the features of an easy-to-use and intuitive dry powder inhaler for asthma and chronic obstructive pulmonary disease therapy: results from a 28-day device handling study, and an airflow resistance study. Expert Opin Drug Deliv. 2014;11(12):1849–1857. doi:10.1517/17425247.2014.949236
- Newhouse MT, Nantel NP, Chambers CB, Pratt B, Parry-Billings M. Clickhaler (a novel dry powder inhaler) provides similar bronchodilation to pressurized metered-dose inhaler, even at low flow rates. Chest. 1999;115(4):952–956. doi:10.1378/chest.115.4.952
- Kocks WH, Wouters J, Bosnic-Anticevich H, et al. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. Npj Prim Care Respir Med. 2022;32(1):18. doi:10.1038/s41533-022-00282-y
- Leving MT, Kocks J, Bosnic-Anticevich S, Dekhuijzen R, Usmani OS. Relationship between peak inspiratory flow and patient and disease characteristics in individuals with COPD:A systematic scoping review. Biomedicines. 2022;10(2):458. doi:10.3390/BIOMEDICINES10020458
- Klijn SL, Hiligsmann M, Evers SMAA, et al. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. Npj Prim Care Resp Med. 2017;27(1). doi:10.1038/s41533-017-0022-1
- Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19(1):1–20. doi:10.1186/S12931-017-0710-Y/TABLES/2
- Torvinen S, Nicolai J, Pulimeno S, et al. The budget impact of Duoresp® Spiromax® compared with commonly prescribed dry powder inhalers for the management of asthma and chronic obstructive pulmonary disease in Italy: estimated impact of inhalation technique. Value Health. 2015;18(7):A496. doi:10.1016/j.jval.2015.09.1391
- Lewis A, Blackney M, Torvinen S, et al. The budget impact of Duoresp® Spiromax® (budesonide + formoterol fumarate dihydrate) compared with Symbicort® Turbohaler® for the management of asthma and chronic obstructive pulmonary disease in the United Kingdom: impact on health care costs and inhalation technique. Value Health. 2014;17(7). doi:10.1016/j.jval.2014.08.2027
- Lewis A, Blackney M, Torvinen S, et al. The potential societal cost benefits of improved inhalation technique with Duoresp® Spiromax® (budesonide + formoterol fumarate dihydrate) compared with Symbicort® Turbuhaler® for the management of asthma and chronic obstructive pulmonary disease in Sweden. Value Health. 2014;17(7). doi:10.1016/j.jval.2014.08.2070
- Lewis A, Blackney M, Garcia Bujalance L, Sánchez-de la Rosa R, Torvinen S, Plich A. The potential for improved inhalation technique with Duoresp® Spiromax® (Budesonide + formoterol fumarate dihydrate) compared with commonly prescribed dry powder inhalers for the management of asthma and chronic obstructive pulmonary disease in Spain: estimated impact on number and cost of unscheduled healthcare events. Value Health. 2015;18(7). doi:10.1016/j.jval.2015.09.638
- Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11(1):597. doi:10.2147/COPD.S103335
- Bijos P, Kaczynski J, Torvinen S, et al. Clinical and economic burden of asthma and chronic obstructive pulmonary disease (COPD) in Poland: estimated impact of poor inhalation technique with inhaled corticosteroid and long-acting beta agonist fixed-dose combinations. Value Health. 2015;18(7):A499. doi:10.1016/J.JVAL.2015.09.1408
- Lewis A, Blackney M, Torvinen S, Plich A. Clinical and economic burden of asthma and chronic obstructive pulmonary disease in the UK: impact of critical inhaler errors with inhaled corticosteroid + long-acting beta agonist fixed-dose combinations. Value Health. 2014;17(3):A174. doi:10.1016/J.JVAL.2014.03.1016
- Cramer JA, Bradley-Kennedy C, Scalera A, Cramer J, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14(1):25–29. doi:10.1155/2007/161652
- Price D, Keininger DL, Viswanad B, Gasser M, Walda S, Gutzwiller FS. Factors associated with appropriate inhaler use in patients with COPD – lessons from the REAL survey. Int J Chron Obstruct Pulmon Dis. 2018;13:695. doi:10.2147/COPD.S149404
- Ramsey SD. Suboptimal medical therapy in COPD: exploring the causes and consequences. Chest. 2000;117(2):33S–37S. doi:10.1378/CHEST.117.2_SUPPL.33S
- Rand CS. Patient adherence with COPD therapy. Eur Respir Rev. 2005;14(96):97–101. doi:10.1183/09059180.05.00009604
- Blackstock FC, Wallack RZ, Nici L, Lareau SC. Why don’t our patients with chronic obstructive pulmonary disease listen to us? The enigma of nonadherence. Ann Am Thorac Soc. 2016;13(3):317–323. doi:10.1513/ANNALSATS.201509-600PS/SUPPL_FILE/DISCLOSURES.PDF
- Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343. doi:10.1164/rccm.201604-0733OC
- Toy EL, Beaulieu NU, Mchale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi:10.1016/j.rmed.2010.09.006
- Simoni-Wastila L, Wei YJ, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all-cause hospitalization and spending in a medicare population. Am J Geriatr Pharmacother. 2012;10(3):201–210. doi:10.1016/J.AMJOPHARM.2012.04.002
- van Boven JFM, Chavannes NH, van der Molen T, Rutten-Van Mölken MPMH, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103–113. doi:10.1016/J.RMED.2013.08.044
- Leving M, Wouters H, de la Hoz A, et al. Impact of PIF, inhalation technique and medication adherence on health status and exacerbations in COPD: protocol of a real-world observational study (PIFotal COPD Study). Pulm Ther. 2021;7(2):591–606. doi:10.1007/s41030-021-00172-7
- Sanders MJ. Guiding inspiratory flow: development of the in-check DIAL G16, a tool for improving inhaler technique. Pulm Med. 2017;2017:1–7. doi:10.1155/2017/1495867
- Plaza V, Fernández-Rodríguez C, Melero C, et al. Validation of the “Test of the Adherence to Inhalers” (TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi:10.1089/JAMP.2015.1212/ASSET/IMAGES/LARGE/FIGURE3.JPEG
- Duarte AG, Tung L, Zhang W, Hsu ES, Kuo YF, Sharma G. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. COPD. 2019;6(3):246. doi:10.15326/JCOPDF.6.3.2018.0163
- Kawamatawong T, Khiawwan S, Pornsuriyasak P. Peak inspiratory flow rate measurement by using In-Check DIAL for the different inhaler devices in elderly with obstructive airway diseases. J Asthma Allergy. 2017;10:17. doi:10.2147/JAA.S127580
- Al-Showair RAM, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395–2401. doi:10.1016/J.RMED.2007.06.008
- Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
- Peché R, Attar‐zadeh D, Scullion J, Kocks J. Matching the inhaler to the patient in COPD. J Clin Med. 2021;10(23):5683. doi:10.3390/JCM10235683
- Harb HS, Laz NI, Rabea H, Abdelrahim MEA. Prevalence and predictors of suboptimal peak inspiratory flow rate in COPD patients. Eur J Pharmaceut Sci. 2020;147:105298. doi:10.1016/J.EJPS.2020.105298
- Wallace AE, Shinde MU, Willey VJ, et al. Health care resource utilization and exacerbation rates in patients with COPD Stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25(2):205–217. doi:10.18553/JMCP.2019.25.2.205
- Fletcher MJ, Upton J, Taylor-Fishwick J, et al. COPD uncovered: an international survey on the impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC Public Health. 2011;11(1):1–13. doi:10.1186/1471-2458-11-612