Abstract
The definition of exacerbation of COPD as a syndrome, as proposed by the Spanish COPD guidelines (GesEPOC) 2021 update, and the consequences that this implies, have direct implications on patient care. This review analyzes this novel vision of the COPD exacerbation syndrome, its rationale, and its clinical implications, as opposed to the traditional symptoms-based or event-based definitions. An exacerbation conceived as a syndrome provides us with an umbrella term to include a set of diverse alterations, which, either in isolation or more frequently in combination, are clinically expressed in a similar way in patients with COPD. In patients with COPD, this occurs as a consequence of worsening expiratory airflow limitation or the underlying inflammatory process, producing a worsening in symptoms with respect to the baseline situation. This definition therefore assumes a worsening in at least one of the two key physiopathological markers, lung function and inflammation. The main features of this new physiopathological proposal include a syndromic approach with narrower differential diagnosis, the use of several biomarkers, treatable traits to better guide treatment, and a new severity classification. Further research is needed to examine the role of eosinophils in this context, but currently, the early results are promising. The evaluation of severity is key in the multidimensional characterization of exacerbation and the GesEPOC 2021 proposes new approaches and also recommends the use of multidisciplinary scores for severity categorization in patients. Finally, another innovation in the GesEPOC 2021 refers to the recurrence of exacerbations, which has implications for disease prognosis or long-term clinical impact which need to be elucidated in further studies.
Keywords:
Introduction
Our understanding of chronic obstructive pulmonary disease (COPD) has advanced apace in recent decades, and the options for a more comprehensive and personalized approach to the disease have led to a paradigm shift that is continually changing.Citation1 Beyond the recent innovations in pharmacological treatments,Citation2,Citation3 various aspects of non-pharmacological treatments, including exercise programs,Citation4 vaccinations, surgical techniques,Citation5 home therapiesCitation6 or the technologies of information and communication,Citation7 have allowed us to provide patients with more satisfactory, multidimensional care.Citation8 Consequently, the recommendations on the management of the disease have been recently updated with these new recommendations in the light of the available evidence.Citation9,Citation10
Recently, the Spanish National Guideline for COPD management (GesEPOC) released the latest 2021 version, in 4 different documents covering the treatment of stable COPD, exacerbations, comorbidities and palliative care, and non-pharmacological interventions.Citation10–13 Altogether, these documents reflect the current challenges in the management of the disease and the possible solutions provided by the group of Spanish experts following evidence-based medical principles. In particular, many proposals are considerably innovative, including the risk stratification of patients, clinical phenotyping for selection of treatment, the concept of disease control in the escalation of treatment, the selection of treatable traits for advanced, complex patients, a new paradigm for the identification and classification of exacerbations, the organization of different comorbidities, the evidence for palliative care and the implementation of non-pharmacological approaches.
One of the key aspects which may be seen as innovative for the scientific community is the GesEPOC 2021’s approach to the concept, categorization, and management of exacerbations. The definition of exacerbation as a syndrome, and the consequences that this involves, have direct implications on the patient that are worth explaining in more detail. In the following lines, we intend to explain this new vision of COPD exacerbation, its rationale, and its clinical implications, in order to help the clinician to identify, manage and prevent these adverse events in real clinical practice.
Current Approaches to a Definition
Within the clinical expression of COPD, its chronic and often progressive clinical course is frequently altered by an acute worsening of the symptoms, usually accompanied by an increase in inflammatory mediators and a functional worsening known as exacerbations. Although clinicians are usually clear about what an exacerbation is, the operational definitions given so far have not yet received unanimous agreement from the scientific community.Citation14–16 The importance of clearly identifying an exacerbation lies not only in its short- and long-term clinical impact: an exacerbation is also in many cases the initial reason why a patient turns to the health services, which allows the case to be diagnosed.Citation17 Consequently, in recent years, different definitions of exacerbation have been proposed (). These definitions are not entirely compatible, contain subtle nuances and have been subject to considerable controversy.
Table 1 Definitions of Exacerbation According to Different Recommendation Documents
Symptoms-Based Vs Event-Based Definitions
According to the 2022 Global Obstructive Lung Disease Initiative (GOLD) document, a COPD exacerbation is defined as an acute worsening of respiratory symptoms that requires additional treatment.Citation18 This definition, mainly based on symptoms and the therapy used to relieve them, is extremely simple and non-specific, but has remained unchanged over the last quarter of a century. In a position paper published in 1995, the American Thoracic Society recognized that an exacerbation was difficult to define, and its pathogenesis was very poorly understood.Citation19 At that time, the document already highlighted the need for a standardized definition that would be universally accepted and useful for clinicians, researchers, and other health providers. Five years later, responding to that challenge, a group of experts defined an exacerbation as a sustained worsening of the clinical condition of the patient with COPD, going beyond the variations of symptoms, and which is acute in its onset and necessitates a change in the habitual medication.Citation20 Since that initial proposal, more than two decades have passed and our knowledge of COPD has substantially improved. It is now understood as a complex and heterogeneous syndrome that requires an increasingly personalized approach.Citation21 Nevertheless, the concept, diagnosis and management of acute exacerbations have seen relatively few changes.
Despite being simple and easy to adopt, the GOLD proposal for a symptom-based definition of exacerbation has several limitations. First, it is highly non-specific, which can lead to it being confused with other diseases it must be differentiated from, such as pneumonia, heart failure, acute ischemic heart disease, arrhythmias, pulmonary embolism, or anxiety, which are some of the other concomitant diseases that may produce similar clinical expression. Of note, differential diagnosis is not easy and frequent overlapping occurs. For example, about one of five patients admitted to hospital with a diagnosis of exacerbation show biomarkers of ventricular dysfunction or ischemic damage.Citation22,Citation23 Pulmonary embolism has been detected in 16% of exacerbations of unknown etiologyCitation24 and up to 27% of patients have shown infiltrates on computed tomography, which are not detected by a chest X-ray.Citation25 Computed tomography is not a routine examination, so many of these infiltrates (in many cases, caused by pneumonia) could be misdiagnosed as COPD exacerbations. Second, the underlying biological or physiopathology mechanism is not taken into account in the GOLD definition, even though inflammation (pulmonary or systemic) and functional changes, such as a worsening of expiratory airflow limitation, air trapping and hyperinflation, seem to be key symptoms.Citation26 Third, it does not recognize the heterogeneity and complexity of the exacerbation, although some different phenotypes or endotypes have been described.Citation27,Citation28 Fourth, although the GOLD definition includes the need for “additional treatment”, some patients suffer from worsening of respiratory symptoms at home without contact with their doctors, or using different medications.Citation29 Despite this, many clinical trials define exacerbations and their severity based on the treatment used (event-based definition).Citation30,Citation31 Finally, the treatment has not changed in decades, probably because this non-specific definition has led to inconsistent research. Bronchodilators, systemic steroids, or antibiotics are prescribed to nearly all patients, regardless of their interindividual differences.
On the other hand, certain initiatives such as the EXAcerbations of Chronic Pulmonary Disease Tool (EXACT) have attempted to standardize the method of studying these events through the development of electronic diaries (again, using a symptoms-based definition).Citation32,Citation33 These types of instruments have been specially developed for use in clinical research, and their transfer to clinical practice entails certain difficulties. Using these diaries, it has been possible to evaluate the clinical course of an exacerbation, observing that about half of the patients have an abrupt onset in a few hours, while the other half show a more insidious onset over a period of a few days or weeks.Citation33 Some intrinsic concepts of exacerbations have also been qualified with this methodology, by differentiating the intensity of symptoms, the duration, and the frequency of the events. Among these, the frequency of exacerbations is the one that has received the most attention. On average, COPD patients experience 1–4 exacerbations per year. However, their distribution is highly variable. While some people do not experience these episodes at all, others experience them repeatedly (termed in the GesEPOC as the exacerbator phenotype).Citation34 In addition, many of these episodes occur in clusters,Citation35 raising the question of whether each one constitutes a new exacerbation or they are simply incomplete resolutions of one single episode, which adds to the confusion.
All in all, using symptoms-based definitions or event-based definitions, scientific progress over the last few decades has been heterogeneous and inconsistent, and has led to unsuccessful clinical outcomes. Today, despite using treatment recommended by current guidelines, there is still a high proportion of patients showing recurrent exacerbations,Citation36 therapeutic failures are observed in one of four patients, the readmission rate at 30 days is 20%, and in-hospital mortality is about 5%, increasing to 11% at 3 months.Citation37–41 Therefore, we urgently need a new strategy for COPD exacerbations, which should start by redefining the existing definition.
The Rome Definition
To overcome the limitations related to this symptoms-based definition, an international group of experts has recently published a new consensus proposal, the so-called Rome definition.Citation40 According to this initiative, an exacerbation in a patient with COPD is defined as
an event characterized by dyspnea and/or cough and sputum that has worsened within the last 14 days, which can be accompanied by tachypnea and/or tachycardia, and is often associated with increased local and systemic inflammation caused by airway infection, pollution, or other airway insults.
The main novelties of this proposal are: (1) a time limit (less than 14 days) has been established; (2) the underlying pathophysiological process has been included, considering tachypnea and tachycardia as important clinical biomarkers; and (3) the main triggering factors are also indicated. The time limit is of interest, since, according to some studies, the time from onset of worsening respiratory symptoms to full exacerbation expression ranged from 0–5 days in 90% of patients, with an overall range of 0–14 days.Citation33
In our opinion, the Rome proposal represents an important step forward in the challenging task of establishing a more precise definition for exacerbation. However, there are still several unresolved issues. First and probably foremost, this is not an operational definition: in other words, it does not establish specific criteria for diagnosis, with the exception of the time limit. The authors have shown that an exacerbation “may be” accompanied by tachypnea and/or tachycardia, as differential pathophysiological elements. However, both clinical signs can appear in many other acute respiratory and non-respiratory diseases, and are therefore non-specific. There are no precise thresholds to distinguish the exacerbation through respiratory or heart rates. The panelists also proposed cutoff points to assess severity classification, but not for the definition itself. Second, the Rome proposal also noted that exacerbations are “often associated” with increased local and systemic inflammation.Citation40 However, some studies have described ‘pauci-inflammatory’ exacerbations in a non-negligible proportion of patients ranging between 14–40%.Citation27,Citation28 This scenario leaves us with uncertainties about whether these non-inflammatory episodes fall outside the Rome definition. Finally, the definition includes precipitating factors as airway infection, pollution or “other insults”, the latter being a very open, non-specific term.
Like the GOLD definition, the Rome proposal does not recognize overlapping with other concomitant diseases which also worsen respiratory symptoms. These other diseases are not considered as exacerbations, and a broad differential diagnosis has been proposed.Citation11 Nonetheless, the panelists acknowledge that on many occasions these other diseases can coincide with the exacerbation and influence the evolution of each one (38).
The Rationale Behind the COPD Exacerbation Syndrome
Probably one of the most relevant conceptual changes in exacerbations is the new definition in the GesEPOC 2021,Citation11 which uses the term COPD exacerbation syndrome (CES). The Spanish guideline defines the CES as an “episode of clinical instability that occurs in a patient with COPD as a consequence of worsening expiratory airflow limitation or the underlying inflammatory process and is characterized by an acute worsening of respiratory symptoms with respect to the patient’s baseline situation”. The GesEPOC recognizes that, from the pathophysiological point of view, an exacerbation is a complex, heterogeneous event that includes a set of diverse alterations, which either in isolation or more frequently in combination, are clinically expressed in a similar way in patients with COPD. Additionally, although not included in the definition, the GesEPOC concept also proposes we consider potential treatable traits in each patient in order to better guide their treatment. Altogether, the new physiopathological proposal, a syndromic approach with a narrower differential diagnosis, and the use of several biomarkers, treatable traits and a new severity classification are some of the major innovations proposed by the GesEPOC 2021.Citation11
Physiopathology
As in the Rome proposal, the underlying physiopathology of exacerbation is also included in the GesEPOC definition, although two key elements are highlighted here: (1) the increase in inflammation (airway or systemic) and/or (2) the worsening of expiratory flow limitation.Citation11 This detail has important consequences for the diagnosis, since to confirm the presence of a CES, one or two key pathophysiological mechanisms must first be documented, as some patients may suffer an exacerbation without a clear increase in inflammation.Citation27,Citation28 In these cases, the worsening of symptoms may be justified by changes in lung mechanics, including the narrowing of the airway, air trapping or hyperinflation.Citation26,Citation41 On other occasions, increased inflammation can be observed without a worsening in expiratory airflow limitation. For this reason, the GesEPOC definition requires that at least one of these two key mechanisms occur. In our opinion, this conceptual change will make it possible to better define what is or what is not an exacerbation, thus increasing the specificity of the diagnosis.
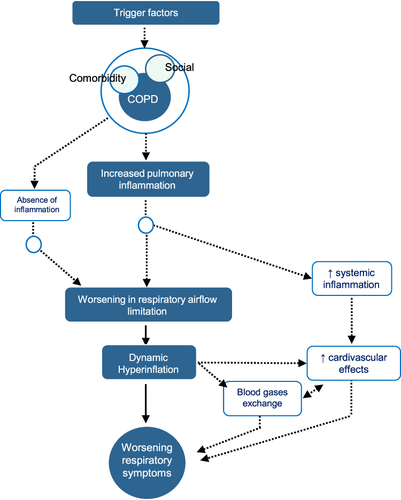
Although inflammation and airflow limitation are key, the new definition does not exclude the co-participation of other pathogenic mechanisms under a syndromic concept. Systemic inflammation has been associated with muscular, nutritional, or cardiovascular manifestations that may also be present in these patients, which condition the final clinical expression.Citation42 Dynamic hyperinflation has also been associated with cardiovascular repercussions. In summary, shows the main pathophysiological mechanisms involved. However, we must bear in mind that, although it is conceptually attractive to have this dual inflammatory and functional vision, in some care settings it may not always be possible to have access the necessary on-site diagnostic tests in order to confirm a greater or lesser degree of involvement of each of these components. This confirms the importance of the differential diagnosis and the evaluation of overlapping comorbidities in this clinical scenario. In addition, thanks to the new syndromic approach, the differential diagnosis is substantially reduced, and the overlapping disorders included under the umbrella of CES have increased. In the absence of both inflammation and expiratory flow limitation, other alternative diagnoses should be evaluated.
Figure 1 Pathophysiology of COPD exacerbation syndrome. Reproduced with permission from Soler-Cataluna JJ, Pinera P, Trigueros JA, et al. Spanish COPD guidelines (GesEPOC) 2021 update diagnosis and treatment of COPD exacerbation syndrome. Arch Bronconeumol. 2022;58(2):159–170.Citation11 Copyright © 2021 SEPAR. Published by Elsevier España, S.L.U. All rights reserved.
Consequences of the New Concept
An approach such as that proposed by the GesEPOC, which implies a conceptual and paradigmatic change in the approach to exacerbations, entails a series of consequences that can directly influence the management of these acute processes. Several other concomitant diseases (pneumonia, heart failure, pulmonary embolism, etc.), which have been considered ‘alternative diagnoses’ in both the GOLD and Rome definitions, can now be included under the umbrella of CES, which is more inclusive than previous category and allows for overlaps between concurrent diseases. One direct consequence of this huge umbrella term is that pneumonia is now considered within CES. Approximately 20% of patients with exacerbation present parenchymal infiltrates on chest X-raysCitation43,Citation44 and slightly over a third of severe cases also present them on computed tomography, despite them not being visible on simple chest x-ray.Citation25 Traditionally, these infiltrates, labeled as pneumonia, have been considered a comorbidity in COPD. However, there is a fine line between COPD exacerbation and pneumonia. The symptoms are practically identical, and the underlying mechanisms are similar, so from a syndromic point of view, both situations fall under CES. In addition, the lung microbiome between COPD patients with or without pulmonary infiltrate does not differ, the triggering factors are similar and so is the treatment.Citation45,Citation46 However, the main difference is the fact that, in the presence of pneumonia, the inflammation is greater and the prognosis worse,Citation44,Citation47 which suggests that we may possibly be facing different expressions of the same pathological process. For this reason, pneumonia, classified in previous editions of the GesEPOC as a comorbidity,Citation48 is currently considered a type of CES. Another consequence of this concept is the better identification and characterization of exacerbations, by including symptoms, lung function and inflammation, the three main pillars of this acute process.
A few years ago, it was proposed that the evaluation of exacerbations should necessarily involve a multidimensional evaluation in which various complementary aspects are taken into consideration. One all these proposals was to evaluate exacerbations on at least two axes,Citation49 with one axis showing the nature (type) of these exacerbations. Today, we know that not all exacerbations are the same, neither endotypically nor phenotypically. It is therefore necessary to identify features that differentiate some exacerbations from others and that make their management different, which have come to be known as treatable traits. On the other axis, the intensity of the exacerbation, measured as its clinical severity, continues to be a mainstay in the management of these acute processes and a challenge for the clinician. Consequently, severity and treatable features are two crucial aspects that are contemplated in the GesEPOC 2021 proposal.
Treatable Traits
The new GesEPOC 2021 proposal also recognizes the heterogeneity and complexity of exacerbations, not only in their pathophysiological mechanisms, but also in their biology (endotypes) or final clinical expression (phenotypes). This opens up the possibility of using different treatments for different patients, ie, providing personalized medical treatment. The GesEPOC proposes identifying different treatable traits, defined as the clinical, physiological, or biological characteristics present in each individual patient, which can be identified by diagnostic tests or biomarkers, each of which has a specific treatment.Citation8,Citation11,Citation50 Studies have identified different treatable traits that can be grouped into different categories ().
Table 2 Treatable Trait Domains to Consider in a Patient with COPD Exacerbation Syndrome
Among the treatable features listed in , eosinophils probably deserve a comment. In recent years, the relationship between peripheral blood eosinophil and stable COPD has been strengthened by several observational studies and post-hoc analyses of clinical trials.Citation51 Leaving aside the debate about its role in stable COPD,Citation52–56 these findings have led to two complementary debates. Firstly, whether peripheral blood eosinophil could be a marker for the choice of treatment during exacerbation. According to the systematic review performed by the GesEPOC 2021, the use of oral corticosteroids is recommended in patients with severe or very severe CES and their use is suggested for moderate CES. The efficacy of systemic steroids is greater in patients with eosinophil counts ≥ 300 cells/µL.Citation11 Accordingly, eosinophils are associated with readmissions after medium-term exacerbation.Citation57 The second debate deals with the relationship between peripheral blood eosinophils during the stable state of the disease and during exacerbations. Some studies have found a relationship between eosinophils in stable and exacerbated COPD.Citation27,Citation58 However, the debate is even more interesting since even if we assume that stable eosinophilic COPD is prone to having eosinophilic exacerbations, an eosinophilic COPD could also suffer a bacterial infection, therefore leading to a neutrophilic type of exacerbation. There are therefore a number of biological mechanisms that need to be unraveled to deepen our understanding of the different relationships of COPD blood eosinophils in its stable state and during exacerbations.
Evaluation of Severity
Once the diagnosis of CES has been established, it is crucial to describe the severity of the episode, which in most cases is the result of the interaction between the underlying disease and the intensity of the acute episode. Traditionally, this assessment of severity has been based on use of the resources or treatment received by the patient: mild, if no specific additional treatment is prescribed; moderate, if oral steroids and/or antibiotics are used; or severe, if the patient is hospitalized.Citation18 However, this approach does not help the attending physician to determine the best treatment and prognosis at the point-of-care. The GesEPOC proposes a new approach which considers baseline status (low or high risk stratification) and several clinical or biological biomarkers ().
Table 3 Severity Classification of COPD Exacerbation, According to GesEPOC Proposal (11)
For severe exacerbations, various prognostic indices have emerged for the evaluation of exacerbations in recent years. The availability of predictive risk scales can be of great interest for stratifying patients and designing care circuits of different intensity and/or complexity.Citation59 Although the GesEPOC 2021 suggests the use of the DECAF index to complete severity assessment in severe exacerbations,Citation60 there are many others currently validated that show an excellent relationship with the prognosis of exacerbation and are easy to apply in emergency departments, such as BAP-65,Citation61 DeCOPDCitation62 or the index by Roche et al,Citation63 among others.Citation64,Citation65
In GesEPOC we aimed to propose a more simple and clinically applicable way of identifying severe cases of COPD exacerbation, beyond the simple verification of acute respiratory failure, but, at the same time, using a limited number of variables that make its application viable in real clinical practice. These different degrees of severity clearly identify different locations from which to deal with these clinical situations. On the other hand, the type of treatment should be more related to the type of exacerbation () than to its severity. Although we have indications of the different impact of the different therapeutic measures in the different types of exacerbations, clinical trials will really be needed to more precisely define the impact of these options in the treatment of exacerbations.
Recurrence of Exacerbations
During the clinical presentation of COPD, it is common to find patients whose exacerbations accumulate in clusters. In this context, it has been noted that the appearance of one exacerbation leads to future exacerbations, which increase in frequency and time proximity.Citation66 For this reason, the GesEPOC 2021 differentiates conceptually between therapeutic failure (a worsening of the symptoms that occurs during an exacerbation and that requires a change in therapeutic strategy), relapse (a new worsening of the symptoms after treatment has ended and less than 4 weeks after the previous episode) and recurrence (when symptoms reappear during the year following an exacerbation). The pathophysiological mechanism behind this perpetuating inflammatory influx,Citation67 the response to different treatment optionsCitation68 and their implications on disease prognosis or clinical impact in the long-termCitation38,Citation69 are yet to be fully elucidated and will become one of the key objectives for research in COPD in the coming years.
Conclusions
Despite the advances made in recent decades, the concept of what is an exacerbation continues to be an ongoing source of debate. The GesEPOC 2021 has sought to provide an updated assessment based on the methodology of evidence-based medicine, bringing to the table an innovative proposal that forces us to think outside the box and accept a definition and a conceptual and practical approach which is closer to the patient and the variability of the disease. Strategies such as the concept of controlCitation41,Citation70,Citation71 or the stratified assessment of these clinical situations will improve clinical decision-making even in complex scenarios. On the other hand, the incorporation of information and communication technologiesCitation72 applied to respiratory medicine will also help us to achieve a more personalized management of the disease.Citation73
Disclosure
JJSC has received research grants from GlaxoSmithKline, speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, FAES, Menarini and Novartis, and consulting fees from Bial, Chiesi, GlaxoSmithKline and Novartis JLLC has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications from (alphabetical order): AstraZeneca, Bial, Boehringer, Chiesi, CSL Behring, Faes, Ferrer, Gebro, Grifols, GSK, Megalabs, Menarini, Novartis, Rovi. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank Simon Armour of the British Academy for his work on improving the English text.
Additional information
Funding
References
- Ancochea J, Soriano JB. COPD in Spain at the start of a new decade. Arch Bronconeumol. 2021;57(1):1–2. doi:10.1016/j.arbres.2020.01.025
- López-Campos JL, Carrasco-Hernández L, Román Rodríguez L, Quintana-Gallego E, Carmona Bernal C, Alcázar Navarrete B. Implicaciones clínicas del uso de la triple terapia en combinación de dosis fija en EPOC: del ensayo al paciente. Archivos de Bronconeumología. 2020;56(4):242–248. doi:10.1016/j.arbres.2019.11.011
- Gea J. COPD therapy: beyond conventional pharmacology. Arch Bronconeumol. 2020;56(6):343–344. doi:10.1016/j.arbres.2019.06.023
- Tekerlek H, Cakmak A, Calik-Kutukcu E, et al. Exercise capacity and activities of daily living are related in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2020;56(4):208–213. doi:10.1016/j.arbres.2019.06.015
- Garrido Ocana AI, Gonzalez Fernandez-Palacios M, Matute de Cárdenas JA, et al. Successful lobectomy in preterm with diffuse persistent interstitial pulmonary emphysema. Arch Bronconeumol. 2020;56(7):461–463. doi:10.1016/j.arbres.2019.10.029
- Lujan M, Ergan B. Guidelines for chronic non-invasive ventilation in COPD: from experience to evidence. Arch Bronconeumol. 2021;57(3):158–159. doi:10.1016/j.arbres.2020.03.016
- Galdiz JB, Gomez A, Rodriguez D, et al. Telerehabilitation programme as a maintenance strategy for COPD patients: a 12-month randomized clinical trial. Arch Bronconeumol. 2021;57(3):195–204.
- Diaz Lopez JM, Giran Gonzalez B, Alcazar-Navarrete B. Personalized medicine in chronic obstructive pulmonary disease: how close are we? Arch Bronconeumol. 2020;56(7):420–421. doi:10.1016/j.arbr.2019.09.013
- Lopez-Campos JL, Soler-Cataluna JJ, Miravitlles M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronconeumol. 2020;56(2):65–67. doi:10.1016/j.arbres.2019.06.001
- Miravitlles M, Calle M, Molina J, et al. Spanish COPD guidelines (GesEPOC) 2021: updated pharmacological treatment of stable COPD. Arch Bronconeumol. 2022;58(1):69–81. doi:10.1016/j.arbres.2021.03.005
- Soler-Cataluna JJ, Pinera P, Trigueros JA, et al. Spanish COPD guidelines (GesEPOC) 2021 update diagnosis and treatment of COPD exacerbation syndrome. Arch Bronconeumol. 2022;58(2):159–170. doi:10.1016/j.arbres.2021.05.033
- Lopez-Campos JL, Almagro P, Gomez JT, et al. Spanish COPD guideline (GesEPOC) update: comorbidities, self-management and palliative care. Arch Bronconeumol. 2022;58(4):334–344. doi:10.1016/j.arbres.2021.08.002
- Cosio BG, Hernandez C, Chiner E, et al. Spanish COPD guidelines (GesEPOC 2021): non-pharmacological treatment update. Arch Bronconeumol. 2022;58(4):345–351. doi:10.1016/j.arbres.2021.08.024
- Cosio BG, Shafiek H, Verdu J, et al. Implementation of an integrated care model for frequent-exacerbator COPD patients: a controlled prospective study. Arch Bronconeumol. 2021;57(9):577–583. doi:10.1016/j.arbres.2021.01.025
- Meeraus WH, DeBarmore BM, Mullerova H, Fahy WA, Benson VS. Terms and definitions used to describe recurrence, treatment failure and recovery of acute exacerbations of COPD: a systematic review of observational studies. Int J Chron Obstruct Pulmon Dis. 2021;16:3487–3502. doi:10.2147/COPD.S335742
- Kim V, Aaron SD. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur Respir J. 2018;52(5):1801261. doi:10.1183/13993003.01261-2018
- Duarte-de-Araújo A, Fonte P, Teixeira P, Hespanhol V, Correia-de-Sousa J. Is an early diagnosis of COPD clinically useful? Archivos de Bronconeumología. 2020;56(6):409–410. doi:10.1016/j.arbres.2019.11.018
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2022.
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121.
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5Suppl 2):398s–401s. doi:10.1378/chest.117.5_suppl_2.398S
- Celli BR, Agusti A. COPD: time to improve its taxonomy? ERJ Open Res. 2018;4(1):00132–2017. doi:10.1183/23120541.00132-2017
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. doi:10.1136/thx.2010.155333
- Nishimura K, Nishimura T, Onishi K, Oga T, Hasegawa Y, Jones PW. Changes in plasma levels of B-type natriuretic peptide with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:155–162. doi:10.2147/COPD.S55143
- Han W, Wang M, Xie Y, Ruan H, Zhao H, Li J. Prevalence of pulmonary embolism and deep venous thromboembolism in patients with acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:732855. doi:10.3389/fcvm.2022.732855
- Park HJ, Kim SH, Kim HC, et al. Utility of computed tomography in a differential diagnosis for the patients with an initial diagnosis of chronic obstructive pulmonary disease exacerbation. Tuberc Respir Dis. 2019;82(3):234–241. doi:10.4046/trd.2018.0087
- Parker CM, Voduc N, Aaron SD, Webb KA, O’Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26(3):420–428. doi:10.1183/09031936.05.00136304
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
- Gao P, Zhang J, He X, Hao Y, Wang K, Gibson PG. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2013;8(5):e57678. doi:10.1371/journal.pone.0057678
- Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi:10.1164/rccm.200708-1290OC
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
- Leidy NK, Wilcox TK, Jones PW, et al. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183(3):323–329. doi:10.1164/rccm.201005-0762OC
- Aaron SD, Donaldson GC, Whitmore GA, Hurst JR, Ramsay T, Wedzicha JA. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67(3):238–243. doi:10.1136/thoraxjnl-2011-200768
- Soler-Cataluna JJ, Rodriguez-Roisin R. Frequent chronic obstructive pulmonary disease exacerbators: how much real, how much fictitious? COPD. 2010;7(4):276–284. doi:10.3109/15412555.2010.496817
- Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. doi:10.1164/rccm.200807-1067OC
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
- Jacobs DM, Noyes K, Zhao J, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thorac Soc. 2018;15(7):837–845. doi:10.1513/AnnalsATS.201712-913OC
- Alqahtani JS, Mandal S, Hurst JR. The impact of re-admissions in COPD. Arch Bronconeumol. 2022;58(2):109–110. doi:10.1016/j.arbres.2021.06.006
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J. 2016;47(1):113–121. doi:10.1183/13993003.01391-2014
- Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP
- Miravitlles M, Sliwinski P, Rhee CK, et al. Changes in control status of COPD over time and their consequences: a prospective international study. Arch Bronconeumol. 2021;57(2):122–129. doi:10.1016/j.arbres.2020.06.003
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi:10.1164/ajrccm.161.5.9908022
- Soler-Cataluna JJ, Miralles C. Exacerbation Syndrome in COPD: a Paradigm Shift. Arch Bronconeumol. 2021;57(4):246–248. doi:10.1016/j.arbr.2020.07.021
- Saleh A, Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. The effect of incidental consolidation on management and outcomes in COPD exacerbations: data from the European COPD audit. PLoS One. 2015;10(7):e0134004. doi:10.1371/journal.pone.0134004
- Williams NP, Ostridge K, Devaster JM, et al. Impact of radiologically stratified exacerbations: insights into pneumonia aetiology in COPD. Respir Res. 2018;19(1):143. doi:10.1186/s12931-018-0842-8
- Monso E. Look at the wood and not at the tree: the microbiome in chronic obstructive lung disease and cystic fibrosis. Arch Bronconeumol. 2020;56(1):5–6. doi:10.1016/j.arbr.2019.04.014
- Huerta A, Crisafulli E, Menendez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. doi:10.1378/chest.13-0488
- Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
- Lopez-Campos JL, Agusti A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposal. Lancet Respir Med. 2015;3(9):729–734. doi:10.1016/S2213-2600(15)00242-8
- Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
- Singh D, Bafadhel M, Brightling CE, et al. Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(5):660–671. doi:10.1164/rccm.201912-2384PP
- Miravitlles M, Monteagudo M, Solntseva I, Alcazar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a population-based study. Arch Bronconeumol. 2021;57(1):13–20. doi:10.1016/j.arbres.2019.12.015
- Schumann DM, Tamm M, Kostikas K, Stolz D. Stability of the blood eosinophilic phenotype in stable and exacerbated cOPD. Chest. 2019;156(3):456–465. doi:10.1016/j.chest.2019.04.012
- Marcos PJ, Lopez-Campos JL. Shall we focus on the eosinophil to guide treatment with systemic corticosteroids during acute exacerbations of chronic obstructive pulmonary disease (COPD)? CON. Med Sci. 2018;6:2.
- Asensio VJ, Tomas A, Iglesias A, et al. Eosinophilic COPD patients display a distinctive serum miRNA profile from asthma and non-eosinophilic COPD. Arch Bronconeumol. 2020;56(4):234–241. doi:10.1016/j.arbres.2019.09.020
- Golpe R, Dacal D, Sanjuan-Lopez P, Martin-Robles I, Perez-de-Llano LA. Plasma eosinophil count and patient-centered events in chronic obstructive pulmonary disease in real-life clinical practice. Arch Bronconeumol. 2020;56(2):129–130. doi:10.1016/j.arbres.2019.09.015
- Hegewald MJ, Horne BD, Trudo F, et al. Blood eosinophil count and hospital readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:2629–2641. doi:10.2147/COPD.S251115
- Kang HS, Kim SK, Kim YH, et al. The association between eosinophilic exacerbation and eosinophilic levels in stable COPD. BMC Pulm Med. 2021;21(1):74. doi:10.1186/s12890-021-01443-4
- Garcia Sanz MT, Gonzalez Barcala FJ. Establishing the prognosis of COPD exacerbations using risk scales from the point of view of the emergency department. Arch Bronconeumol. 2020;56(2):63–64. doi:10.1016/j.arbr.2019.04.017
- Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. doi:10.1136/thoraxjnl-2012-202103
- Tabak YP, Sun X, Johannes RS, Gupta V, Shorr AF. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602. doi:10.1001/archinternmed.2009.270
- Quintana JM, Esteban C, Unzurrunzaga A, et al. Predictive score for mortality in patients with COPD exacerbations attending hospital emergency departments. BMC Med. 2014;12:66. doi:10.1186/1741-7015-12-66
- Roche N, Chavaillon JM, Maurer C, Zureik M, Piquet J. A clinical in-hospital prognostic score for acute exacerbations of COPD. Respir Res. 2014;15:99. doi:10.1186/s12931-014-0099-9
- Eccles SR, Subbe C, Hancock D, Thomson N. CREWS: improving specificity whilst maintaining sensitivity of the National Early Warning Score in patients with chronic hypoxaemia. Resuscitation. 2014;85(1):109–111. doi:10.1016/j.resuscitation.2013.08.277
- Echevarria C, Steer J, Bourke SC. Comparison of early warning scores in patients with COPD exacerbation: DECAF and NEWS score. Thorax. 2019;74(10):941–946. doi:10.1136/thoraxjnl-2019-213470
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
- García-Quero C, García-Río F. Smoking-induced small airway dysfunction. an early marker of future COPD? Arch Bronconeumol. 2021;57(1):3–4. doi:10.1016/j.arbres.2020.02.006
- Soler-Cataluna JJ, Novella L, Soler C, et al. Clinical characteristics and risk of exacerbations associated with different diagnostic criteria of asthma-COPD overlap. Arch Bronconeumol. 2020;56(5):282–290. doi:10.1016/j.arbr.2020.03.003
- Fernandez-Garcia S, Represas-Represas C, Ruano-Ravina A, Botana-Rial M, Martinez-Reglero C, Fernandez Villar A. Dependence in performing activities as a predictor of mortality following hospitalization for chronic obstructive pulmonary disease exacerbation. Arch Bronconeumol. 2020;56(5):291–297. doi:10.1016/j.arbr.2020.03.004
- Erro Iribarren M, Alonso Perez T, Soriano JB, Ancochea Bermudez J. Adjusting the level of intervention in patients with chronic obstructive pulmonary disease according to the risk stratification proposed by the Spanish COPD guidelines (GesEPOC) version 2017. Arch Bronconeumol. 2020;56(3):183–185. doi:10.1016/j.arbres.2019.09.016
- Soler-Cataluña JJ, Alcázar B, Miravitlles M. Clinical control in COPD: a new therapeutic objective? Arch Bronconeumol. 2020;56(2):68–69. doi:10.1016/j.arbres.2019.06.004
- Marcos PJ, Represas Represas C, Ramos C, et al. Impact of a home telehealth program after a hospitalized COPD exacerbation: a propensity score analysis. Arch Bronconeumol. 2022;58(6):474–481. doi:10.1016/j.arbres.2020.05.030
- Montes de Oca M, Lopez Varela MV, Acuna A, et al. Incorporating new evidence on inhaled medications in COPD. The Latin American Chest Association (ALAT) 2019. Arch Bronconeumol. 2020;56(2):106–113. doi:10.1016/j.arbres.2019.09.023
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2022. Available from: https://goldcopd.org/. Accessed December 20, 2022.
