Abstract
Background
Assessment of airway inflammation in the clinical course of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) may advance our understanding of the pathogenesis and treatment.
Objectives
To assess airway inflammation in patients during the course of AECOPD by serial analyses of their exhaled breath condensates (EBC).
Methods
Twenty-six patients with AECOPD (22 males, mean[SD] percentage predicted forced expiratory volume in one second (FEV1) 44.8 [14.3]), 11 with stable COPD, and 14 age and sex-matched healthy controls were studied. Patients with AECOPD were treated with systemic steroid and antibiotic for 7 days. EBC was collected from each patient with AECOPD on Day 5, 14, 30, and 60 post-hospitalization using EcoScreen (VIASYS Healthcare, USA) during tidal breathing over 10 minutes. Concentrations of tumor necrosis factor-α (TNF-α), leukotriene B4 (LTB4), and interleukin-8 (IL-8) were measured by enzyme-linked immunosorbent assay.
Results
The median (IQR) of TNF-α level on Day 5 was 5.08 (3.80–6.32) pg/ml, which was lower than on Day 14 (5.84 [4.91–9.14] pg/ml, p = 0.017), Day 30 (6.14 [3.82–7.67] pg/ml, p = 0.045), and Day 60 (5.60 [4.53–8.80] pg/ml, p = 0.009). On Day 60, subjects receiving inhaled corticosteroid (ICS) had a lower level of TNF-α than those who were not (4.82 [4.06–5.65] vs 7.66 [5.48–10.9] pg/ml, p = 0.02). EBC LTB4 level did not change significantly during recovery from AECOPD whereas IL-8 was mostly undetectable.
Conclusions
EBC TNF-α level was low in patients receiving systemic steroid and antibiotic therapy for AECOPD. These findings suggest a potential role for serial EBC TNF-α for non-invasive monitoring of disease activity.
Background
Assessment of airway inflammation in the clinical course of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) may advance our understanding of the pathogenesis and the treatment effects in patients with AECOPD. Exhaled breath condensate (EBC) analysis has been developed in recent years as a novel tool to sample the lower airway lining fluid (CitationEffros et al 2004; CitationHunt 2002; CitationMontuschi and Barnes 2002; CitationKo et al 2007). Its noninvasive nature offers many advantages over invasive techniques such as bronchoalveolar lavage, bronchial biopsy and sputum induction, which are not so well tolerated by AECOPD patients. Because of the noninvasive nature of EBC collection, repeated measurements are feasible in the longitudinal follow-up of patients with AECOPD. Our group has previously demonstrated the repeatability of certain markers in the EBC of stable asthma and COPD patients (CitationKo et al 2006; CitationKo et al 2006; CitationLeung et al 2005).
Tumor necrosis factor-α (TNF-α) is a pleiotropic inflammatory cytokine produced by many different cell types. The main sources in vivo are stimulated monocytes, fibroblasts, and endothelial cells (CitationMukhopadhyay et al 2006). Leukotriene B4 (LTB4) is a potent chemoattractant of neutrophils, and it may be released by macrophages, epithelial cells and activated neutrophils (CitationFord-Hutchinson et al 1980). Interleukin 8 (IL-8), on the other hand, is largely produced by neutrophils and macrophages (CitationBaggiolini et al 1989). Previous studies found significant increases in neutrophils and concentrations of TNF-α and IL-8 in spontaneous or induced sputum samples from patients with COPD when compared to smoking and non-smoking controls (CitationKeatings et al 1996; CitationYamamoto et al 1997). During AECOPD, the concentrations of TNF-α and IL-8 may increase further in the induced sputum samples (CitationAaron et al 2001). In patients having AECOPD related to bacterial infection, increases in the concentration of neutrophil products such as myeloperoxidase and elastase, and the neutrophil chemoattractants (IL-8 and LTB4) have been observed in expectorated sputum, and their levels fell subsequently after antibiotic therapy (CitationCrooks et al 2000). Limited data, however, are available on the changes in the EBC levels of TNF-α, LTB4, and IL-8 during AECOPD. This study aimed to assess the levels of these inflammatory mediators during the course of recovery from AECOPD by serial analyses of EBC.
Material and methods
Subject recruitment
Patients who had been admitted to the Prince of Wales Hospital with AECOPD were recruited for this study. AECOPD was defined when a patient with background COPD (CitationNational Heart, Lung and Blood Institute, World Health Organization 2006) presented with at least two of the following major symptoms (increased dyspnoea, increased sputum purulence, increased sputum volume) or one major and one minor symptom (nasal discharge/congestion, wheeze, sore throat, cough) for at least two consecutive days (CitationSeemungal et al 1998; CitationPatel et al 2002; CitationKo et al 2007). Subjects with pneumonic changes on the chest radiographs were excluded. Age and sex-matched COPD subjects who were clinically stable (without AECOPD for at least 10 weeks) and healthy, nonsmoking subjects were recruited as controls. Patients with AECOPD were managed by our standard care with administration of antibiotic (oral amoxicillin/clavulanic acid for 7 days) and systemic steroid (oral prednisolone 30mg daily for 7 days). All patients also received bronchodilators via a spacer device and if needed, oxygen therapy for respiratory failure. Current smokers (defined as cigarette smoking in the last 6 months) were excluded from this study. Informed written consent was obtained from each subject and the study was approved by the research ethics committee of the Chinese University of Hong Kong.
Collection of exhaled breath condensate
EBC was collected using the EcoScreen (VIASYS Healthcare, Conshohochen, PA, USA) according to the manufacturer’s instructions. The collection was carried out from 9–10 am. After rinsing their mouths, the recruited subjects breathed tidally through a mouthpiece that was connected through a unique one-way valve into a cooled collection tube where vapors, aerosols and moisture in the breath condensed along the walls of the tube. The design of the system prevented salivary contamination of EBC. Each subject was asked to breathe through the device, while wearing a nose-clip, for 10 minutes, and more than 1 mL of EBC could be collected from each subject. EBC was stored in 250 μL aliquots immediately at −70 °C until analysis. EBC was collected on Days 5, 14, 30, and 60 after hospitalization for subjects with AECOPD. For the stable COPD patients and normal controls, EBC collection was performed once.
Lung function measurement
Spirometry (post-bronchodilator) was performed on all subjects before each EBC collection with the Vitalograph (Model S, Buckingham, UK) spirometer according to the American Thoracic Society standards (CitationAmerican Thoracic Society. Standardization of spirometry – 1987). The updated predicted spirometry values for Hong Kong Chinese were adopted (CitationIp et al 2006).
Measurement of TNF-α, LTB4, and IL-8
The concentrations of TNF-α, LTB4, and IL-8 in EBC were measured in one batch by high-sensitivity sandwich enzyme immunoassays (TNF-α and IL-8 from BioSource International, Camarillo, CA, USA; LTB4 from Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturers’ instructions. The detection limits of TNF-α, LTB4, and IL-8 were 0.09, 4, and 0.02 pg/mL, respectively, and the intra-assay and inter-assay variability of all three markers were <10%.
Intra-subject repeatability of TNF-α, LTB4, and IL-8 was assessed by collection of EBC in 6 stable COPD subjects. EBC was collected as described above at the same time (9–10 am) on 2 consecutive days.
Statistical analysis
Data were analyzed by the Statistical Package of the Social Science (SPSS) for Windows, Version 11.5 (SPSS Inc, Chicago, IL, USA). Demographic data of the subjects were presented as means ± standard deviation (SD). The levels of TNF-α, LTB4, and IL-8 were expressed as median and interquartile range (IQR). When the levels of these biomarkers were below the respective detection limits of the assays, the samples were assigned values at half of the lower detection limits for statistical analysis. Results were analyzed by two-tailed Students t-test or ANOVA for parametric variables and Mann-Whitney U-test or Kruskal-Wallis test for nonparametric numerical variables. The serial changes of TNF-α, LTB4, and IL-8 levels were compared at the different time points post-admission for AECOPD by Wilcoxon Signed Ranks test and Friedman test as appropriate. Correlation of lung function with the levels of EBC markers were assessed by Spearman’s rank correlation test. The p values of <0.05 were considered as statistically significant. Bland and Altman’s method was used to assess repeatability of TNF-α, LTB4, and IL-8 in the EBC (CitationBland and Altman 1986).
Results
Altogether 32 patients with AECOPD were initially recruited for this study. Two patients refused to return for EBC collection after Day 14 whereas 4 patients had another exacerbation before Day 60 and thus were excluded from the study. Finally, 26 subjects with AECOPD, 11 stable COPD patients, and 14 controls were included in the analysis. The demographics of the subjects are shown in . The AECOPD, stable COPD and control subjects were age and sex-matched. Twelve (46.1) and 5 (45.5%) subjects with AECOPD and stable COPD were on inhaled corticosteroid (ICS) whereas the median (IQR) beclomethasone-equivalent doses were 1000 (950–2000) and 1000 (1000–1500) μg/day, respectively. None were on long term home oxygen therapy or oral steroid therapy when stable.
Table 1 Clinical characteristics of the subjects
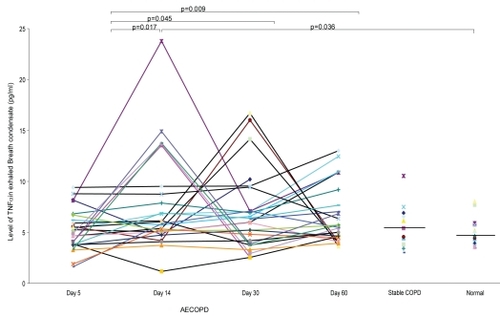
The detection rates of TNF-α, LTB4, and IL-8 in EBC of the subjects are shown in . The EBC levels of TNF-α and LTB4 are summarized in . The level of TNF-α in EBC was the lowest on Day 5 post-hospitalization after commencement of treatments with both systemic steroid and antibiotic. The levels of TNF-α at Day 5 was significantly lower than at Day 14 (p = 0.017), Day 30 (p = 0.045) and Day 60 (p = 0.009). There was however no significant difference in the levels of TNF-α between Day 14, Day 30, and Day 60 (p = 0.89). The level of TNF-α at Day 14 post-hospitalization for AECOPD was significantly higher than the normal controls (p = 0.036), but not different from the stable COPD subjects (p = 0.26). The EBC TNF-α level at the start of exacerbation (Day 5 post-exacerbation) was not significantly different from that during stable COPD (p = 0.39). There was no difference in the TNF-α level between the stable COPD and normal controls (p = 0.41). illustrates the serial levels of TNF-α during the course of AECOPD versus TNF-α levels for stable COPD and control subjects based on their single EBC collection.
Table 2 Detection rates of TNF-α, LTB4 and IL-8 in exhaled breath condensate
Table 3 Levels of the biomarkers
Figure 1 Levels of TNF-α in the subjects with AECOPD, stable COPD and normal controls
The levels of LTB4 on Day 5, Day 14, Day 30, and Day 60 of AECOPD showed no statistically significant difference. In addition, there was no difference in the level of LTB4 at the different time points of the AECOPD when compared to the stable COPD patients or normal controls. IL-8 was undetectable in most of the subjects and thus their results were not presented.
The levels of TNF-α and LTB4 did not correlate with the absolute and normal predicted values of forced expiratory volume in one second (FEV1) at different time points of AECOPD. The levels of TNF-α and LTB4 on Day 60 post-hospitalization showed no significant difference in their levels when comparing those with FEV1 ≥ 50% against those with FEV1 < 50% predicted normal, whereas the same observation was made among the stable state COPD subjects. On Day 60 post-hospitalization for AECOPD, subjects receiving ICS therapy had a lower level of TNF-α than those who were not on ICS (median [IQR] 4.82 [4.06–5.65] vs 7.66 [5.48–10.9] pg/ml, p = 0.02). The ICS was started before AECOPD and continued throughout the study. For those patients who were not on ICS before AECOPD, ICS was not introduced during the study period. The reduction of TNF-α as noted in the AECOPD patients on ICS, however, was not observed in the stable COPD patients, who had no change in their medications for 8 weeks before recruitment into this study. With reference to LTB4, there was no difference in its levels between patients on ICS and those without ICS therapy for both the AECOPD subjects at Day 60 post-exacerbation and the stable COPD subjects.
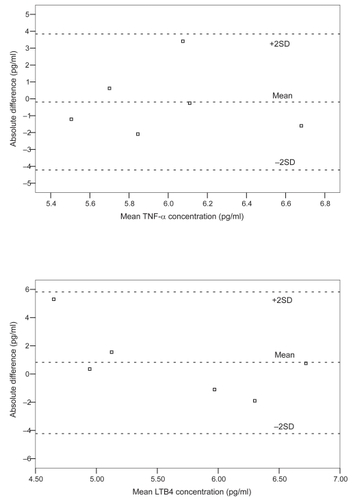
The results of serial spirometry are shown in . There were no significant changes in the subjects’ lung function in the course of AECOPD. Six stable COPD subjects (5 males) aged 66 to 74 years had EBC collected and measured for repeatability testing of the biomarkers. For TNF-α and LTB4 repeatability testing, the differences between most of the paired measurements laid within ± 2SDs (mean differences −0.19 ± 2.01, −0.83 ± 2.23 pg/ml for TNF-α and LTB4, respectively). Bland and Altman’s plots of the repeatability tests for TNF-α and LTB4 are shown in . IL-8 was only measurable in 1 of the 6 subjects and thus the results are not presented.
Table 4 Serial spirometry results of the subjects with acute exacerbation of COPD (n = 26)
Discussion
To the best of our knowledge, this study is the first to assess inflammatory markers in the EBC serially over 60 days in patients hospitalized with AECOPD. We have shown that the TNF-α level in EBC was lower on Day 5 post-hospitalization for AECOPD when compared to the levels on Day 14, Day 30, and Day 60. The lower TNF-α level on Day 5 was likely secondary to the anti-inflammatory effect of systemic steroid and antibiotic. In contrast, there was no significant change in the serial LTB4 levels in EBC. Repeatability measurements of TNF-α and LTB4 among our stable COPD patients were satisfactory. Interestingly, the subjects receiving ICS had a lower EBC TNF-α level at Day 60 post-hospitalization for AECOPD than those not on ICS treatment. Previous studies have either measured EBC biomarkers at the start of exacerbation and compared against stable COPD and normal subjects (CitationGessner et al 2005), or measured the level of biomarkers serially during the first week of AECOPD (CitationGerritsen et al 2005; CitationOudijk et al 2006).
As our patients were dyspneic on admission to hospital, EBC assessment was only commenced on Day 5 when they became more stable and able to comply with EBC collection. Ideally, systemic steroids should have been withheld as it could suppress inflammation in the airway. As previous studies (CitationDavies et al 1999; CitationWood-Baker et al 2005) have consistently shown that systemic steroid can reduce treatment failure and need for additional medical treatment in subjects with severe AECOPD, we felt that it was unethical to withhold the therapy, which was strongly recommended in the GOLD Guideline (CitationNational Heart, Lung and Blood Institute, World Health Organization 2006), for research purposes.
Previous studies have found that TNF-α level was raised in both EBC (CitationGessner et al 2005) and sputum (CitationAaron et al 2001) of subjects with AECOPD. However, the changes in circulating levels of TNF-α during AECOPD were less consistent. Some studies showed that its level correlated negatively with the changes in FEV1 during the exacerbation (CitationPinto-Plata et al 2007), whereas others reported no change when comparing the stable baseline level with the AECOPD level (CitationHurst et al 2006). It is interesting to note that the level of TNF-α was similar from Day 14 onwards during the course of AECOPD in our study. It remains unclear why the TNF-α level was similar between the stable state COPD and the normal healthy controls. A previous study has suggested that markers such as LTB4 and 8-isoprostane might take 2 months to return to normal state with antibiotic (without systemic steroid) treatment (CitationBiernacki et al 2003). The 1-week course of systemic steroid administered to our patients with AECOPD has possibly sped up the recovery of TNF-α.
TNF-α could be measured in the EBC in every subject in this current study, and similar observation was noted in our previous study involving asthmatic subjects (CitationLeung et al 2005). This finding was also consistent with another study assessing TNF-α in EBC using cytometric bead array rather than ELISA (CitationGessner et al 2005). Our level of TNF-α was lower than that measured by cytometric bead array assay and this might be explained by the different methods of biomarker measurement (CitationGessner et al 2005). An experimental study with animal models showed that over-expression of TNF-α induced the pathological changes similar to emphysema and pulmonary fibrosis (CitationLundblad et al 2005). It is likely that TNF-α plays an important role in the pathogenesis of COPD and its level may be altered during the course of AECOPD.
We have noted that TNF-α level was lower in the EBC on Day 60 post-hospitalization for AECOPD in subjects who were on ICS therapy than those who were not. A previous study has shown that some cytokines in EBC such as IL-1β, IL-6, IL-8, IL-12, and IL-10, but not TNF-α, were lower in stable COPD patients on ICS compared to those who were not on this therapy (CitationGessner et al 2005). Induced sputum study also failed to show the effect of ICS therapy on TNF-α or other inflammatory indices in the sputum of COPD patients (CitationKeatingset al 1997). Several studies have suggested that ICS could reduce the frequency of AECOPD in subjects with severe COPD (CitationBurge et al 2000). Whether TNF-α or other biomarkers are involved in the mechanisms of AECOPD will require further exploration.
LTB4 in the COPD airway is likely mediating its effects via activation of neutrophils. Assessment of chemotactic activity of LTB4 during AECOPD using the LTB4 receptor antagonist LY293111 revealed that LTB4 contributed significantly (~30%) to neutrophil influx into the airway in COPD subjects (CitationCrooks et al 2000). A previous study found that LTB4 level in EBC was higher in COPD patients (for both steroid naïve and those on ICS) than among controls (CitationMontuschi et al 2003). In our study, the changes in the level of LTB4 were not apparent during the course of AECOPD and this might be related to the treatment with both systemic steroid and antibiotic. IL-8 was not measurable in the majority of the EBC samples in this study. Previous studies on IL-8 in the airway have been inconclusive. One study found no difference in the IL-8 level in induced sputum specimen in subjects with stable or AECOPD (CitationBhowmik et al 2000), whereas Gessner and colleagues noted that EBC IL-8 level was higher in subjects with AECOPD admitted to the general ward or to the intensive care unit for mechanical ventilation (CitationGessner et al 2005). Studies on serum IL-8 level in patients with AECOPD treated with systemic steroid showed a gradual fall in its level in the first 7 days of the AECOPD (CitationGerritsen et al 2005). It is uncertain why we could not measure IL-8 reliably in our study. In the study by Gessner and colleagues, the IL-8 level, measured by the multiplex fluorescent bead immunoassay cytometric bead array technique, was well above the detection limit of the ELISA kit used in our current study, despite the fact that their levels of IL-8 in the stable COPD patients and controls were also quite low (CitationGessner et al 2005). It is possible that we might have missed the peak of IL-8 at the start of the AECOPD and thus could not detect any IL-8 after 5-days of systemic steroid.
The limitations of this study included a relatively small sample size and the ex-smoker status of the subjects was not confirmed objectively by urine cotinine assay. As smoking may affect EBC markers, we did not include active smokers in this study (CitationGessner et al 2005; CitationCarpagnano et al 2003; CitationGareyet al 2004). Ideally age- and sex-matched ex-smokers with normal lung function would serve as better controls than nonsmoking age and sex-matched subjects. In addition, we had not collected baseline EBC samples before AECOPD and thus the levels of biomarkers before therapy were unknown. Though the repeatability test of the TNF-α and LTB4 appeared satisfactory, as reflected by the differences between most of the paired measurements laying within ± 2SDs (), the relatively large SD values when compared to the absolute measured values of TNF-α and LTB4 would warrant cautions when interpreting the results. EBC assessment is still a relatively new tool in respiratory research with many methodological pitfalls (CitationEffros et al 2004; CitationHorvath et al 2005), including the variability of the measurement and the low concentrations of mediators measured (CitationEffros et al 2004; CitationHorvathet al 2005). There are suggestions that biomarker concentrations depend on other factors such as the dilution of the samples (CitationEffros et al 2004; CitationHorvathet al 2005). Adjusting the concentrations using dilutional indicator (eg, conductivity of lyonphized EBC) in future studies may improve the accuracy of EBC measurement. As many factors were interplaying that could affect the levels of biomarkers (eg, the assay method of the biomarkers and the treatment given during the exacerbations), it is difficult to conclude whether the changes in the levels of the biomarkers were, in fact, due to “cause and effect” with respect to exacerbations and remissions.
Conclusion
In conclusion, it is feasible to obtain serial EBC in patients following AECOPD. EBC TNF-α level was lower on Day 5 post-hospitalization for AECOPD than the levels at other time points up to 60 days, and this was likely due to the effect of treatment with systemic steroid and antibiotic. The level of LTB4 did not change significantly during the course of AECOPD whereas IL-8 was mostly undetectable in the EBC. Research in EBC is a constantly evolving field and more sensitive assays and novel biomarkers may be identified in the future (CitationBarnes et al 2006). As in the case of measuring exhaled nitric oxide level in asthma management (CitationSmith et al 2005), prospective randomized trials are needed to evaluate if the measurement of TNF-α in EBC may guide treatment and improve outcome in patients with COPD.
Acknowledgments
This study was supported by the Respiratory Research Fund of the Chinese University of Hong Kong. The authors would like to thank Miss Mabel Tong for performing the spirometry, Miss Brenda Li and Miss HY Chu for performing the ELISA experiments, and Miss Doris Chan for assisting in the statistical analysis. The authors report no conflicts of interest in this work.
References
- AaronSDAngelJBLunauM2001Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1633495511179105
- American Thoracic SocietyStandardization of spirometry – 1987 update. Statement of the American Thoracic SocietyAm Rev Respir Dis1361285983674589
- BaggioliniMWalzAKunkelSL1989Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophilsJ Clin Invest84104592677047
- BarnesPJChowdhuryBKharitonovSA2006Pulmonary biomarkers in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med17461416556692
- BhowmikASeemungalTASapsfordRJ2000Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbationsThorax551142010639527
- BiernackiWAKharitonovSABarnesPJ2003Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPDThorax58294812668789
- BlandJMAltmanDG1986Statistical methods for assessing agreement between two methods of clinical measurementLancet1307102868172
- BurgePSCalverleyPMJonesPW2000Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ320129730310807619
- CarpagnanoGEKharitonovSAFoschino-BarbaroMP2003Increased inflammatory markers in the exhaled breath condensate of cigarette smokersEur Respir J215899312762340
- CrooksSWBayleyDLHillSL2000Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4Eur Respir J152748010706491
- DaviesLAngusRMCalverleyPM1999Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trialLancet3544566010465169
- EffrosRMDunningMB3rdBillerJ2004The promise and perils of exhaled breath condensatesAm J Physiol Lung Cell Mol Physiol287L10738015531756
- Ford-HutchinsonAWBrayMADoigMV1980Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytesNature28626456250050
- GareyKWNeuhauserMMRobbinsRA2004Markers of inflammation in exhaled breath condensate of young healthy smokersChest12522614718416
- GerritsenWBAsinJZanenP2005Markers of inflammation and oxidative stress in exacerbated chronic obstructive pulmonary disease patientsRespir Med99849015672854
- GessnerCScheibeRWotzelM2005Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary diseaseRespir Med9912294016140223
- HorvathIHuntJBarnesPJ2005Exhaled breath condensate: methodological recommendations and unresolved questionsEur Respir J265234816135737
- HuntJ2002Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung diseaseJ Allergy Clin Immunol110283412110814
- HurstJRDonaldsonGCPereraWR2006Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1748677416799074
- IpMSKoFWLauAC2006Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilizationChest1293849216478856
- KeatingsVMCollinsPDScottDM1996Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthmaAm J Respir Crit Care Med15353048564092
- KeatingsVMJatakanonAWorsdellYM1997Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPDAm J Respir Crit Care Med15554289032192
- KoFWIpMChanPK2007A 1-Year Prospective Study of the Infectious Etiology in Patients Hospitalized With Acute Exacerbations of COPDChest131445217218555
- KoFWLauCYLeungTF2006Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary diseaseRespir Med100630816213701
- KoFWLauCYLeungTF2006Exhaled breath condensate levels of eotaxin and macrophage-derived chemokine in stable adult asthma patientsClin Exp Allergy36445116393265
- KoFWLeungTFHuiDS2007Are exhaled breath condensates useful in monitoring asthma?Curr Allergy Asthma Rep7657117504663
- LeungTFWongGWKoFW2005Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic childrenInt Arch Allergy Immunol137667215832052
- LundbladLKThompson-FigueroaJLeclairT2005Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotypeAm J Respir Crit Care Med17113637015805183
- MontuschiPBarnesPJ2002Analysis of exhaled breath condensate for monitoring airway inflammationTrends Pharmacol Sci23232712008001
- MontuschiPKharitonovSACiabattoniG2003Exhaled leukotrienes and prostaglandins in COPDThorax58585812832671
- MukhopadhyaySHoidalJRMukherjeeTK2006Role of TNFalpha in pulmonary pathophysiologyRespir Res712517034639
- National Heart, Lung and Blood Institute, World Health OrganizationGlobal Initiative for chronic obstructive lung diseaseGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease Updated 2006NHLB/WHO2006
- OudijkEJGerritsenWBNijhuisEH2006Expression of priming-associated cellular markers on neutrophils during an exacerbation of COPDRespir Med1001791916531033
- PatelISSeemungalTAWilksM2002Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbationsThorax577596412200518
- Pinto-PlataVMLivnatGGirishM2007Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPDChest131374317218554
- SeemungalTADonaldsonGCPaulEA1998Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1571418229603117
- SmithADCowanJOBrassettKP2005Use of exhaled nitric oxide measurements to guide treatment in chronic asthmaN Engl J Med35221637315914548
- Wood-BakerRRGibsonPGHannayM2005Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst RevCD00128815674875
- YamamotoCYonedaTYoshikawaM1997Airway inflammation in COPD assessed by sputum levels of interleukin-8Chest112505109266891