Abstract
Background
Antibiotic treatment is one of the major pharmacologic treatments for acute exacerbation of chronic obstructive pulmonary disease (AECOPD). However, the choice of antibiotic depends on the local resistance pattern. A multicenter, randomized, controlled trial was done in patients with AECOPD to compare the efficacy of levofloxacin with that of cefuroxime axetil.
Methods
Patients with AECOPD and without radiographic evidence of pneumonia were enrolled and randomized to either levofloxacin 500 mg daily or cefuroxime 250 mg twice daily in the mildmoderate exacerbation group, or 500 mg twice daily in the severe exacerbation group, for seven days. Clinical efficacy and microbiologic response were evaluated 5–7 days after the last dose.
Results
Treatment was clinically successful in 90.4% of patients in the levofloxacin group, and in 90.6% of patients in the cefuroxime group (95% confidence interval −9.40 to 10.91), within a noninferiority margin of 10%. The microbiologic response appeared to be higher in the levofloxacin group, but the difference was not statistically significant. The safety profile was similar in both groups.
Conclusion
Levofloxacin is not inferior to cefuroxime with regard to clinical efficacy in treating AECOPD.
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a serious event. It is related to decreased health status, increased medical and social costs, and increased mortality.Citation1–Citation3 The mainstay of pharmacologic treatment for AECOPD includes antibiotics and systemic steroids.Citation4 Antibiotics have been shown to be beneficial in patients with increased or purulent sputum and in those with severe exacerbations requiring ventilatory support.Citation5–Citation9 A recent report showed that antibiotics in addition to systemic steroids in AECOPD have a short-term effect on clinical outcome and microbiologic success.Citation10
However, the choice of antibiotic is a more complicated issue. Organisms frequently isolated from patient with AECOPD are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, so-called atypical pathogens, and respiratory virusesCitation11–Citation16. In more severe exacerbations and in patients with risk factors, it is known that Pseudomonas aeruginosa and Enterobacteriaceae can be pathogenic organisms.Citation17–Citation21 Moreover, the prevalence of each pathogen can be different according to the regional microbiologic environment, so it is crucial to consider individual characteristics and regional prevalence of micro-organisms as well when choosing antibiotics in AECOPD. Drug resistance of S. pneumoniae is a serious issue in some countries. In Korea, for example, 60%–75% of isolates of S. pneumoniae show intermediate or high resistance to penicillin. Therefore, it is important to investigate if other antibiotics are as useful as conventional choices in some countries.
Levofloxacin is a fluoroquinolone antibiotic agent with activity against various micro-organisms causing AECOPD, and is widely recommended for the treatment of AECOPD, along with macrolides, combinations of ß-lactam/ß-lactamase inhibitors, and second-generation or third-generation cephalosporins. Although widely used in clinical practice to treat AECOPD,Citation22,Citation23 the efficacy and safety of levofloxacin in comparison with other antibiotics is seldom investigated. Levofloxacin has been demonstrated to be effective and safe compared with cefuroxime in three randomized clinical trials, but the subjects in those studies were patients with chronic bronchitis, not COPD.Citation24,Citation25 Therefore, we designed a randomized clinical trial to investigate whether or not levofloxacin is inferior to cefuroxime with regard to its clinical efficacy and safety in the treatment of AECOPD.
Materials and methods
Study design and patients
This prospective, multicenter, randomized, open-label, parallel-group clinical trial was carried out at seven hospitals in South Korea from November 2006 to June 2009. In total, 141 men and women older than 18 years with AECOPD were enrolled. Exacerbation of COPD was defined as recently increased cough or dyspnea, recent change in color or amount of sputum, and a diagnosis of COPD on spirometry, ie, post-bronchodilator forced expiratory volume in one second/forced vital capacity <0.7.
Initial plain chest radiography was reviewed by two chest radiologists, and those patients who had radiographic evidence of pneumonia, bronchiectasis, cystic fibrosis, tuberculosis, or lung cancer were excluded. Patients with serious renal dysfunction, a history of seizure, a history of allergy or other side effects to quinolone, cephalosporins, or penicillins, a history of antibiotic therapy during the previous 48 hours, pregnancy or possibility of pregnancy, and lactating women were also excluded.
All patients were judged to have mild, moderate, or severe AECOPD by specialists, and randomized to receive levofloxacin or cefuroxime in a ratio of 1:1. A simple randomization was performed at the study coordinating center by a single person who was not otherwise involved in the study. A central computer was used to randomize patients stratified by site.
Patients in the levofloxacin group were treated with a once-daily dose of 500 mg for seven days, and those in the cefuroxime group were treated for seven days with 250 mg twice daily (mild to moderate exacerbations) or 500 mg twice daily (severe exacerbations). All patients provided their informed consent, and the study protocol was approved by the local institutional review board for all hospitals.
Outcomes and follow-up
On the first return visit (3–5 days after the initial visit) and the second return visit 2 (5–7 days after final dose), all patients were evaluated for clinical response, side effects, and compliance with medication. Plain chest radiography was obtained at the initial visit, sputum for Gram stain and microbiologic culture was obtained at all visits, and laboratory tests for blood cell counts, serum chemistry, and urinalysis were also performed at the initial and second visits. Two sets of blood cultures were obtained at the initial visit for patients in whom bacteremia was suspected, and at subsequent visits in cases of proven bacteremia.
The primary outcome was clinical success of antibiotic therapy. Clinical response was graded as cure, improved, or failure, and a clinical response of cure and improved at the second visit were defined as clinical success. The secondary outcome was microbiologic efficacy. Microbiologic response was graded as eradication (disappearance of pathogenic bacteria on the second visit), presumed eradication (inability to produce sputum due to improvement), persistence (persistence of initial pathogenic bacteria), presumed persistence (detection of pathogenic bacteria only on the second visit with clinical evidence of persistence), or superinfection (appearance of pathogenic bacteria other than initial ones) at the second visit. A microbiologic response of eradication, presumed eradication, or superinfection was defined as effective.
Statistical analysis
Based on a previously reported clinical success rate of 75% for levofloxacin and 76% for cefuroxime,Citation24 we estimated that 62 patients would be needed in each group to demonstrate that clinical success in the levofloxacin group was not lower than 10% compared with the cefuroxime group, with a statistical power of 80%. Therefore, we enrolled 70 patients in each treatment group, assuming a 12% dropout rate.
We used SAS version 9.1.2 (SAS Corporation, Cary, NC, USA) for data management and statistical analysis. The lower limit of the 95% confidence interval for a difference in clinical success (success rate of levofloxacin group – success rate of cefuroxime group) of −10% was defined as the criterion for noninferiority. The intention-to-treat population was used for all analyses. The clinical success rate in the two groups was compared by Chi-square test.
Results
Baseline characteristics
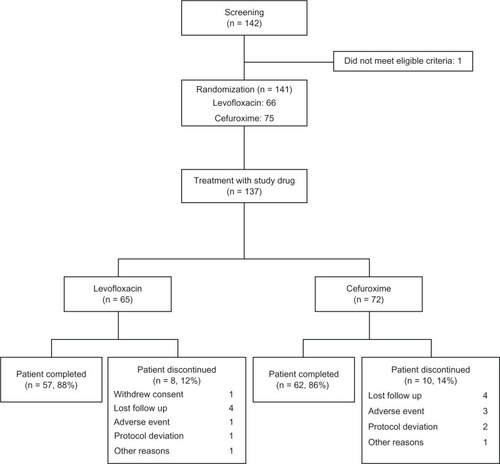
Of 142 patients screened, 141 were eligible for the study. Four patients refused to give their consent, and 137 were randomized to receive levofloxacin (n = 65) or cefuroxime (n = 72). Eighty-eight percent of patients in the levofloxacin group and 86% of those in the cefuroxime group completed therapy. There was no statistically significant difference in dropout rates between the groups (). shows the baseline characteristics of the subjects. There was no notable difference between the two groups in terms of gender, age, smoking status, and severity of exacerbation.
Table 1 Characteristics of enrolled patients
Clinical success rates
Clinical response as evaluated by respiratory specialists at the second visit was comparable in both groups. Clinical success was achieved in 90.4% of patients in the levofloxacin group and 90.6% of those in the cefuroxime group. The minimum and maximum value for the 95% confidence interval of the difference between the groups was −9.40 and 10.91, respectively, confirming that the clinical success rate of levofloxacin was not inferior to that of cefuroxime.
Microbiologic efficacy rates
Microbiologic efficacy rates were 85.7% in the levofloxacin group and 68.8% in the cefuroxime group (). However, because of the small number of patients who were available for evaluation, the difference was not statistically significant.
Table 2 Clinical success rates of both groups
Table 3 Microbiologic efficacy rates of both groups
Micro-organisms identified
shows the organisms identified in each group. Thirty-seven pathogens from 12 species were isolated. Of those, S. pneumoniae accounted for 40.5% (15 isolates), M. catarrhalis for 13.5% (five isolates), and Klebsiella pneumoniae for 10.8% (four isolates).
Table 4 Pathogens identified in sputum
Safety
The safety evaluation was performed in all patients who were randomized and took the test medication. Side effects related to medication were found in three cases from each group. There were four cases of mild dyspepsia, one of headache, and one of insomnia. None were serious, and no death was reported because of side effects.
Discussion
We found that levofloxacin was not inferior to cefuroxime in terms of clinical response rate and safety in the treatment of AECOPD. The findings of this study suggest that levofloxacin 500 mg daily for seven days is at least as effective as cefuroxime 500 mg daily for mild to moderate AECOPD and 1000 mg daily for severe AECOPD. The microbiologic response seemed higher in the levofloxacin group, but because of the small number of patients in whom analysis was possible, this result was not statistically significant. The safety profiles of both groups were also comparable.
To our knowledge, this is the first study comparing the efficacy of levofloxacin and cefuroxime in the treatment of AECOPD. Previous studies comparing levofloxacin versus cefuroxime in treating exacerbations of chronic bronchitis have reported similar results. Shar et al have reported the results of their randomized double-blind study of levofloxacin versus cefuroxime in acute exacerbations of chronic bronchitis.Citation24 In their study, three groups of patients (receiving levofloxacin 250 mg or 500 mg daily or cefuroxime 250 mg twice daily) showed similar clinical success rates and tolerability. Petitpretz et al reported the results of another randomized clinical trial of levofloxacin 500 mg daily versus cefuroxime 250 mg twice daily in patients with exacerbation of chronic obstructive bronchitis.Citation25 The results of these studies are quite similar to ours, but our study demonstrated noninferiority of levofloxacin compared with cefuroxime also in AECOPD. Lastly, Ruiz-Gonzalez et al reported randomized trial of levofloxacin versus standard antibiotic therapy in AECOPD.Citation26 The standard therapy included clarithromycin, cefuroxime, and amoxicillin-clavulanic acid. Patients in the levofloxacin group showed a reduction in hospitalization rate compared with those in the standard antibiotic group. Therefore, the results of our study not only confirm previous studies but confirm the noninferiority of levofloxacin over cefuroxime in AECOPD.
This was a multicenter study done in seven university-affiliated hospitals, and all subjects were patients with COPD who had been previously diagnosed by spirometry.
However, it also has some limitations, in particular its nonblinded design. We cannot exclude the possibility that open-label treatment might have influenced our judgment of clinical efficacy and safety. A relatively small sample size is another shortcoming, in that studies of similar design to ours have had 600–800 subjects.Citation24,Citation25 Our study was underpowered with regard to the primary outcome, so we cannot reach any conclusions regarding microbiologic response. The lack of a placebo arm is also problematic; however, because our study included patients with severe exacerbations, it would have been unsafe and unethical to include a placebo group. Having adopted a subjective measure of success, nonblinding could be another limitation.
The efficacy of an antimicrobial agent is determined by many factor, including regional antimicrobial resistance. South Korea, where this study was conducted, has a very high rate of antimicrobial-resistant respiratory pathogens. For instance, pneumococcal resistance to penicillin ranges from 60% to 75%.Citation27–Citation29 Considering the fact that S. pneumoniae is the most frequent pathogen implicated in AECOPD, levofloxacin could be a more appropriate choice than macrolides or beta-lactams in treating AECOPD in Korea. In conclusion, levofloxacin is not inferior to cefuroxime in regard to clinical efficacy when treating AECOPD.
Acknowledgments
This study has been financially supported by Daiichi Sankyo Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
- SeemungalTARDonaldsonGCBhowmikAJeffriesDJWedzichaJATime course and recovery of exacerbations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001611608161310806163
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax20025784785212324669
- PietilaMPThomasCFInflammation and infection in exacerbations of chronic obstructive pulmonary diseaseSemin Respir Infect20031891612652449
- AlbertsonTELouieSChanALThe diagnosis and treatment of elderly patients with acute exacerbation of chronic obstructive pulmonary disease and chronic bronchitisJ Am Geriatr Soc20105857057920398122
- RamFSRodriguez-RoisinRGranados-NavarreteAGarcia-AymerichJBarnesNCAntibiotics for exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20062CD00440316625602
- McCroryDCBrownCGelfandSEBachPBManagement of acute exacerbations of COPD: a summary and appraisal of published evidenceChest20011191190120911296189
- SaintSBentSVittinghoffEGradyDAntibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysisJAMA19952739579607884956
- AnthonisenNRManfredaJWarrenCPHershfieldESHardingGKNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med19871061962043492164
- NouiraSMarghliSBelghithMBesbesLElatrousSAbrougFOnce daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trialLancet20013582020202511755608
- DanielsJMSnijdersDde GraaffCSVlaspolderFJansenHMBoersmaWGAntibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201018115015719875685
- WoodheadMBlasiFEwigSGuidelines for the management of adult lower respiratory tract infectionsEur Respir J2005261138118016319346
- MonsoERuizJRosellABacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brushAm J Respir Crit Care Med1995152131613207551388
- PelaRMarchesaniFAgostinelliCAirways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigationMonaldi Arch Chest Dis1998532622679785808
- SeemungalTHarper-OwenRBhowmikARespiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011641618162311719299
- BlasiFDamatoSCosentiniRChlamydia pneumoniae and chronic bronchitis: association with severity and bacterial clearance following treatmentThorax20025767267612149525
- SeemungalTAWedzichaJAMacCallumPKJohnstonSLLambertPAChlamydia pneumoniae and COPD exacerbationThorax20025710881089
- KolodziejMAJensenLRoweBSinDSystematic review of noninvasive positive pressure ventilation in severe stable COPDEur Respir J20073029330617459893
- SolerNTorresAEwigSBronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilationAm J Respir Crit Care Med1998157149815059603129
- FagonJYChastreJTrouilletJLCharacterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patientsAm Rev Respir Dis1990142100410082240819
- MiravitllesMEspinosaCFernandez-LasoEMartosJAMaldonadoJAGallegoMRelationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPDChest1999116404610424501
- EllerJEdeASchabergTNiedermanMSMauchHLodeHInfective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung functionChest1998113154215489631791
- SinDDMcAlisterFAManSFAnthonisenNRContemporary management of chronic obstructive pulmonary disease: scientific reviewJAMA20032902301231214600189
- QuonBSGanWQSinDDContemporary management of acute exacerbations of COPD: a systematic review and metaanalysisChest200813375676618321904
- ShahPMMaesenFPVDolmannAVetterNFissEWeschRLevofloxacin versus cefuroxime axetil in the treatment of acute exacerbation of chronic bronchitis: results of a randomized, doubleblind studyJ Antimicrob Chemother19994352953910350383
- PetitpretzPChoneCTremolieresFLevofloxacin 500 mg once daily versus cefuroxime 250 mg twice daily in patients with acute exacerbations of chronic obstructive bronchitis: clinical efficacy and exacerbation-free intervalInt J Antimicrob Agents200730525917512704
- Ruiz-GonzalezAGimenezAGomez-ArbonesXOpen-label, randomized comparison trial of long-term outcomes of levofloxacin versus standard antibiotic therapy in acute exacerbations of chronic obstructive pulmonary diseaseRespirology20071211712117207036
- ChongYLeeKParkYJKorean Nationwide Surveillance of Antimicrobial Resistance of bacteria in 1997Yonsei Med J19983956957710097685
- SongJHEmergence and spread of antimicrobial resistance of Streptococcus pneumoniae in KoreaYonsei Med J19983954655310097682
- BaqueroFPneumococcal resistance to beta-lactam antibiotics: a global geographic overviewMicrob Drug Resist199511151209158743