Abstract
Adult-onset asthma and chronic obstructive pulmonary disease (COPD) are major public health burdens. This review presents a comprehensive synopsis of their epidemiology, pathophysiology, and clinical presentations; describes how they can be distinguished; and considers both established and proposed new approaches to their management. Both adult-onset asthma and COPD are complex diseases arising from gene–environment interactions. Early life exposures such as childhood infections, smoke, obesity, and allergy influence adult-onset asthma. While the established environmental risk factors for COPD are adult tobacco and biomass smoke, there is emerging evidence that some childhood exposures such as maternal smoking and infections may cause COPD. Asthma has been characterized predominantly by Type 2 helper T cell (Th2) cytokine-mediated eosinophilic airway inflammation associated with airway hyperresponsiveness. In established COPD, the inflammatory cell infiltrate in small airways comprises predominantly neutrophils and cytotoxic T cells (CD8 positive lymphocytes). Parenchymal destruction (emphysema) in COPD is associated with loss of lung tissue elasticity, and small airways collapse during exhalation. The precise definition of chronic airflow limitation is affected by age; a fixed cut-off of forced expiratory volume in 1 second/forced vital capacity leads to overdiagnosis of COPD in the elderly. Traditional approaches to distinguishing between asthma and COPD have highlighted age of onset, variability of symptoms, reversibility of airflow limitation, and atopy. Each of these is associated with error due to overlap and convergence of clinical characteristics. The management of chronic stable asthma and COPD is similarly convergent. New approaches to the management of obstructive airway diseases in adults have been proposed based on inflammometry and also multidimensional assessment, which focuses on the four domains of the airways, comorbidity, self-management, and risk factors. Short-acting beta-agonists provide effective symptom relief in airway diseases. Inhalers combining a long-acting beta-agonist and corticosteroid are now widely used for both asthma and COPD. Written action plans are a cornerstone of asthma management although evidence for self-management in COPD is less compelling. The current management of chronic asthma in adults is based on achieving and maintaining control through step-up and step-down approaches, but further trials of back-titration in COPD are required before a similar approach can be endorsed. Long-acting inhaled anticholinergic medications are particularly useful in COPD. Other distinctive features of management include pulmonary rehabilitation, home oxygen, and end of life care.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are both chronic inflammatory diseases of the airways that induce airflow limitation. Asthma often starts in childhood, in such cases being commonly associated with allergies. It may remit and recur in adulthood,Citation1 or symptoms may continue throughout adolescence into adult life. Asthma may also develop de novo at any age, in some cases apparently triggered by a severe respiratory tract infection.Citation2 Asthma is characterized by intermittent and variable wheeze, chest tightness, and shortness of breath. COPD becomes apparent in middle to older age, but is now considered to have origins in early life.Citation3 COPD is characterized predominantly by gradually increasing dyspnea. Clinical features common to both include cough, mucus hypersecretion, wheeze, and intermittent exacerbations or “flare-ups”.
Asthma and COPD are usually considered to be distinct diseases and up until recently were associated with distinct approaches to diagnosis and management.Citation4–Citation6 However, it has become increasingly evident that differentiating asthma from COPD can be difficult, particularly in older populations. This is because older patients frequently exhibit features of more than one disease.Citation7–Citation9 This is commonly referred to as asthma–COPD overlap, and includes the coexistence of asthma, and emphysema or chronic bronchitis.Citation10,Citation11
Distinguishing between adult-onset asthma and COPD is a vigorously debated topic in respiratory medicine. This review compares and contrasts the current evidence on epidemiology, pathophysiology, diagnosis, and management of these two diseases.
Epidemiology of asthma and COPD
Burden of disease related to asthma and COPD
Adult-onset asthma and COPD have become much more common in the recent past and are now major public health problems in many countries.Citation12,Citation13 Asthma prevalence has increased in epidemic proportions over the last few decades and continues to rise in most parts of the world.Citation13 COPD burden is also set to increase during the next few decades, especially with the aging of the population and continued use of tobacco.
Adult-onset asthma differs from childhood asthma in that it is more often nonatopic and severe and has a lower remission rate.Citation14 Although asthma has a relatively low mortality in younger adults, in the elderly, it is associated with substantial morbidity, healthcare utilization,Citation15 and mortality.Citation16 The prevalence of current asthma in Australian adults is around 10%, which includes both childhood and adult-onset disease.Citation17 Substantial variation in the prevalence of adult asthma across 25 countries has been reported by the European Community Respiratory Health Survey, the largest international study of asthma in young adults.Citation18 This variation has been attributed more to differences in potential environmental risk factors than to genetics, as variation was observed even across countries with similar ethnic populations.
COPD is the fourth leading cause of death worldwide and expected to be the third leading cause by 2030.Citation19 However a systematic review of the health burden of COPD has shown that mortality has now started to decrease for men in some Western countries, whereas rates have continued to rise or plateau for women.Citation20
There is substantial variation in COPD prevalence across countries, and the use of various diagnostic classifications has been suggested as one reason. However, the findings of the Burden of Obstructive Lung Disease Study (BOLD) that used similar methodology across 12 countries suggest otherwise. BOLD estimated overall 11.8% of middle-aged to elderly men and 8.5% of similarly aged women as having COPD, identified using spirometric criteria,Citation12 but the prevalence varied significantly across study centers. Perhaps not surprisingly, the variation followed a similar geographical distribution to the variation in the prevalence of smoking.
Both diseases have substantial economic and social costs. These include both direct costs related to medical management and indirect costs related to disability, lost revenue from not attending work, and caring for patients. In Europe, 6% of the health budget is used for direct costs related to respiratory diseases, just over half of which are related to COPD.Citation21 A recent study in the UK found the annual per patient cost of COPD management, excluding medications, to be £2,108 for all patients, while this ranged from £1,523 to £3,396 for mild to severe COPD.Citation22 Annual per person costs for an adult with asthma have also been found to vary from $2,646 to $12,813 USD for mild to severe asthma in a sample of patients in California.Citation23
Definitions used in epidemiology
The demarcation between childhood-onset and adult-onset asthma is somewhat arbitrary, but adult-onset has been defined as from as young as 16 years.Citation24 The combination of airway hyperresponsiveness (AHR) and current asthma symptoms is a reliable indicator of clinically important asthma across age groups.Citation25 However, the presence of wheeze alone within the last 12 months has been used by many large epidemiological studies to determine current asthma prevalence regardless of the age of onset.Citation26–Citation28
Post-bronchodilator (BD) airflow obstruction is fundamental to the definition of COPD.Citation21 A clinical diagnosis of COPD also relies on the presence of coexistent symptoms and risk factors.Citation21 However, epidemiological studies have mainly adopted only the spirometric criterion.Citation12,Citation29,Citation30
The Global initiative for Obstructive Lung Disease (GOLD) endorses the spirometry criterion of post-BD forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70, with spirometric staging by the BOLD study also including an FEV1 less than 80% predicted.Citation12,Citation29 This fixed cut-off is susceptible to the risk of overdiagnosing a substantial number of older symptomatic individuals as having COPD,Citation31 which might contribute to the relative underdiagnosis of asthma in the elderly, particularly females. An alternative approach has been proposed to overcome this problem, whereby the cut-off is based on the lower limit of predicted normal.Citation32 This cut-off assumes the “usual progressive” lung function decline of COPD is greater than the physiological age-related decline of the FEV1/FVC ratio.
Risk factors for adult-onset asthma and COPD
Both asthma and COPD are acknowledged as complex diseases related to both genetic and environmental risk factors, but environment seems to play the stronger role. Although asthma has a high heritability, genetic studies to date have been able to explain only a small proportion of the variability. The evidence for a genetic etiology of adult-onset asthma is even less convincing, with neither family history nor atopy being clearly linked to this phenotype.Citation14 Homozygous α-1-antitrypsin deficiency (PiZZ) is the most common genetic risk factor for COPD, and the odds are also increased among heterozygous alpha-1-antitrypsin deficient (PiMZ) smokers.Citation30 Very recently, an interaction has been demonstrated between the PiMZ genotype and occupational exposures to vapors, gas, dust, and fumes, on annual decline in lung function.Citation33
A growing body of evidence suggests that early life and childhood factors are important in both adult-onset asthma and COPD.Citation34,Citation35 This concept is based on data that show associations between various adverse perinatal outcomes and harmful environmental stimuli, with slowing of lung function growth for susceptible children and adolescents.Citation36–Citation39 This effectively reduces peak lung function and impacts on the corresponding trajectory of lung function decline in adulthood. Such prenatal and postnatal factors include intrauterine growth restriction, prematurity, second-hand smoke exposure, air pollution, recurrent respiratory infections, and personal smoking.Citation35
Occupational exposures are major preventable risk factors for adult-onset asthma.Citation40,Citation41 During an individual’s “productive working years”, occupational asthma can frequently be overlooked, and from middle-age, symptoms may be attributed erroneously to COPD. A systematic inquiry into past and current occupational exposures is often required to confirm the diagnosis, as asthma might be still present after the exposure ceases.Citation4 A combined effect of occupational exposure and personal smoking has been shown to further increase the likelihood of airflow obstruction.Citation42
Nonoccupational risk factors for adult-onset asthma are heterogeneous and play a lesser role. These can be either childhood or adult exposures. Longitudinal studies have shown that childhood infections, tobacco smoke exposure, obesity, and allergic phenotypes influence adult-onset asthma.Citation43 In addition, female reproductive history, especially contraception,Citation44 menopause,Citation45 and hormone replacement therapyCitation46 is emerging as a strong risk factor for adult-onset asthma.
The patterns of COPD risk factors differ between geographical areas. In high- and middle-income countries, tobacco smoke is the biggest risk factor. However, in low-income countries, exposure to indoor air pollution, such as through the use of biomass fuels for cooking and heating, and occupational exposures, are more important.Citation47,Citation48 Personal tobacco smoking is the principal risk factor for COPD in industrialized countries; however, a meta-analysis has also shown an independent association for second-hand tobacco smoke exposure, which may be causal.Citation49 Traffic-related air pollution has been associated with COPD mortality,Citation50 as have decrements in lung function among people with asthma.Citation51,Citation52
Introduction to the pathophysiology of asthma and COPD
Asthma pathophysiology
Asthma is predominantly an airway disease, generally without involvement of lung parenchyma. It is viewed as a condition characterized by Type 2 helper T cell (Th2) cytokine-mediated eosinophilic airway inflammation. Bronchial biopsies from subjects with asthma demonstrate infiltration with eosinophils, activated mast cells, and Th2 predominant T cells.Citation53 There is also significant thickening due to collagen deposition in the basement membrane underpinning the bronchial epithelium. The concept of a variety of asthma syndromes is longstanding.Citation2 However, recent studies have highlighted the potential for the presence of a variety of different pathological asthma phenotypes, with some studies demonstrating neutrophilic or paucigranulocytic findings in sputum rather than eosinophilia in patients with a range of asthma severities.Citation2
Asthma is associated with AHR, which may be evident though reversibility to BD on spirometric testing or through responses to a variety of challenge tests. Asthma symptoms classically relapse and remit over time and are responsive to corticosteroids. However, over time, a proportion of individuals with asthma may develop some degree of persistent airflow obstruction, which does not remit with anti-inflammatory therapy.Citation54 In this case, differentiation from COPD may be very difficult. As around 30% of people with asthma smoke, a proportion of these will develop chronic airflow limitation, which is likely to be indistinguishable from COPD. Recent studies suggest that 13%–20% of patients with COPD have an overlap phenotype with asthma; this is as high as 50% in patients over 50 years.Citation54–Citation57
COPD pathophysiology
In COPD, repeated exposure to noxious stimuli triggers an inflammatory response, which appears to continue even after removal of the inciting stimulus.Citation58 Early inflammation in young smokers is associated with mononuclear cells in the airway wall and macrophages in the small airway lumen. In patients with established COPD, the inflammatory cell infiltrate in small airways comprises predominantly neutrophils, cluster of differentiation (CD)8 positive lymphocytes, and mast cells.Citation59 There may be associated “spill-over” of the inflammatory response from the lungs into the systemic circulation, leading to potential downstream effects such as arterial stiffness and its consequences.Citation60,Citation61
Although, as with asthma, there has been a tendency to “lump” patients with COPD into a homogeneous group, clinicians have for decades recognized variations in clinical phenotype of patients with COPD, which is underpinned, to an extent, by variations in the underlying pathology. Pathological features of small airways disease may predominate, with such patients having relatively minimal parenchymal destruction. Other patients may have relative sparing of airways and a more prominent picture of acinar destruction (emphysema). Parenchymal destruction is associated with loss of lung tissue elasticity, and small airways collapse during exhalation, leading to so-called “gas trapping”. Goblet cell metaplasia and impaired mucociliary function contribute to excess mucus accumulation and worsening obstruction.
An approach to grading and treating COPD based on clinical phenotypes aligned to pathology has been described in the recent Spanish COPD guidelines, which propose four different phenotypes including 1) infrequent exacerbators with either chronic bronchitis or emphysema, 2) asthma–COPD overlap, 3) frequent exacerbators with emphysema predominant, and 4) frequent exacerbators with chronic bronchitis predominant.Citation62 It has been proposed that such clinical phenotypes may help clinicians identify patients that respond to specific pharmacological interventions.
The role of age in both diseases
Age-related features
Asthma of an earlier onset is closely linked to other allergic diseases and to skin test sensitivity.Citation63 Most children with the persistent asthma phenotype have current symptoms as adults; however, around half are in remission by mid-adulthood.Citation64 Compared to childhood-onset asthma, asthma of adult onset is more likely among nonatopic females and features a greater fall in lung function despite a shorter duration of disease.Citation63,Citation65 A history of allergic rhinitis, and less so, atopic dermatitis, were associated with retrospectively recalled history of physician-diagnosed asthma from age 16 years for young to middle-aged adults in the European Community Respiratory Health Survey.Citation24 However, there was no association between skin test positivity and adult-onset asthma for middle-aged adults followed for 14 years in the Busselton Health Study.Citation40
The diagnostic value of atopy in distinguishing adult-onset asthma from COPD decreases with advancing age despite increasing levels of sensitization until mid-adult life, which plateau after the age of 55 years.Citation66 The adult-onset asthma phenotype shares similar clinical features with the noneosinophilic asthma phenotype and like COPD is associated with greater corticosteroid resistance when compared with younger individuals with eosinophilic asthma.Citation67
For those with childhood asthma who become asymptomatic by mid-adolescence, a recurrence of asthma symptoms in adulthood may be misclassified as “adult-onset asthma” if the past childhood history cannot be recalled.Citation68,Citation69 One epidemiological study that prospectively documented childhood asthma and/or wheezy breathing when participants were aged 7 years, identified two-thirds of young adults as being unable to accurately recall their childhood history, and this was mostly for those without severe eczema or allergic rhinitis.Citation69
COPD generally manifests clinically from mid-adult life, with increasing prevalence and disease severity with advancing age.Citation12 The rate of disease progression varies between individuals, with an accelerated FEV1 decline more common for current smokers and those with BD-reversibility, emphysema, and/or acute exacerbations.Citation70 The link between advanced age and COPD may be weakening for populations of developed countries given that the probability of asthma as the cause for underlying symptoms for an older individual seems to have increased, particularly for countries of high asthma prevalence. This requires validation by prospective cohorts that use consistent asthma and COPD definitions over time.
Apart from respiratory infection, adults with asthma appeared to have less comorbidity than those with COPD.Citation71 COPD is associated with multiple comorbidities including ischemic heart disease, cardiac failure, osteoporosis and muscle weakness, obesity and the metabolic syndrome, depression and anxiety, lung cancer, pneumonia,Citation71 and pulmonary embolism.Citation72 Although these may be due in part to the common risk factor of smoking, the development of the comorbidities may also be linked by systemic inflammation driven by the elevation of proinflammatory mediators and their receptors, including tumor necrosis factor alpha, interleukin (IL)-6, and IL-1 receptor antagonist IL-1Ra, leading to an increased production of diverse chemokines (RANTES, MIP-1α, IL-8, MCP-1) and an increase in C-reactive protein.Citation61,Citation73–Citation75
Altered perception of airway obstruction
Some data have suggested that for people over 65 years, those with adult-onset asthma of a longer duration may have more severe airway obstruction than those with a more recent asthma diagnosis, yet they report fewer asthma symptoms.Citation76 This might adversely influence the implementation of an asthma action plan, which relies on the accurate interpretation of asthma symptoms.Citation77 Older individuals might attribute respiratory symptoms to bronchitis, respiratory tract infection, obesity, poor fitness, or even to the “aging process”Citation77 and be more accepting of a progressive decline in mobility and activity.Citation4
Differential diagnosis of adult asthma and COPD
Dyspnea due to cardiac failure is a common and important differential diagnosis for adult-onset asthma and COPD in the elderly, including acute exacerbations of COPD.Citation72 Bronchiectasis is another important differential diagnosis for nonsmoking individuals with post-BD airflow obstruction and a productive cough, particularly for females. Bronchiectasis can complicate severe asthma in the form of allergic bronchopulmonary aspergillosis, where identifying proximal bronchial dilatation on high resolution computed tomography (HRCT) chest scan may be used to confirm the diagnosis. An accelerated FEV1 decline has been observed for those with clinically apparent bronchiectasis despite medical therapy, averaging 49 mL per year over an 8 year period.Citation78 Around half of older COPD patients with moderate to severe airflow obstruction have been documented to have radiologically-defined bronchiectasis when screened with HRCT chest scans.Citation79,Citation80 As bronchiectasis is frequently associated with AHRCitation81 and/or BD-reversibility, it seems possible that this entity could contribute to the overlap between asthma and COPD. Bronchiectasis in the presence of COPD is also a poor prognostic marker, where its severity has been independently associated with increases in all-cause mortality.Citation80
Asthma
Clinical practice guidelines propose diagnostic criteria to assist clinicians in distinguishing between asthma and other obstructive airway diseases.Citation4 A diagnosis of asthma is made following a clinical assessment of symptoms and demonstration of variable airflow obstruction. This hallmark feature of asthma can be assessed by performing pre-BD and post-BD spirometry.Citation4 An improvement of FEV1 and/or FVC of greater than 12% or 200 mL is considered a significant BD response, consistent with asthma.Citation82 However, such BD responses may also be seen in patients with COPD,Citation83 and a subgroup of patients with long standing asthma do not demonstrate reversible airflow obstruction,Citation84 which creates diagnostic challenges for clinicians.
Another diagnostic measure used in the assessment of asthma is AHR, a term that describes the ability of the airways to narrow excessively after exposure to bronchoconstrictor agonists.Citation85 AHR plays a crucial role in the pathogenesis of asthma,Citation85 and the severity of AHR predicts the response to inhaled corticosteroids.Citation86 However, in an older population, AHR is a common and important determinant of accelerated decline in lung function.Citation87 This makes AHR a risk factor for the progression of airflow limitation in COPD,Citation88 and while AHR is a common physiological component of asthma, it is also frequently present in patients with COPD.
COPD
As previously mentioned, there are disease-specific guidelines and recommendations that propose diagnostic criteria to assist clinicians in the diagnosis of COPD.Citation89 The GOLD recommendations define a post-BD FEV1/FVC of less than 0.7 as diagnostic of COPD.Citation5,Citation21 These recommendations also define spirometric indexes for grading severity, and treatment recommendations are based on this severity grading scale.Citation5,Citation21
A normal gas transfer factor in symptomatic smokers with airflow obstruction is an inexpensive and simple measurement to exclude significant morphological emphysema, whereas variable amounts of emphysema can be present for those with low gas transfer factor levels.Citation90 The use of HRCT can identify individuals with a predominant emphysematous phenotype, where the extent of low attenuation areas has been linked to increased respiratory mortality.Citation91 These data suggest that the identification of COPD-associated emphysema and/or bronchiectasis has some prognostic value, although HRCT scans are not currently recommended in the routine clinical management of COPD.Citation21
Differentiating adult asthma and COPD
In differentiating the two diseases a number of factors are often considered (), including
Table 1 Common symptoms, physiology, and pathology used to distinguish asthma and COPD and the common problems with this approach
accurate medical history involving assessment of age of symptom onset and variability of symptoms;
reversibility of airflow obstruction and BD response;
smoking and occupational history;
response to treatment trials;
and atopic status.
Each of these, however, is associated with error due to the overlap and convergence of symptoms and clinical characteristics. We have outlined the traditional approach to differentiating asthma and COPD and the problems associated with this approach in .
Recently, airway inflammation has been frequently but erroneously considered in the differential diagnosis of asthma and COPD. While the assessment of airway inflammation can assist in the guidance of treatment decisions, it does not differentiate between diagnoses due to the heterogeneity of inflammation across and within disease groups.
The question that must be asked then is, does it matter? One argument is to justify alternative management approaches. However, treatments for asthma and COPD have tended to converge, and there is a large degree of overlap for treatment recommendations in these diseases.Citation10 This is discussed further in the next section.
As a counter argument, COPD prognosis differs significantly from that of asthma, and this may also apply to asthma–COPD overlap. In a recent longitudinal study, Fu et al evaluated and compared clinical outcomes of patients with asthma, COPD, and asthma–COPD overlap at baseline and 4 years.Citation103 The results showed significantly greater decline in 6 minute walk distance at 4 years in the COPD group compared with the asthma and the overlap groups. In another cross sectional study, patients with overlap of asthma and COPD had significantly poorer quality of life and were more likely to have experienced a severe exacerbation (odds ratio 3.55, 95% confidence interval 1.76, 7.15) of their airways disease in the last year, than those with COPD alone.Citation104
The differentiation of asthma and COPD is particularly difficult in older people, where the common pathophysiological components of airflow obstruction, AHR, and airway inflammation overlap and converge and where, in patients over the age of 55 years, overlap of asthma and COPD occurs in up to 50% of individuals.Citation10,Citation55,Citation56
New approaches using inflammometry
In recognition of this difficulty with traditional approaches, alternative approaches to the assessment and management of obstructive airway diseases in adults have been proposed.Citation9–Citation11,Citation97,Citation105 These new approaches challenge the rigid categorization of patients into existing diagnostic labels of either asthma or COPD and suggest the importance of multiple clinical, functional, immunological, and molecular assessments that may be used to tailor and optimize treatments.Citation9,Citation105–Citation108
Central to these approaches is inflammometry: the measurement of inflammation to guide treatment decisions.Citation97 Importantly, in this management approach, the presence of inflammatory cells in the airways does not diagnose asthma or COPD, but their measurement can be useful for clinical assessment in guiding treatment decisions and in long-term monitoring. High quality evidence from many well-designed clinical trialsCitation109–Citation111 and a systematic reviewCitation112 report the superior effects of targeting airway eosinophilic inflammation on exacerbation reduction in both asthma and COPD. Airway inflammation in both asthma and COPD is heterogeneous, with four different airway inflammatory phenotypes having been described ():
Figure 1 Airway inflammation phenotypes present in sputum from asthma and COPD patients.
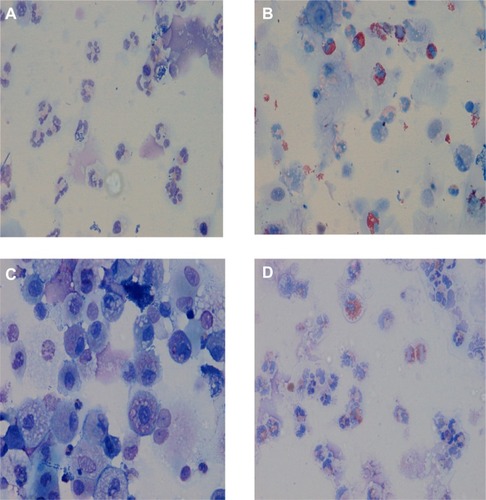
Eosinophilic
Neutrophilic
Paucigranulocytic
Mixed eosinophilic and neutrophilic.
Although traditionally asthma is considered an eosinophilic disease and COPD is associated with airway neutrophilia, the heterogeneity of sputum cellularity in these diseases is now increasingly recognized, with many studies reporting airway eosinophilia in COPD and airway neutrophilia in asthma.Citation96,Citation102,Citation107,Citation113,Citation114
In a large cross sectional study of patients with airways disease, D’Silva et al reported the cellular profile of over 4,000 induced or spontaneous sputum samples.Citation101 In the group with asthma–COPD overlap, eosinophilic bronchitis was seen in 35%, neutrophilic bronchitis in 19%, and a mixed inflammatory pattern in 10%. In COPD, the phenotypes were respectively 18%, 34%, and 7%, and in asthma alone 26%, 14%, and 6%. These data demonstrate the heterogeneous nature of airway inflammation in asthma and COPD. The assessment of airway inflammation in airways disease may be important in order to tailor treatment more effectively.
Gonem et al proposed an approach that tailors treatment using a classification system that takes into account a number of pathophysiological components rather than disease labels.Citation105 The A to E system includes assessment and management of AHR, Bronchitis (airway inflammatory phenotype), Cough reflex sensitivity, Damage (relating to BD and corticosteroid-resistant airflow obstruction resulting from airway and parenchymal damage), and Extra pulmonary factors. This system remains an important current research area.
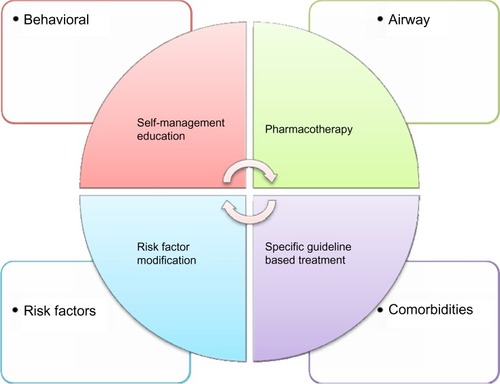
Gibson et al,Citation10 McDonald et al,Citation11 and McDonald and GibsonCitation115 propose an approach targeted at older patients and those with difficult airways disease. They suggest a model of multidimensional assessment and individualized management focusing on the four domains: airways, comorbidity, self-management, and risk factors (). In a pilot study comparing management using this approach to usual care, the intervention achieved significant improvements in health status as well as in both airway and systemic inflammation. This study suggested that a personalized approach was feasible and could result in superior outcomes, but larger studies are required.Citation107
Figure 2 Model of disease components and individualized treatment approach.
Differential management of adult asthma and COPD
Guidance for clinicians around the world is provided by the Global Initiative for AsthmaCitation4 and GOLD.Citation21 However, clinical practice guidelines need to be adapted to national and local conditions.Citation89,Citation116 The detailed management of acute exacerbations is beyond the scope of this review.
There are features common to the management of both adult-onset asthma and COPD. Both conditions require the development of a partnership between patients and their health care providers. Both conditions also require the identification and reduction of exposure to risk factors.Citation4 A short-acting beta-agonist such as salbutamol or terbutaline provides effective symptom relief in both asthma and COPD and may be the only medication that is required in mild intermittent asthma.
Optimal inhaler technique involving accurate coordination of device actuation and inhalation are necessary for appropriate delivery of any inhaled drug. These require adequate cognitive function, manual dexterity, and strength of the intrinsic muscles of the hand. Elderly individuals can have poor device technique although competence can improve through the use of education as well as breath-actuated devicesCitation117 or large volume spacers.Citation118 Given that device selection can also be driven by convenience, cost, and patient preference, regular objective monitoring of inhaler technique and adherence is essential.Citation117,Citation119
Combination inhalers containing a long-acting beta-agonist (LABA) and corticosteroid are widely used both in asthma and COPD. Preventer (or controller) medication is re commended for adults who report asthma symptoms twice or more during the past month, waking due to asthma symptoms once or more during the past month, or an asthma flare-up in the previous 12 months.Citation116 A single fixed dose combination inhaler may enhance adherence to inhaled corticosteroids in asthma,Citation120 and several randomized controlled trials suggest that single inhaler therapy reduces asthma exacerbations requiring oral corticosteroids, hospitalization, or emergency visits.Citation121 The TOwards a Revolution in COPD Health (TORCH) trial found a modest reduction in mortality among COPD patients who were randomized to the combination of salmeterol and fluticasone compared to placebo.Citation122 However, those who received the inhaled corticosteroid appeared to have an increased risk of pneumonia, although this was not radiologically confirmed. A meta-analysis of 24 randomized controlled trials suggested that the increased risk of pneumonia might be a class effect of inhaled corticosteroids.Citation123
Systemic corticosteroids are generally recommended in patients with moderate or severe exacerbations of asthma. Oral corticosteroids are as effective as injected corticosteroids, provided they can be swallowed and retained.Citation116 Prednisolone 40–50 mg daily (or parenteral hydrocortisone 400 mg daily) is as effective as higher doses in hospitalized patients with severe exacerbations of asthma.Citation124 Similarly, systemic corticosteroids reduce the risk of treatment failure and the need for additional treatment for an exacerbation of COPD, as well as shortening hospital stay.Citation125 A short course of 40 mg prednisone daily for 5 days was recently shown to have non-inferior outcomes to a 2-week course at the same dosage.Citation126 Because of the risk of adverse effects, long-term use of oral corticosteroids is not recommended in either asthma or COPD. Specific complications include osteoporosis, weight gain, immobility, insomnia, emotional lability, glucose intolerance and diabetes mellitus, susceptibility to infection, skin atrophy, impaired wound healing, fluid retention, and increased cardiovascular risk.Citation127
Distinctive features of asthma management
Written asthma action plans are a cornerstone of asthma management in adults. A systematic review found that optimal self-management allowing for optimization of asthma control by adjustment of medications may be conducted by either self-adjustment with the aid of a written action plan or by regular medical review.Citation128 Having a written action plan was also associated with a reduced risk of death from asthma.Citation129 Although widely recommended in guidelines, the evidence for self-management in COPD is less compelling. A systematic review of 29 randomized controlled trials of self-management education (SME) compared to usual care found that SME in COPD led to a significant reduction in respiratory related hospital admission and improved health related quality of life and dyspnea. While the authors concluded that SME improves these outcomes, due to the heterogeneity of the interventions, study populations, follow-up time, and outcome measures data are still insufficient to formulate clear recommendations regarding the form and contents of SME programs in COPD.Citation130 Another systematic review concluded that giving patients an action plan and limited SME for the management of COPD exacerbations could not be recommended as the standard of care.Citation131
The stepwise approach
The current management of asthma in adults is based on achieving asthma control and involves a stepwise approach,Citation4 titrating the inhaled corticosteroid dose, and adding other medications such as LABA, leukotriene modifier, oral corticosteroid, and monoclonal antibody therapy as necessary. When control improves, the dose of inhaled corticosteroid can be reduced and/or other medications ceased (). However, there are currently few published trials of back-titration in COPD, so once commenced, many patients with COPD typically remain on high doses of inhaled corticosteroids. Back-titration studies are difficult given the challenges of reconciling the numerous COPD phenotypes that reflect the marked heterogeneity of the disease.Citation132,Citation133 A systematic review of three adequate quality trials of back-titration in COPD found that patients who had medication withdrawn were 1.11 (95% confidence interval 0.84, 1.46) times more likely to have an exacerbation in the following year.Citation134 The results of the Withdrawal of Inhaled Steroids During Optimised bronchodilator Management (WISDOM) TrialCitation135 are awaited with interest.
Figure 3 Stepped approach to adjusting asthma medication in adults.
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting beta-agonist; SABA, short-acting beta-agonist.
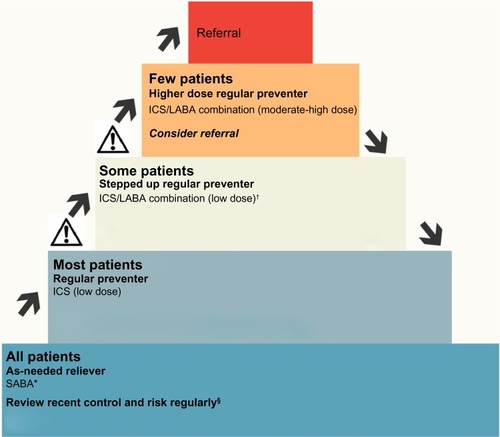
Leukotriene receptor antagonists such as montelukast are an alternative oral preventer in mild to moderate asthma. They probably only have a limited role in adult patients, particularly those with aspirin or exercise-induced asthma.Citation136 A systematic review of 56 randomized controlled trials found that monotherapy with inhaled corticosteroids was superior to leukotriene antagonists in reducing the requirement for rescue systemic corticosteroids or hospital admission, especially in adult patients with moderate airflow obstruction.Citation137
As asthma is primarily an allergic disease, even in adults, new biological agents are being trialed targeting key molecules in the inflammatory pathways. Omalizumab is a monoclonal antibody directed against immunoglobulin E. A recent systematic review of 25 randomized controlled trials found that compared to placebo, subcutaneous omalizumab reduced the risk of exacerbations and permitted many patients to reduce or withdraw from inhaled corticosteroids.Citation138 Mepolizumab is another monoclonal antibody directed against the cytokine IL-5. There are clinical trials showing that mepolizumab reduces exacerbations and improves quality of life in patients with refractory eosinophilic asthma.Citation139 However, the high cost of these biological agents means that they can only be considered for patients with difficult to control asthma managed in specialist facilities, where patient selection and management is based on targeted phenotypes.Citation140
Distinctive features of COPD management
Stable COPD requires both nonpharmacological and pharmacological management. illustrates a stepwise approach to guide treatment decisions.
Figure 4 Stepwise management of Stable COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; COPD-X, Confirm diagnosis, Optimise function, Prevent deterioration, Develop support, manage eXacerbations; FEV1, forced expiratory volume in 1 second; GP, general practitioner; ICS, inhaled corticosteroids; LABA, long-acting beta-agonist.

Smoking cessation
The major risk factor for COPD is active tobacco smoking,Citation21 so particular emphasis needs to be given to smoking cessation and maintenance of abstinence. There are now a wide range of interventions including telephone counseling,Citation141 nicotine replacement therapies,Citation142 and newer pharmacological agents such as bupropion/nortriptylineCitation143 and varenicline,Citation144 which are effective particularly when combined with behavioral support.Citation145 New methods of delivery such as pharmacist-led interventions are being trialed in hospitalized patients.Citation146 Of course, smoking cessation is also important in asthma as continued smoking can lead to fixed airflow limitation in susceptible patients.
Antimuscarinic agents
Long-acting inhaled antimuscarinic medications such as tiotropium, aclidinium, and glycopyrronium are particularly useful in COPD. Randomized controlled trials have demonstrated improvements in dyspnea, lung function, and quality of life compared to placebo or LABA.Citation147 Tiotropium is also associated with a reduction in and prolongation of time to exacerbations.Citation148 However, there does not appear to be any reduction in the rate of decline in lung function.Citation149 The benefits come with an increase in adverse effects such as dry mouth and urinary retention.Citation150 Some evidence is now emerging regarding the benefit of tiotropium in asthma.Citation151,Citation152
Pulmonary rehabilitation
Pulmonary rehabilitation programs involve patient assessment, exercise training, education, nutritional intervention, and psychosocial support. Systematic reviews have shown that in patients with COPD, these programs reduce dyspnea, fatigue, anxiety and depression; improve exercise capacity, emotional function, and health-related quality of life; and enhance patients’ sense of control over their condition.Citation153 Pulmonary rehabilitation also reduces the length of hospitalization in COPD patientsCitation154 and is cost effective.Citation155 In patients with asthma–COPD overlap, exercise training can improve symptoms and health status.Citation156
Although access to comprehensive pulmonary rehabilitation varies with location, exercise training is an effective option in the management of patients with COPD if multidisciplinary education cannot be offered.Citation157
Action plans and self-management
Action plans for exacerbations are effective in asthma,Citation158 allowing patients to develop coping skills, anticipate early exacerbation symptoms, self-initiate appropriate treatment, and seek medical advice prior to significant deterioration. Trials assessing the effects of action plans in COPD management have shown conflicting results, with variable adjuncts to patient care likely contributors. Those with positive results, such as expedited exacerbation recovery and reduced hospital admissions, have included additional supports, such as intensive education and case management.Citation159–Citation161 In contrast, action plans with limited or no SME and no case management have little beneficial effect.Citation131
A recent randomized controlled trial that suggested an unexpected increase in all-cause and COPD-specific mortality with a comprehensive care management program including a COPD action plan for US veterans,Citation162 highlighted the value of identifying those with adult-onset asthma for whom the benefits are well-documented. Putting this disturbing finding into the context of the COPD literature is important, and identifying factors predisposing to a poor outcome will be a challenge for those involved in developing clinical practice guidelines. While the association did not appear related to increasing age or COPD severity,Citation162 these findings suggest that self-management programs may not be appropriate for all patients with COPD.
Home oxygen
The use of domiciliary oxygen is common at the more severe end of the COPD spectrum. Indications to consider referral for assessment of need for long-term continuous oxygen therapy include severe airflow obstruction (FEV1 <30% predicted), presence of cyanosis, polycythemia, signs of right heart failure, saturation of ≤92% breathing room air.Citation163 Long-term continuous oxygen therapy has been proven to offer survival benefits and improved quality of life in patients with COPD and severe hypoxemia (arterial blood partial pressure of O2 ≤55 mmHg or 55–59 mmHg with evidence of end-organ damage).
However, the role of oxygen therapy in patients with exertional desaturation, nocturnal hypoxemia, or resting mild-moderate hypoxemia is less clear. Recent studies suggest an absence of long-term effects on dyspnea or quality of life from the use of ambulatory oxygen therapy in normoxemic or mildly hypoxemic patients with COPD who desaturate with exertion, even though they may demonstrate small acute benefits during laboratory-based exercise tests.Citation164,Citation165 Occasional so-called “n-of-1 trials” may be of use in some individuals.Citation165
Isolated nocturnal hypoxemia is not uncommon in COPD patients, particularly during rapid eye movement sleep. However, it has not been shown to lead to worse quality of life, daytime hypoxemia, or pulmonary hypertension. Limited studies have not consistently shown beneficial effects in sleep quality, pulmonary hemodynamics, or survival over 2 years with nocturnal supplemental oxygen.Citation166–Citation168 Similarly, patients with COPD and resting mild-moderate hypoxemia have not shown a survival benefit with domiciliary oxygen therapy. The currently recruiting US Long-term Oxygen Treatment Trial (NCT0692198) may provide more data regarding the effects of domiciliary oxygen in the latter patient subgroup.
End of life care
The disease trajectory for COPD is one of deteriorating health status and increasing morbidity, usually over a period of time. This differs from the more predictable trajectory of most cancers and as such, the most appropriate timing for discussions around end of life issues in COPD and referral time for palliative care are not clear.Citation169 Despite this difficulty, primary care providers and specialist COPD teams have an integral role in developing partnerships with patients and carers to provide information about prognosis, and over time, should introduce topics regarding intubation, admission to intensive care unit, and patients’ views on “not for resuscitation” or medical treatment orders. This involves a discussion regarding quality of life and choices they wish to consider.Citation89 A useful resource to guide patients in making these choices is the Advance Care Planning website (http://www.respectingpatientchoices.org.au). Opioids may have a role in patients with severe intractable dyspnea.Citation170
Qualitative data suggest that there is a need for clinicians to improve their approach to end of life care in COPD. In one study, participants discussed their expectations about the future; this may have been fear about their future outcome or an idealistic expectation that they would not get any worse. These data suggest that participants did not have a clear understanding of their disease or of how it would progress.Citation171 This is in accord with a study that interviewed 16 COPD patients within the last year of life. Even at this late stage, half of the participants expressed a desire for more information about their disease and its prognosis.Citation172
Areas for further research
Although the importance and management of asthma–COPD overlap remains a strongly debated topic, there are few data to inform practice and policy. This is because large scale randomized trials have largely excluded patients with asthma from COPD trials and those with COPD from asthma studies.Citation173,Citation177 Furthermore, epidemiological research to date has mainly treated these as two distinct groups of diseases and, therefore, not much evidence is available on the overlapping or distinctly separate groups. A number of clinical and epidemiological research questions remain outstanding.
An area that needs research is the current classifications for obstructive lung diseases. These classifications are complex, with asthma regarded as multiple overlapping syndromes,Citation174 and the heterogeneity of both stable COPD and its acute exacerbations being increasingly recognized.Citation133 The GOLD strategy document has progressively acknowledged the overlap between asthma and COPD, and progress is being made on a joint Global Initiative for Asthma/GOLD asthma–COPD overlap document. Other factors besides current or past smoking which may contribute to persistent airflow obstruction in asthma include severe symptoms, long-standing disease, old age at onset, frequent exacerbations, ongoing exposures to allergens and occupational irritants, aspirin sensitivity, elevated serum immunoglobulin E, and ongoing airway inflammation and hyperresponsiveness.Citation175 With increasing recognition of this overlap phenotype, further revisions of the current taxonomy are likely to be developed.Citation175 Given the limited data available for this subgroup,Citation173,Citation176 developing targeted management-specific guidelines poses challenges.
Clinical questions that need research include whether the unopposed use of LABA in asthma–COPD overlap is problematic, as it is in asthma without COPD.Citation177 Likewise, does inhaled corticosteroid therapy in asthma–COPD overlap cause the same pneumonia risk as in COPD alone? Furthermore, back-titration of therapy is recommended in asthma as disease control improves. The approach to management involving back-titration in asthma–COPD overlap warrants further investigation. Finally, further clinical studies recruiting the population with asthma–COPD overlap and testing novel and personalized approaches are needed. The newly proposed system of the tailored treatment approach, which uses a classification system based on a number of pathophysiological components, remains an important current research area.Citation105 A pilot study has shown that a personalized approach improved health status as well as both systemic and airway inflammation, but larger studies are required to confirm these findings.Citation107
There has been increasing interest in understanding how long-standing asthma can lead to irreversible airflow limitation.Citation34,Citation49,Citation65,Citation84 In this context, distinguishing between adult-onset asthma and COPD can be especially difficult for the high proportion of adults with asthma who have smoked. It has been suggested that risk factors for the multiple phenotypes related to overlaps between asthma and COPDCitation57 could vary.Citation7 Understanding risk factors for these multiple phenotypes is critical to advancing knowledge of causal mechanisms and modifiable risk factors for both conditions.
Conclusion
Current clinical practice is based upon distinguishing between adult-onset asthma and COPD. There are separate guidelines for each condition. However, it is not always possible to clearly differentiate between these obstructive lung diseases because of common risk factors, such as tobacco smoking, and the convergence of symptoms and other clinical features. Studies based on newer approaches, for example, inflammometry, may result in better clinical outcomes. Further randomized controlled trials of these and other novel approaches, for example, a multidimensional approach targeting individualized management, should provide important data to inform future guidelines. The management of asthma and COPD is increasingly converging. This has already occurred for pharmacological therapies such as combination inhalers. Written action plans and self-management currently seem to be more useful in asthma than COPD. Further research is needed to examine back-titration of inhaled corticosteroids in COPD and to determine the best management of the common asthma–COPD overlap syndrome.
Disclosure
The authors report no conflicts of interest in this work.
References
- BurgessJAMathesonMCGurrinLCFactors influencing asthma remission: a longitudinal study from childhood to middle ageThorax201166650851321450787
- PavordIDNon-eosinophilic asthma and the innate immune responseThorax200762319319417329554
- SvanesCSunyerJPlanaEEarly life origins of chronic obstructive pulmonary diseaseThorax2010651142019729360
- Global Initiative for Asthma [homepage on the Internet]Global Strategy for Asthma Management and Prevention2011 Available from: http://www.ginasthma.org/GINA-Report,-Global-Strategy-for-Asthma-Management-and-PreventionAccessed March 21, 2014
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGOLD Scientific CommitteeGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med200116351256127611316667
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- MarshSETraversJWeatherallMProportional classifications of COPD phenotypesThorax200863976176718728201
- GibsonPGSimpsonJLThe overlap syndrome of asthma and COPD: what are its features and how important is it?Thorax200964872873519638566
- GuerraSAsthma and chronic obstructive pulmonary diseaseCurr Opin Allergy Clin Immunol20099540941619638929
- GibsonPGMcDonaldVMMarksGBAsthma in the older adultsLancet2010374974380381320816547
- McDonaldVMHigginsIGibsonPGManaging older patients with coexistent asthma and chronic obstructive pulmonary disease: diagnostic and therapeutic challengesDrugs Aging201330111723229768
- BuistASMcBurnieMAVollmerWMBOLD Collaborative Research GroupInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- MasoliMFabianDHoltSBeasleyRGlobal Initiative for Asthma (GINA) ProgramThe global burden of asthma: executive summary of the GINA Dissemination Committee reportAllergy200459546947815080825
- de NijsSBVenekampLNBelEHAdult-onset asthma: is it really different?Eur Respir Rev201322127445223457164
- National Heart Lung and Blood Institute [homepage on the Internet]Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung and Blood Diseases; 2012 [updated 2013]Washington, DCNational Institutes of Health, US Department of Health and Human Services Available from: http://www.nhlbi.nih.gov/resources/docs/cht-book.htmAccessed March 21, 2014
- McDonaldVMGibsonPGAsthma mortality and management in older Australians: time for a new approach?Australas J Ageing200827421519032626
- Australian Centre for Asthma MonitoringAsthma in Australia 2011: with a focus chapter on chronic obstructive pulmonary disease [webpage on the Internet]CanberraAustralian Institute of Health and Welfare2011 Available from: http://www.aihw.gov.au/publication-detail/?id=10737420159Accessed May 9, 2014
- BurneyPChinnSJarvisDLuczynskaCLaiEEuropean Community Respiratory Health SurveyVariations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS)Eur Respir J1996946876958726932
- LopezADMathersCDEzzatiMJamisonDTMurrayCJGlobal and regional burden of disease and risk factors, 2001: systematic analysis of population health dataLancet200636795241747175716731270
- RycroftCEHeyesALanzaLBeckerKEpidemiology of chronic obstructive pulmonary disease: a literature reviewInt J Chron Obstruct Pulmon Dis2012745749422927753
- Global strategy for the diagnosis, management, and prevention of COPD [webpage on the Internet]Global Initiative for Chronic Obstructive Lung Disease2014 Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.htmlAccessed March 21, 2014
- PunekarYSShuklaAMüllerovaHCOPD management costs according to the frequency of COPD exacerbations in UK primary careInt J Chron Obstruct Pulmon Dis20149657324426781
- CisternasMGBlancPDYenIHA comprehensive study of the direct and indirect costs of adult asthmaJ Allergy Clin Immunol200311161212121812789219
- TorénKHermanssonBAIncidence rate of adult-onset asthma in relation to age, sex, atopy and smoking: a Swedish population-based study of 15813 adultsInt J Tuberc Lung Dis19993319219710094318
- ToelleBGPeatJKSalomeCMMellisCMWoolcockAJToward a definition of asthma for epidemiologyAm Rev Respir Dis199214636336371519839
- ZhangXMorrison-CarpenterTHoltJBCallahanDBTrends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009BMC Public Health201313115624325173
- PearceNAït-KhaledNBeasleyRISAAC Phase Three Study GroupWorldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC)Thorax200762975876617504817
- McHughMKSymanskiEPompeiiLADelclosGLPrevalence of asthma among adult females and males in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2001–2004J Asthma200946875976619863277
- ToelleBGXuanWBirdTERespiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) studyMed J Aust2013198314414823418694
- MolloyKHershCPMorrisVBClarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotesAm J Respir Crit Care Med2014189441942724428606
- HnizdoEGlindmeyerHWPetsonkELEnrightPBuistASCase definitions for chronic obstructive pulmonary diseaseCOPD2006329510017175672
- QuanjerPHPrettoJJBrazzaleDJBorosPWGrading the severity of airways obstruction: new wine in new bottlesEur Respir J201443250551223988764
- MehtaAThunGAImbodenMSERPINA1 PiMZ genotype, environmental and occupational exposure, and change in lung functionPaper presented at: ISEE Environment and HealthAugust 19–23, 2013Basel, Switzerland
- SternDAMorganWJHalonenMWrightALMartinezFDWheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort studyLancet200837296431058106418805334
- StocksJHislopASonnappaSEarly lung development: lifelong effect on respiratory health and diseaseLancet Respir Med20131972874224429276
- GaudermanWJVoraHMcConnellREffect of exposure to traffic on lung development from 10 to 18 years of age: a cohort studyLancet2007369956157157717307103
- LandauLITobacco smoke exposure and tracking of lung function into adult lifePaediatr Respir Rev2008913943 quiz 4318280978
- GaudermanWJAvolEGillilandFThe effect of air pollution on lung development from 10 to 18 years of ageN Engl J Med2004351111057106715356303
- GillilandFDBerhaneKLiYFRappaportEBPetersJMEffects of early onset asthma and in utero exposure to maternal smoking on childhood lung functionAm J Respir Crit Care Med2003167691792412480608
- JamrozikEKnuimanMWJamesADivitiniMMuskAWRisk factors for adult-onset asthma: a 14-year longitudinal studyRespirology200914681482119703063
- HoyRFBurgessJABenkeGOccupational exposures and the development of new-onset asthma: a population-based cohort study from the ages of 13 to 44 yearsJ Occup Environ Med201355323523923439266
- BlancPDIribarrenCTrupinLOccupational exposures and the risk of COPD: dusty trades revisitedThorax200964161218678700
- SvanesCWhat has the ECRHS told us about the childhood risks of asthma, allergy and lung function?Clin Respir J20082Suppl 1344420298348
- JenkinsMADharmageSCFlanderLBParity and decreased use of oral contraceptives as predictors of asthma in young womenClin Exp Allergy200636560961316650045
- RealFGSvanesCOmenaasERLung function, respiratory symptoms, and the menopausal transitionJ Allergy Clin Immunol200812117280. e318028993
- RomieuIFabreAFournierAPostmenopausal hormone therapy and asthma onset in the E3N cohortThorax201065429229720142267
- PoJYFitzGeraldJMCarlstenCRespiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysisThorax201166323223921248322
- KurmiOPSempleSSimkhadaPSmithWCAyresJGCOPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysisThorax201065322122820335290
- EisnerMDAnthonisenNCoultasDCommittee on Nonsmoking COPD, Environmental and Occupational Health AssemblyAn official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010182569371820802169
- DimakopoulouKSamoliEBeelenRAir pollution and nonmalignant respiratory mortality in 16 cohorts within the ESCAPE projectAm J Respir Crit Care Med2014189668469624521254
- McCreanorJCullinanPNieuwenhuijsenMJRespiratory effects of exposure to diesel traffic in persons with asthmaN Engl J Med2007357232348235818057337
- BalmesJREarnestGKatzPPExposure to traffic: lung function and health status in adults with asthmaJ Allergy Clin Immunol2009123362663119152968
- BarnesPJThe cytokine network in asthma and chronic obstructive pulmonary diseaseJ Clin Invest2008118113546355618982161
- BroekemaMTimensWVonkJMPersisting remodeling and less airway wall eosinophil activation in complete remission of asthmaAm J Respir Crit Care Med2011183331031620813885
- SorianoJBDavisKJColemanBVisickGManninoDPrideNBThe proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United KingdomChest2003124247448112907531
- WeatherallMTraversJShirtcliffePMDistinct clinical phenotypes of airways disease defined by cluster analysisEur Respir J200934481281819357143
- PirasBMiravitllesMThe overlap phenotype: the (missing) link between asthma and COPDMultidiscip Respir Med201271822958436
- RutgersSRPostmaDSten HackenNHOngoing airway inflammation in patients with COPD who do not currently smokeThorax2000551121810607796
- BarnesPJImmunology of asthma and chronic obstructive pulmonary diseaseNat Rev Immunol20088318319218274560
- BarnesPJCelliBRSystemic manifestations and comorbidities of COPDEur Respir J20093351165118519407051
- SindenNJStockleyRASystemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidenceThorax2010651093093620627907
- MiravitllesMSoler-CataluñaJJCalleMA new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC)Prim Care Respir J201322111712123443227
- MirandaCBusackerABalzarSTrudeauJWenzelSEDistinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammationJ Allergy Clin Immunol2004113110110814713914
- TaiATranHRobertsMOutcomes of childhood asthma to the age of 50 yearsJ Allergy Clin Immunol2014133615721578. e324495434
- PerretJLDharmageSCMathesonMCThe interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle ageAm J Respir Crit Care Med20131871424823155143
- JamesALKnuimanMWDivitiniMLChanges in the prevalence of asthma in adults since 1966: the Busselton health studyEur Respir J201035227327819643935
- HaldarPPavordIDNoneosinophilic asthma: a distinct clinical and pathologic phenotypeJ Allergy Clin Immunol2007119510431052 quiz 105317472810
- PhelanPDThe natural history of asthma from childhood to adult lifeWallsRSJenkinsCRUnderstanding Asthma. A Management CompanionEastgardens, NSWMacLennan and Petty20002025
- BurgessJAWaltersEHByrnesGBWho remembers whether they had asthma as children?J Asthma2006431072773017169822
- VestboJEdwardsLDScanlonPDECLIPSE InvestigatorsChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med2011365131184119221991892
- SorianoJBVisickGTMuellerovaHPayvandiNHansellALPatterns of comorbidities in newly diagnosed COPD and asthma in primary careChest200512842099210716236861
- ZvezdinBMilutinovSKojicicMA postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbationChest2009136237638019318666
- ArnsonYShoenfeldYAmitalHEffects of tobacco smoke on immunity, inflammation and autoimmunityJ Autoimmun2010343J258J26520042314
- ShaabanRKonySDrissFChange in C-reactive protein levels and FEV1 decline: a longitudinal population-based studyRespir Med2006100122112212016650972
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- WeinerPMagadleRWaizmanJWeinerMRabnerMZamirDCharacteristics of asthma in the elderlyEur Respir J19981235645689762780
- GoemanDPDouglassJAUnderstanding asthma in older Australians: a qualitative approachMed J Aust2005183Suppl 1S26S2715992317
- KingPTHoldsworthSRFreezerNJVillanuevaEGallagherMHolmesPWOutcome in adult bronchiectasisCOPD200521273417136958
- PatelISVlahosIWilkinsonTMBronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170440040715130905
- Martínez-GarcíaMAde la Rosa CarrilloDSoler-CataluñaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- PangJChanHSSungJYPrevalence of asthma, atopy, and bronchial hyperreactivity in bronchiectasis: a controlled studyThorax198944119489512595637
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- TashkinDPCelliBDecramerMBronchodilator responsiveness in patients with COPDEur Respir J200831474275018256071
- VonkJMJongepierHPanhuysenCISchoutenJPBleeckerERPostmaDSRisk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow upThorax200358432232712668795
- O’ByrnePMInmanMDAirway hyperresponsivenessChest2003123Suppl 3411S416S12629006
- JuniperEFFrithPAHargreaveFEAirway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthmaThorax19813685755797031972
- ScichiloneNMessinaMBattagliaSCatalanoFBelliaVAirway hyperresponsiveness in the elderly: prevalence and clinical implicationsEur Respir J200525236437515684304
- TashkinDPAltoseMDConnettJEKannerRELeeWWWiseRAMethacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research GroupAm J Respir Crit Care Med19961536 Pt 1180218118665038
- The COPD-X Plan. Australian and New Zealand Guidelines for the management of Chronic Obstructive Pulmonary Disease 2013 [web-page on the Internet]Lung FoundationAustralia2013 Available from: http://www.copdx.org.au/Accessed July 25, 2014
- CummingGHuntLBForm and function in the human lung: Proceedings of a Symposium held in the University of Birmingham, Birmingham, UK, 5–7 April 1967Edinburgh, ScotlandE & S Livingstone Ltd1968
- JohannessenASkorgeTDBottaiMMortality by level of emphysema and airway wall thicknessAm J Respir Crit Care Med2013187660260823328525
- FattahiFten HackenNHLöfdahlCGAtopy is a risk factor for respiratory symptoms in COPD patients: results from the EUROSCOP studyRespir Res2013141023356508
- ShaabanRZureikMSoussanDRhinitis and onset of asthma: a longitudinal population-based studyLancet200837296431049105718805333
- CalverleyPMBurgePSSpencerSAndersonJAJonesPWBronchodilator reversibility testing in chronic obstructive pulmonary diseaseThorax200358865966412885978
- ReidDWSoltaniAJohnsDPBronchodilator reversibility in Australian adults with chronic obstructive pulmonary diseaseIntern Med J2003331257257714656230
- McDonaldVMSimpsonJLHigginsIGibsonPGMultidimensional assessment of older people with asthma and COPD: clinical management and health statusAge Ageing2011401424921087988
- PavordIDGibsonPGInflammometry: the current state of playThorax201267319119222344396
- NairPUpdate on clinical inflammometry for the management of airway diseasesCan Respir J201320211712023616969
- SalviSSBarnesPJChronic obstructive pulmonary disease in non-smokersLancet2009374969173374319716966
- VozorisNTStanbrookMBSmoking prevalence, behaviours, and cessation among individuals with COPD or asthmaRespir Med2011105347748420850288
- D’SilvaLHassanNWangHYHeterogeneity of bronchitis in airway diseases in tertiary care clinical practiceCan Respir J201118314414821766077
- SimpsonJLScottRBoyleMJGibsonPGInflammatory subtypes in asthma: assessment and identification using induced sputumRespirology2006111546116423202
- FuJJGibsonPGSimpsonJLMcDonaldVMLongitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndromeRespiration2014871637424029561
- HardinMSilvermanEKBarrRGCOPDGene InvestigatorsThe clinical features of the overlap between COPD and asthmaRespir Res20111212721951550
- GonemSRajVWardlawAJPavordIDGreenRSiddiquiSPhenotyping airways disease: an A to E approachClin Exp Allergy201242121664168323181785
- BainesKJSimpsonJLWoodLGScottRJGibsonPGInduced sputum differential gene expression implicates increased p38 signalling activity in severe asthmaPaper presented at: Annual Meeting of the European Respiratory Society CongressSeptember 24–28, 2011Amsterdam, Netherlands
- McDonaldVMHigginsIWoodLGGibsonPGMultidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense?Thorax201368769169423503624
- PavordIDComplex airway disease: an approach to assessment and managementLancet Respir Med201311849024321807
- GreenRHBrightlingCEMcKennaSAsthma exacerbations and sputum eosinophil counts: a randomised controlled trialLancet200236093471715172112480423
- JayaramLPizzichiniMMCookRJDetermining asthma treatment by monitoring sputum cell counts: effect on exacerbationsEur Respir J200627348349416507847
- SivaRGreenRHBrightlingCEEosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trialEur Respir J200729590691317301099
- PetskyHLCatesCJLassersonTJA systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils)Thorax201267319920820937641
- BainesKJSimpsonJLWoodLGScottRJGibsonPGTranscriptional phenotypes of asthma defined by gene expression profiling of induced sputum samplesJ Allergy Clin Immunol20111271153160 160.e1–e921211650
- GorskaKKrenkeRKorczynskiPKosciuchJDomagala-KulawikJChazanREosinophilic airway inflammation in chronic obstructive pulmonary disease and asthmaJ Physiol Pharmacol200859Suppl 626127019218650
- McDonaldVMGibsonPGExacerbations of severe asthmaClin Exp Allergy201242567067722515389
- National Asthma Council AustraliaAustralian asthma handbook, Version 1.0MelbourneNational Asthma Council Australia2014 Available from: http://www.asthmahandbook.org.auAccessed March 20, 2014
- ChapmanKRLoveLBrubakerHA comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjectsChest19931045133213378222783
- ConnollyMJInhaler technique of elderly patients: comparison of metered-dose inhalers and large volume spacer devicesAge Ageing19952431901927645436
- BarronsRPegramABorriesAInhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary diseaseAm J Health Syst Pharm201168131221123221690428
- SovaniMPWhaleCIOborneJPoor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?Br J Gen Pract200858546374318186995
- KewKMKarnerCMindusSMFerraraGCombination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and childrenCochrane Database Syst Rev201312CD00901924343671
- CalverleyPMAndersonJACelliBTORCH investigatorsSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- SinghSLokeYKRisk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and updateCurr Opin Pulm Med201016211812219926996
- ManserRReidDAbramsonMJCorticosteroids for acute severe asthma in hospitalised patientsCochrane Database Syst Rev20011CD00174011279726
- WaltersJAGibsonPGWood-BakerRHannayMWaltersEHSystemic corticosteroids for acute exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20091CD00128819160195
- LeuppiJDSchuetzPBingisserRShort-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trialJAMA2013309212223223123695200
- McDonoughAKCurtisJRSaagKGThe epidemiology of glucocorticoid-associated adverse eventsCurr Opin Rheumatol200820213113718349741
- GibsonPGPowellHCoughlanJSelf-management education and regular practitioner review for adults with asthmaCochrane Database Syst Rev20031CD00111712535399
- AbramsonMJBaileyMJCouperFJVictorian Asthma Mortality Study GroupAre asthma medications and management related to deaths from asthmaAm J Respir Crit Care Med20011631121811208619
- ZwerinkMBrusse-KeizerMvan der ValkPDSelf management for patients with chronic obstructive pulmonary diseaseCochrane Database Syst Rev20143CD00299024665053
- WaltersJATurnockACWaltersEHWood-BakerRAction plans with limited patient education only for exacerbations of chronic obstructive pulmonary diseaseCochrane Database Syst Rev20105CD00507420464737
- FriedlanderALLynchDDyarLABowlerRPPhenotypes of chronic obstructive pulmonary diseaseCOPD20074435538418027163
- AgustiACalverleyPMCelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigatorsCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- NadeemNJTaylorSJEldridgeSMWithdrawal of inhaled corticosteroids in individuals with COPD – a systematic review and comment on trial methodologyRespir Res20111210721838890
- MagnussenHWatzHKirstenAStepwise withdrawal of inhaled corticosteroids in COPD patients receiving dual bronchodilation: WISDOM study design and rationaleRespir Med2014108459359924477080
- National Asthma Council AustraliaAsthma Management Handbook 2006MelbourneNational Asthma Council Australia2006 Available from: http://www.nationalasthma.org.au/uploads/handbook/370-amh2006_web_5.pdfAccessed March 20, 2014
- ChauhanBFDucharmeFMAnti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and childrenCochrane Database Syst Rev20125CD00231422592685
- NormansellRWalkerSMilanSJWaltersEHNairPOmalizumab for asthma in adults and childrenCochrane Database Syst Rev20141CD00355924414989
- HaldarPBrightlingCEHargadonBMepolizumab and exacerbations of refractory eosinophilic asthmaN Engl J Med20093601097398419264686
- McDonaldVMVertiganAEGibsonPGHow to set up a severe asthma serviceRespirology201116690091121692918
- SteadLFHartmann-BoyceJPereraRLancasterTTelephone counselling for smoking cessationCochrane Database Syst Rev20138CD00285023934971
- SteadLFPereraRBullenCNicotine replacement therapy for smoking cessationCochrane Database Syst Rev201211CD00014623152200
- HughesJRSteadLFHartmann-BoyceJCahillKLancasterTAntidepressants for smoking cessationCochrane Database Syst Rev20141CD00003124402784
- CahillKSteadLFLancasterTNicotine receptor partial agonists for smoking cessationCochrane Database Syst Rev20124CD00610322513936
- SteadLFLancasterTBehavioural interventions as adjuncts to pharmacotherapy for smoking cessationCochrane Database Syst Rev201212CD00967023235680
- ThomasDAbramsonMJBonevskiBA pharmacist-led system-change smoking cessation intervention for smokers admitted to Australian public hospitals (GIVE UP FOR GOOD): study protocol for a randomised controlled trialTrials20131414823693155
- YohannesAMWillgossTGVestboJTiotropium for treatment of stable COPD: a meta-analysis of clinically relevant outcomesRespir Care201156447748721255503
- VogelmeierCHedererBGlaabTPOET-COPD InvestigatorsTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med2011364121093110321428765
- TashkinDPCelliBSennSUPLIFT Study InvestigatorsA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- KestenSJaraMWentworthCLanesSPooled clinical trial analysis of tiotropium safetyChest200613061695170317166984
- KerstjensHAEngelMDahlRTiotropium in asthma poorly controlled with standard combination therapyN Engl J Med2012367131198120722938706
- BefekaduEOnofreiCColiceGLTiotropium in asthma: a systematic reviewJ Asthma Allergy20147112124600237
- LacasseYBrosseauLMilnePulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20023CD003793 [updated in Cochrane Database Syst Rev. 2006;4: CD003793]12137716
- GriffithsTLBurrMLCampbellIAResults at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trialLancet2000355920136236810665556
- GriffithsTLPhillipsCJDaviesSBurrMLCampbellIACost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programmeThorax2001561077978411562517
- TurnerSEastwoodPCookAJenkinsSImprovements in symptoms and quality of life following exercise training in older adults with moderate/severe persistent asthmaRespiration201181430231020501982
- BlackstockFCWebsterKEMcDonaldCFHillCJComparable improvements achieved in chronic obstructive pulmonary disease through pulmonary rehabilitation with and without a structured educational intervention: a randomized controlled trialRespirology201419219320224261584
- GibsonPGPowellHWilsonASelf-management education and regular practitioner review for adults with asthmaCochrane Database of Systematic Reviews20023CD001117
- TrappenburgJCMonninkhofEMBourbeauJEffect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trialThorax2011661197798421785156
- BischoffEWHamdDHSedenoMEffects of written action plan adherence on COPD exacerbation recoveryThorax2011661263121037270
- BourbeauJJulienMMaltaisFChronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du QuébecReduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management interventionArch Intern Med2003163558559112622605
- FanVSGazianoJMLewRA comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trialAnn Intern Med20121561067368322586006
- Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update) [webpage on the Internet]BethesdaNational Institute for Health and Care Excellence Available from: http://publications.nice.org.uk/chronic-obstructive-pulmonary-disease-cg101Accessed March 20, 2014
- MooreRPBerlowitzDJDenehyLA randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemiaThorax2011661323720880870
- NonoyamaMLBrooksDGuyattGHGoldsteinRSEffect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemiaAm J Respir Crit Care Med2007176434334917446339
- McKeonJLMurree-AllenKSaundersNASupplemental oxygen and quality of sleep in patients with chronic obstructive lung diseaseThorax19894431841882650011
- ChaouatAWeitzenblumEKesslerRA randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patientsEur Respir J19991451002100810596681
- FletcherECLuckettRAGoodnight-WhiteSMillerCCQianWCostarangos-GalarzaCA double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mmHgAm Rev Respir Dis19921455107010761586049
- DeanMMEnd-of-life care for COPD patientsPrim Care Respir J2008171465018264648
- JenningsALDaviesANHigginsJPGibbsJSBroadleyKEA systematic review of the use of opioids in the management of dyspnoeaThorax2002571193994412403875
- McDonaldVMHigginsIGibsonPGInsight into older peoples’ healthcare experiences with managing COPD, asthma, and asthma-COPD overlapJ Asthma201350549750423638867
- JonesIKirbyAOrmistonPThe needs of patients dying of chronic obstructive pulmonary disease in the communityFam Pract200421331031315128695
- TraversJMarshSCaldwellBExternal validity of randomized controlled trials in COPDRespir Med200710161313132017113277
- WenzelSEAsthma: defining of the persistent adult phenotypesLancet2006368953780481316935691
- LangePPersistent airway obstruction in asthmaAm J Respir Crit Care Med201318711223281346
- BeasleyRWeatherallMTraversJShirtcliffePTime to define the disorders of the syndrome of COPDLancet2009374969167067219716947
- NelsonHSWeissSTBleeckerERYanceySWDorinskyPMSMART Study GroupThe Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterolChest20061291152616424409
