Abstract
Background
Although proven to be associated with bronchial obstruction, chest signs are not listed among cues that should prompt spirometry in the early diagnosis of chronic obstructive pulmonary disease (COPD) in established guidelines.
Aims
We aimed to explore how chest findings add to respiratory symptoms and a history of smoking in the diagnosis of COPD.
Methods
In a cross-sectional study, patients aged 40 years or older, previously diagnosed with either asthma or COPD in primary care, answered questionnaires and underwent physical chest examination and spirometry.
Results
Among the 375 patients included, 39.7% had forced expiratory volume in 1 second/forced vital capacity <0.7. Hyperresonance to percussion was the strongest predictor of COPD, with a sensitivity of 20.8, a specificity of 97.8, and likelihood ratio of 9.5. In multivariate logistic regression, where pack-years, shortness of breath, and chest findings were among the explanatory variables, three physical chest findings were independent predictors of COPD. Hyperresonance to percussion yielded the highest odds ratio (OR = 6.7), followed by diminished breath sounds (OR = 5.0), and thirdly wheezes (OR = 2.3). These three chest signs also gave significant diagnostic information when added to shortness of breath and pack-years in receiver operating-characteristic curve analysis.
Conclusion
We found that chest signs may add to respiratory symptoms and a history of smoking in the diagnosis of COPD, and we conclude that chest signs should be reinstated as cues to early diagnosis of COPD in patients 40 years or older.
Introduction
Spirometry is the recommended method for assessing lung function in patients with suspected chronic obstructive pulmonary disease (COPD).Citation1 Although spirometry testing is performed increasingly often and spirometers are about to become standard equipment at GPs’ offices in some countries, easy access does not apply to all. Also, lung-function assessment by spirometry testing is not only a question of availability. Even with a spirometer at hand, the GP has to select the right patients for this examination to be cost-effective.Citation2
Spirometry screening has been proposed as a measure for early detection of COPD in smokers.Citation3,Citation4 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a somewhat different approach, suggesting that COPD should be considered and spirometry performed if the patient is older than 40 years, and one or more of the following is present: dyspnea, chronic cough or sputum production, history of exposure to risk factors (eg, tobacco smoking), or a family history of COPD.Citation1
Chest signs are not on this list of cues that should prompt spirometry in the early diagnosis of COPD, although several studies published between 1970 and 2002 have demonstrated associations between chest findings and bronchial obstruction.Citation5–Citation11 This study aimed to clarify how chest signs are associated with bronchial obstruction and to what degree chest signs may add to respiratory symptoms and smoking history in the diagnosis of COPD.
Methods
Subjects
Patients were recruited from seven GP offices in Norway. Of 43,241 patients listed at these offices, 18,931 were aged ≥40 years. Among these, 1,784 subjects had been registered with a diagnosis of asthma or COPD by a GP within 5 years before the start of the study. A random sample of these (n = 1,111) were mailed an invitation to participate in our study, and 380 patients (34%) consented and were included. The patients were examined between April 2009 and March 2010. The examinations, carried out by a total of 20 GPs during a stable phase of the disease, included auscultation and percussion of the chest, questionnaires, and spirometry.Citation12 The study was approved by the Regional Committee for Medical and Health Research Ethics.
Chest examination
Percussion and auscultation were performed bilaterally, and the GPs carried out the examinations as usual. The findings were recorded in a form integrated in the medical record that assured complete recordings. The form also included several items on the participants’ obstructive disease, comorbidity, and medications. Notably, the GPs were not told that the diagnostic usefulness of chest findings was among the objectives of our study. Due to different practice routines, the chest examinations were carried out before spirometry in four of the GPs’ offices, and after spirometry in the remaining three.
Questionnaires
Each patient answered a clinical COPD questionnaire (CCQ),Citation13 scoring their respiratory symptoms and limitations in daily activities on scales from 0 to 6. Scores on shortness of breath, cough, and sputum were included as variables in the present study (). Smoking habits in terms of quantity and duration were recorded in a separate questionnaire. Pack-years (the number of cigarettes smoked daily multiplied by the number of total years of smoking, divided by 20) were calculated for each patient. Sporadic smokers were registered with 0.5 pack-years.
Table 1 Questions on shortness of breath, cough, and phlegm in the clinical COPD questionnaire (CCQ)
Spirometry
Spirometry was carried out by trained health-care workers, who followed the guidelines of the European Respiratory Society/American Thoracic Society.Citation14 The patients were seated during the examination, and did not wear a nose clip. They were told not to take their regular medications on the examination day. All offices used the Spirare SPS310 spirometer (Diagnostica, Oslo, Norway). Postbronchodilator spirometry was obtained 20 minutes after inhalation of 0.4 mg salbutamol. Norwegian reference values were applied.Citation15
Statistical analyses
The main outcome in the analysis was postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.7, which according to the GOLD criteria indicates a presence of COPD, although asthma may be the primary disease. A secondary outcome was postbronchodilator FEV1% predicted <50 in addition to FEV1/FVC < 0.7, indicating severe COPD (GOLD stage III–IV). The diagnostic properties of chest signs, symptoms, and pack-years with respect to COPD and severe COPD were evaluated by calculating sensitivity, specificity, predictive value, and likelihood ratio (LR). LRs with 95% confidence intervals were calculated using online software.Citation16
Statistically significant predictors of COPD and severe COPD in bivariate analysis were entered into logistic regression models, with the two categories of reduced lung function as outcome variables. To account for clustering of data, we used mixed-effect two-level models with the patient as first level and the examining GP as second level. A backward stepwise method was applied, in which only significant variables (P < 0.05) were retained in the final models.
Added diagnostic value of independent predictors in the multivariable logistic regression was evaluated by receiver operating-characteristic (ROC) curve analysis. A first curve was constructed by computing the sensitivity and specificity of the CCQ score of the significant respiratory symptom, a second curve by adding pack-years graded into five categories to the symptom score, and a third curve by adding the score for chest findings, reflecting the odds ratios of these findings, to the symptom score and the pack-year score. SPSS (version 19; IBM, Armonk, NY, USA) and STATA (version 12.0; College Station, TX, USA; two-level logistic regression) were used in the statistical analyses. A 5% level of statistical significance was applied.
Sensitivity analyses
The GPs were not blinded for clinical information about the patients, and we checked whether chest examination after spirometry was associated with improved estimates of diagnostic value compared to chest examination before spirometry. Then we compared the sensitivity, specificity, and LRs of “any chest sign” obtained in practices where chest examination was carried out before spirometry with that obtained where chest examination was done after spirometry. In practices where chest examination was carried out before spirometry, we also checked whether the diagnostic values of “any chest sign” differed dependent on whether the evaluation was done against prebronchodilator spirometry or postbronchodilator spirometry.
Results
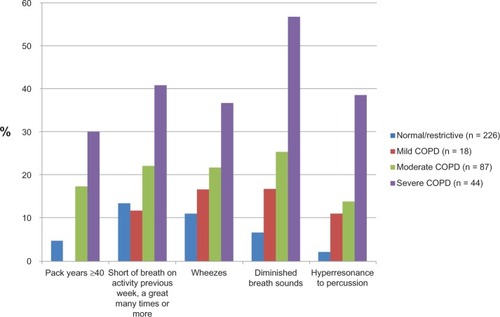
Of the 380 patients who took part in the study, two were excluded from analysis due to ongoing exacerbation and three due to missing data. The mean (± standard deviation) age of the 375 included patients was 61.4 ± 11.5 years, and 61.9% were women; 74.4% were current or previous smokers, and 39.1% had smoked ≥20 pack-years (). Mild or moderate COPD (GOLD I–II) was registered in 28.0%, and severe COPD (GOLD III–IV) in 11.7% of the subjects (). The most frequently reported respiratory symptoms were shortness of breath when doing physical activities and coughing (). At least one chest sign was recorded in 38.7% of the patients. Wheezes and diminished breath sounds were the chest signs most frequently recorded (). The frequency of heavy smoking, severe dyspnea, and chest signs all increased significantly by decreasing lung function (P < 0.001 for all variables, ).
Table 2 Sex, age, diagnosis, smoking history, lung function (by GOLD stages), respiratory symptoms, and chest signs in 375 patients aged ≥40 years diagnosed with asthma or COPD in primary care
Figure 1 Frequency of heavy smoking, shortness of breath, and chest findings by level of lung function in 375 patients aged ≥40 years diagnosed with asthma or chronic obstructive pulmonary disease (COPD) in primary care. The patients were in a stable phase of illness.
Diagnostic values
The probability of COPD increased by the number of pack-years the patients had been smoking (). With more than 40 pack-years, the LR was 4.1. To have ever smoked had the highest sensitivity of all findings (0.83), but the specificity was low (0.35). Among the respiratory symptoms, shortness of breath doing physical activities, occurring a great many times the previous week, had the highest LR, whereas cough and phlegm were not significant predictors of COPD. The strongest COPD predictors were found among the chest signs, and hyperresonance to percussion had the highest LR (9.5) and specificity (0.98) (). There was no statistically significant association between crackles and COPD.
Table 3 Values of smoking habit, respiratory symptoms, and chest signs in predicting COPD (postbronchodilator FEV1/FVC < 0.7) in 375 patients diagnosed with asthma or COPD
When the predictors were evaluated against severe COPD, sensitivity was generally higher and specificity lower, whereas the LRs were only slightly changed ().
Table 4 Diagnostic values of smoking habit, respiratory symptoms, and chest signs in predicting severe COPD (postbronchodilator FEV1/FVC < 0.7 and FEV1% predicted <50) in 375 patients diagnosed with asthma or COPD
Multivariate analysis
In the multivariate regression models, any degree of COPD could be independently predicted by pack-years but not by shortness of breath, whereas the opposite was the case for severe COPD (). Age and chest signs were significant predictors for both outcomes. Among the chest findings, diminished breath sounds and hyperresonance to percussion were the two strongest independent predictors.
Table 5 Independent predictive values of smoking, respiratory symptoms, and chest signs for COPD (FEV1/FVC < 0.7) and severe COPD (FEV1/FVC < 0.7 and FEV1% predicted <50), as evaluated by multivariate logistic regression in 351 patients diagnosed with asthma or COPD
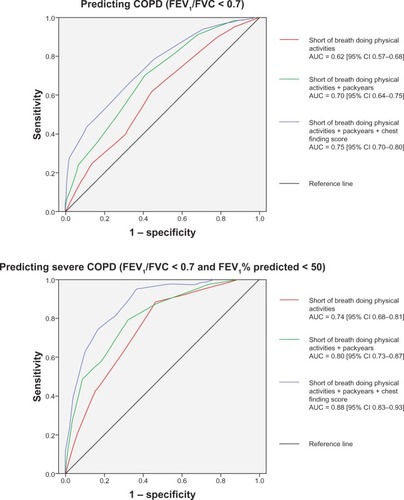
ROC-curve analyses displaying diagnostic values for COPD of dyspnea score (CCQ score for shortness of breath during physical activities), pack-years, and chest-finding score showed added diagnostic value when the three scores were used together (). The added score predicted severe COPD more strongly (area under the curve [AUC] = 0.88) than it predicted COPD of any degree of severity (AUC = 0.75). The AUC obtained with all three scores in the model was higher than the upper limit of the 95% confidence interval of the AUC when only pack-years and dyspnea score were included.
Figure 2 Receiver operating-characteristic curves showing predictive value of three different scores in predicting COPD (FEV1/FVC < 0.7) and severe COPD (FEV1/FVC < 0.7 and FEV1% predicted <50): dyspnea score (short of breath doing physical activities in the clinical COPD questionnaire), summing up of dyspnea score and pack-years (0–10, 1; 10–20, 2; 20–40, 3; ≥40, 4), and summing up of dyspnea score, pack-years, and chest-finding score (addition of wheezes, 1; diminished breath sound, 2; hyperresonance to percussion, 3).
Sensitivity analyses
COPD was somewhat more prevalent and chest signs less frequently recorded in the four practices where auscultation was done before spirometry, compared to the other three practices (). The less frequent recording of abnormal chest findings was associated with somewhat lower sensitivity and considerably higher specificity and LR. This was the case whether the diagnostic value of any chest finding was evaluated against prebronchodilator or postbronchodilator spirometry.
Table 6 Sensitivity analysis comparing lung function, the prevalence of any chest sign, and the sensitivity, specificity, and likelihood ratio of any chest sign for COPD in practices where patients were examined before spirometry and after spirometry
Discussion
Main findings
Presence of prolonged expiration, wheezes, hyperresonance to percussion, and diminished breath sounds by physical chest examination significantly increased the probability of bronchial obstruction in our study sample. Hyperresonance to percussion and diminished breath sounds got higher like-lihood ratios than did heavy smoking and any respiratory symptoms. These two chest signs, and also wheezes, were found with similar frequency in the patients with moderate COPD (GOLD II), as were ≥40 pack-years and frequent dyspnea on physical activities. The three chest signs gave significant diagnostic information when added to dyspnea and pack-years, as shown by both logistic regression and ROC-curve analysis. A model including pack-years, shortness of breath, and chest signs had good test characteristics by ROC-curve analysis in predicting COPD and even better in predicting severe COPD. The findings indicate that abnormal chest findings, as well as a history of shortness of breath and smoking, are cues that should prompt spirometry in patients 40 years or older with no established diagnosis of COPD.
Strengths and weaknesses
Our study is among the largest studies carried out to evaluate chest findings as signs of bronchial obstruction. For comparison of our results with previous studies, it was an advantage to evaluate against both “any” COPD and severe COPD, since reductions in lung function somewhere in between our two cutoff levels have usually been used as outcomes in previous studies. Although the sensitivity of the chest findings was higher and specificity lower when using severe obstruction as the reference standard compared to when FEV1/FVC < 0.7 was used, we observed that the LR showed little variation ( and ), as can be expected when both the numerator and the denominator in the fraction (sensitivity/1 – specificity) decreases. This supports comparisons with previous studies.
A main concern in studies like this is the selection of patients studied and whether the results are applicable in other settings. Like in most previous studies, our study sample consisted of patients with known or possible obstructive lung disease. The patients had at least once during the preceding 5 years been labeled with a diagnosis of asthma or COPD, but we do not know to what degree the registered diagnoses were based on established criteria.Citation12 Overrepresentation of severe cases among the COPD patients and overrepresentation of asthma among those with normal spirometry have most probably led to increased sensitivity and decreased specificity of the chest signs. The LRs, however, were probably less affected. Ideally, we should have recruited unselected patients above 40 years of age. Only a few studies have focused on adults without known bronchial obstruction.Citation11,Citation17,Citation18 There is a tendency of decreasing frequency of chest signs in COPD patients (sensitivity) with decreasing prevalence of COPD in the study sample. The lower sensitivity in such samples is usually counterweighted by a higher specificity,Citation11 resulting in the LR being less affected.Citation17 This makes it likely that our results are applicable also in patient populations with a lower prevalence of COPD.
The computerized form the GPs had to fill in during the consultation led to complete recording of chest findings. Since all patients had previously been labeled with a diagnosis of asthma or COPD by a GP, one could expect that the chest examination was carried out with similar attention in all patients. However, chest findings only made up a small part of the registrations, and the main topic of the study was exacerbations of asthma and COPD. With this focus, the GPs have probably been less prone to “adjust” their recordings to the severity of the patient’s disease than if evaluation of chest examination had been the aim. A rigorous design with the GPs blinded for all clinical information was probably not of fundamental importance. At the three GP practices where chest examination was carried out after the spirometry, the GPs could in theory check the spirogram before examining the patient and possibly expect more chest findings in patients with bronchial obstruction and fewer findings when the FEV1/FVC was above 0.7. From the results, this does not appear to have occurred. More findings were recorded in patients without obstruction, and the lowest specificity and LRs of chest findings were found in the practices where the patients were examined after spirometry.
This inconsistency suggests sources of flaw that cannot be addressed by blinding the observing doctor. Previous studies have demonstrated considerable interobserver variation when patients have been examined by more than one doctor.Citation19 This has particularly been the case for hyperresonance to percussion and diminished breath sounds.Citation19 Percussion of the chest is somewhat demanding, and the skills needed to elicit a hyperresonant sound may vary between doctors.Citation20 To differentiate between normal and diminished breath sounds, proper instructions to the patient, clinical experience, and attentive listening are needed. The terms used for abnormal lung sound vary between clinicians.Citation21,Citation22 Improved skills among the GPs in examining the chest and more standardized use of terms when describing the findings are likely to improve the diagnostic value of the findings.
The most important strength of the study is that we evaluated chest signs together with smoking history and shortness of breath, the symptoms regarded as the best cues to an early diagnosis of COPD. We found chest signs to be independent predictors that add to these established cues, and we see no good reason why these strong findings should not be applicable also in unselected adults, at least to some significant degree.
Comparisons with previous studies
Hyperresonance to percussion
Similar sensitivity and specificity of hyperresonance to percussion have been found in previous studies when evaluated against bronchial obstruction among adults with a possible lung diseaseCitation11 or a history of smoking.Citation6 Our study confirms that hyperresonance to percussion is a strong predictor, with an LR above 5.
Diminished breath sounds
Diminished breath sounds have also previously been shown to predict bronchial obstruction, with LRs between 2 and 6.Citation11 In a recent study from primary care of patients with persistent cough, diminished breath sounds significantly predicted FEV1/FVC < 0.7 in univariate analysis (sensitivity 0.16 and specificity 0.91), but not in multivariate analysis.Citation18 The considerable variation in diagnostic properties of this variable probably reflects that it is particularly susceptible to interobserver variability.
Wheezes/rhonchi and crackles
Associations between wheezes/rhonchi and bronchial obstruction have been demonstrated in several studies,Citation9,Citation10,Citation17,Citation18,Citation23,Citation24 and LRs between 2 and 3, as in the present study, have been found, also among patients without known bronchial obstruction.Citation11,Citation17,Citation18 Crackles were not a significant predictor in our study, in contrast to what has been found in previous studies.Citation7,Citation11
Smoking and respiratory symptoms
Smoking history, shortness of breath, and cough with phlegm are well-known predictors of COPD.Citation17,Citation24–Citation27 In a recent study of adults aged 40 years or more with a COPD prevalence of only 9.6%, all these variables were significant predictors of COPD.Citation28 The ROC-curve analysis of a diagnostic model including age, smoking status, and breathlessness obtained an AUC of 0.77.Citation28 This is close to the AUC of 0.80 for pack-years and dyspnea when evaluated against severe COPD in our study.
Implications for practice
Based on our findings and supported by previous studies, we suggest reinstating chest findings as cues to the early diagnosis of COPD. At present, their relevance is not reflected in established guidelines. Smoking and respiratory symptoms, but not chest findings, are listed as information that should lead to spirometry in a search for COPD. GOLD argues that “Physical signs of airflow limitation are usually not present until significant impairment of lung function has occurred, and their detection has a relatively low sensitivity and specificity.”Citation1 In the National Institute for Health and Care Excellence guidelines, it is stated that the diagnosis of COPD “is suspected on the basis of symptoms and signs and supported by spirometry.” Physical chest findings are not listed among the clinical information that should raise suspicion for COPD, and are considered less predictive than symptoms in COPD assessment.Citation29 A similar understanding of the value of chest signs in the early diagnosis of COPD is presented by Rennard.Citation30
The predictive value of chest signs for COPD is only one reason chest findings should be reinstated in early diagnosis. In practice, chest examination is carried out in all kinds of patients, such as those with hypertension, heart disease, and respiratory tract infections. The examination is done quickly and carried out frequently, also during busy consultations, and it has high standing (face value) among patients. To elicit a thorough history on smoking and respiratory symptoms, which of course often should be done, takes longer time and is probably less frequently carried out. COPD questionnaires are not easily implemented in a busy practice, and they have not been evaluated as elements of ordinary consultations. So far, we do not know whether the diagnostic properties of such questionnaires in disclosing mild-to-moderate COPD are better than those of chest signs.
Acknowledgments
The authors thank the participating patients and Nordbyen legesenter in Tromsø, Allmed legesenter in Hammerfest, Alta Helsesenter, Skedsmokorset legesenter, Langbølgen legesenter in Oslo, and Gransdalen legesenter in Oslo for taking part in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of COPD2011 Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed June 29, 2013
- EnrightPWhitePDetecting mild COPD: don’t waste resourcesPrim Care Respir J2011206821311843
- EnrightPLKaminskyDAStrategies for screening for chronic obstructive pulmonary diseaseRespir Care2003481194120114651760
- StratelisGJakobssonPMolstadSZetterstromOEarly detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 yearsBr J Gen Pract20045420120615006126
- PardeeNEMartinCJMorganEHA test of the practical value of estimating breath sound intensity. Breath sounds related to measured ventilatory functionChest197670341344954460
- BadgettRGTanakaDJHuntDKCan moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone?Am J Med1993941881968430714
- van SchayckCPvan WeelCHarbersHJvan HerwaardenCLDo physical signs reflect the degree of airflow obstruction in patients with asthma or chronic obstructive pulmonary disease?Scand J Prim Health Care199192322381792447
- HollemanDRJrSimelDLGoldbergJSDiagnosis of obstructive airways disease from the clinical examinationJ Gen Intern Med1993863688441077
- ShimCSWilliamsMHJrRelationship of wheezing to the severity of obstruction in asthmaArch Intern Med19831438908926679232
- MariniJJPiersonDJHudsonLDLakshminarayanSThe significance of wheezing in chronic airflow obstructionAm Rev Respir Dis197912010691072507523
- MelbyeHThe usefulness of clinical pulmonary examination in the diagnosis of bronchial obstructionTidsskr Nor Laegeforen199811819992003 Norwegian9656782
- MelbyeHDrivenesEDalbakLGLeinanTHøegh-HenrichsenSOstremAAsthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or moreInt J Chron Obstruct Pulmon Dis2011659760322135492
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- QuanjerPHTammelingGJCotesJEPedersenOFPeslinRYernaultJCLung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory SocietyEur Respir J Suppl1993165408499054
- LanghammerAJohnsenRGulsvikAHolmenTLBjermerLForced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trøndelag StudyEur Respir J20011877077911757626
- MK Anders ConsultingConfidence interval – likelihood ratio Available from: http://mkanders.com/CIforLR.phpAccessed June 29, 2013
- StrausSEMcAlisterFASackettDLDeeksJJThe accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. CARE-COAD1 Group. Clinical Assessment of the Reliability of the Examination-Chronic Obstructive Airways DiseaseJAMA20002831853185710770147
- BroekhuizenBDSachsAPVerheijTJAccuracy of symptoms, signs, and C-reactive protein for early chronic obstructive pulmonary diseaseBr J Gen Pract201262e632e63822947584
- BenbassatJBaumalRNarrative review: should teaching of the respiratory physical examination be restricted only to signs with proven reliability and validity?J Gen Intern Med20102586587220349154
- YernaultJCBohadanaABChest percussionEur Respir J19958175617608586135
- WilkinsRLDexterJRMurphyRLJrDelBonoEALung sound nomenclature surveyChest1990988868892209143
- FrancisNAMelbyeHKellyMJVariation in family physicians’ recording of auscultation abnormalities in patients with acute cough is not explained by case mix. A study from 12 European networksEur J Gen Pract201319778423544624
- MelbyeHBronchial airflow limitation and chest findings in adults with respiratory infectionScand J Prim Health Care1995132612678693210
- StrausSEMcAlisterFASackettDLDeeksJJAccuracy of history, wheezing, and forced expiratory time in the diagnosis of chronic obstructive pulmonary diseaseJ Gen Intern Med20021768468812220364
- FletcherCPetoRThe natural history of chronic airflow obstructionBr Med J1977116451648871704
- LindbergAJonssonACRönmarkELundgrenRLarssonLGLundbäckBTen-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohortChest20051271544155215888826
- MedboAMelbyeHWhat role may symptoms play in the diagnosis of airflow limitation? A study in an elderly populationScand J Prim Health Care200826929818570007
- RaghavanNLamYMWebbKAComponents of the COPD Assessment Test (CAT) associated with a diagnosis of COPD in a random population sampleCOPD2012917518322409441
- National Institute for Health and Care ExcellenceChronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update)2010 Available from: http://publications.nice.org.uk/chronic-obstructive-pulmonary-disease-cg101Accessed June 29, 2013
- RennardSIChronic obstructive pulmonary disease: definition, clinical manifestations, diagnosis, and staging2013 Available from: http://www.uptodate.com/contents/chronic-obstructive-pulmonary-disease-definition-clinical-manifestations-diagnosis-and-staging?source=search_result&search=copd+diagnosis&selectedTitle=1%7E150#H6 (07.01.13)Accessed June 29, 2013