Abstract
Background
Chronic obstructive pulmonary disease (COPD) is present in approximately one-third of all congestive heart failure (CHF) patients, and is a key cause of underprescription and underdosing of β-blockers, largely owing to concerns about precipitating respiratory deterioration. For these reasons, the aim of this study was to evaluate the impact of β-blockers on the long-term outcomes in CHF patients with COPD. In addition, we compared the effects of two different β-blockers, carvedilol and bisoprolol.
Methods
The study was a retrospective, non-randomized, single center trial. Acute decompensated HF patients with COPD were classified according to the oral drug used at discharge into β-blocker (n=86; carvedilol [n=52] or bisoprolol [n=34]) and non-β-blocker groups (n=46). The primary endpoint was all-cause mortality between the β-blocker and non-β-blocker groups during a mean clinical follow-up of 33.9 months. The secondary endpoints were the differences in all-cause mortality and the hospitalization rates for CHF and/or COPD exacerbation between patients receiving carvedilol and bisoprolol.
Results
The mortality rate was higher in patients without β-blockers compared with those taking β-blockers (log-rank P=0.039), and univariate analyses revealed that the use of β-blockers was the only factor significantly correlated with the mortality rate (hazard ratio: 0.41; 95% confidence interval: 0.17–0.99; P=0.047). Moreover, the rate of CHF and/or COPD exacerbation was higher in patients treated with carvedilol compared with bisoprolol (log-rank P=0.033). In the multivariate analysis, only a past history of COPD exacerbation significantly increased the risk of re-hospitalization due to CHF and/or COPD exacerbation (adjusted hazard ratio: 3.11; 95% confidence interval: 1.47–6.61; P=0.003).
Conclusion
These findings support the recommendations to use β-blockers in HF patients with COPD. Importantly, bisoprolol reduced the incidence of CHF and/or COPD exacerbation compared with carvedilol.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is present in approximately one-third of all congestive heart failure (CHF) patients, and is a key cause of underprescription and underdosing of β-blockers, owing largely to concerns about precipitating respiratory deterioration in these patients.Citation1–Citation4 Despite increasing evidence that β-blockers are safe and could be beneficial in patients with COPD, most large-scale CHF trials exclude patients with COPD.Citation5 The National Institute for Health and Care Excellence and European Society of Cardiology guidelines state that COPD is not a contraindication to the use of β-blockers, and mild deterioration in pulmonary function and symptoms should not result in prompt discontinuation. Low-dose initiation and gradual uptitration is recommended.Citation6,Citation7 In addition, some studies have suggested differences in the pulmonary effects between cardioselective and non-cardioselective β-blockers, but the results of these studies are not fully conclusive.Citation8–Citation13 Of the β-blocker class of drugs, carvedilol is a non-selective β-adrenergic receptor (AR) blocker that also blocks α1-AR signaling and that has been shown to improve the state of HF patients in many studies.Citation14–Citation18 However, some patients are not able to continue or uptitrate the drug because of various adverse symptoms and signs, which seem to be mainly related to the β2-AR blocking effect. On the other hand, bisoprolol, a highly selective β1-AR blocker, has also been shown to be effective for HF patients in several studies.Citation19–Citation21 Although it has been reported that cardioprotective β-blockers do not worsen the pulmonary function in CHF patients with COPD, the impact of β-blocker selectivity on the long-term outcomes has not yet been well evaluated in these patients. In a sub-analysis of the OPTIMIZE-HF study, patients receiving non-cardioselective β-blockers were found to have a lower event rate compared to patients receiving cardioselective β-blockers.Citation22 Thus, taking this into consideration, the aim of the present study was to evaluate the impact of β-blockers on the long-term outcomes in HF patients with COPD. In addition, we also compared the effects of carvedilol and bisoprolol in this patient population.
Materials and methods
Study design
This study was a retrospective, non-randomized, single center trial based on data collected over a 48-month period (January 1, 2009 to December 31, 2012) from 212 consecutive acute decompensated HF patients with COPD who were admitted to our hospital with HF categorized as New York Heart Association Class 3 or 4. Patients with acute coronary syndromes (n=34), including acute myocardial infarction, or with end-stage renal disease that required dialysis therapy (n=14) as well as patients who did not finish the treatment course after discharge (n=32) were excluded from the study. The diagnosis of HF was made on the basis of the criteria recommended in the Framingham Heart Study.Citation23 The diagnosis of COPD was made on the basis of the global initiative for chronic obstructive lung disease criteria.Citation24 At the time of registration, the COPD of all patients was in the stable phase. Finally, of the identified 212 patients, 132 met all inclusion criteria and were enrolled in this study.
The patients were divided into two groups according to the agents administered after admission and followed-up for a mean period of 33.9 months. The first group was treated without any β-blockers (non-β-blocker group, n=46), whereas the second group was administered β-blockers before discharge (β-blocker group, n=86; carvedilol [n=52] or bisoprolol [n=34]). After discharge, the patients continued treatment with either carvedilol or bisoprolol, with the dose being carefully increased to tolerance. The subjects were followed-up at intervals of 1–2 months in the outpatient department of our hospital from January 2009 through December 2013. The patients’ health status was checked during each follow-up, and was recorded using electronic clinical records; furthermore, all-cause death and hospitalization due to CHF and/or COPD exacerbation were also verified using these electronic clinical records.
This retrospective study was conducted using data from a large university hospital. The protocol used complies with the Declaration of Helsinki and was approved by our Institutional Ethics Committee, which waived the need for patient consent because of the retrospective nature of the study.
Relevant factors
The heart rate (HR) and systolic blood pressure (SBP) were measured at rest, simultaneously with the electrocardiogram measurements. Height and weight were measured at the time of discharge, and the body mass index (kg/m2) was calculated as an index of obesity. Hypertension was defined as an SBP of 140 mmHg or greater, a diastolic blood pressure of 90 mmHg or greater, or the current use of antihypertensive agents. Chronic kidney disease was defined as a glomerular filtration rate (GFR) ≤60 mL/min/1.73 m2. The GFR was estimated using the simplified prediction equation derived from the Modification of Diet in a Renal Disease study.Citation25 During each echocardiographic study, the left ventricular ejection fraction (LVEF) was calculated using the modified Simpson’s method. The tricuspid regurgitation velocity was obtained by continuous wave Doppler from the right ventricular inflow or the apical four-chamber view position. The tricuspid regurgitation pressure gradient (TRPG) was calculated as follows: TRPG =4× tricuspid regurgitation velocity.Citation2 Clinical data were obtained just prior to discharge when the hemodynamic conditions of the patients had stabilized. Twelve months after the treatment initiation, all parameters were re-measured and compared with the initial data.
The primary endpoint was the differences in all-cause mortality between the β-blocker use and non-use groups during the mean clinical follow-up of 33.9 months. The secondary endpoints were the differences in composite events, including the all-cause mortality and hospitalization rates for CHF and/or COPD exacerbation, between patients receiving carvedilol and bisoprolol.
Statistical analysis
The SPSS software package Version 20.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. All continuous values are expressed as means ± standard deviations, and data of categorical variables are expressed as the number and percentage of patients. Survival and cardiac event-free curves were created using the Kaplan–Meier method, and differences in the survival and cardiac event-free rates among the groups were analyzed using the log-rank test. The relative risks in each group were calculated using Cox regression analyses, and P-values of less than 0.05 were considered statistically significant.
Results
We divided the patients into two groups according to whether a β-blocker was prescribed at discharge or not (β-blocker group, n=86; non-β-blocker group, n=46). The baseline characteristics of the patients are shown in . There were no significant differences between the two groups in terms of age, sex, prevalence of hypertension, atrial fibrillation, chronic kidney disease or ischemic heart disease, brain natriuretic peptide (BNP) levels, forced expiratory volume in 1 second (FEV1), or medical treatment at the time of discharge. However, patients not receiving β-blockers had a significantly higher HR at the 1-year follow-up compared with patients receiving β-blockers. In the β-blocker group, 52 patients (60.5%; mean dose: 6.69 mg/day) received carvedilol at discharge, whereas 34 patients (39.5%; mean dose: 2.96 mg/day) received bisoprolol. Regarding the different β-blockers, the clinical characteristics, excluding the HR and LVEF, did not significantly differ between the carvedilol and bisoprolol groups (). Higher HR and ejection fraction (EF) were observed in the patients receiving bisoprolol. The mean baseline FEV1 values were 1.70 L and 1.52 L in patients receiving carvedilol and bisoprolol, respectively (P=0.344).
Table 1 Clinical characteristics of the study population
Clinical outcomes
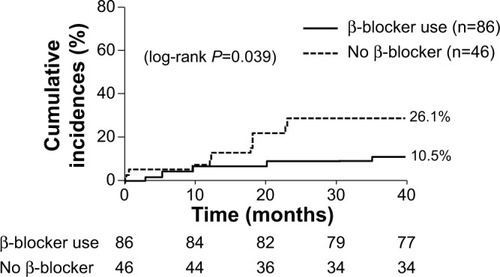
During the mean follow-up period of 33.9 months, the primary endpoint event (all-cause mortality) occurred in 9 patients (10.5%) in the β-blocker group and 12 patients (26.1%) in the non-β-blocker group (log-rank P=0.039) (). β-blocker therapy significantly reduced the risk of all-cause death in the univariate analysis (unadjusted hazard ratio [HR]: 0.41; 95% confidence interval [CI]: 0.17–0.99; P=0.047) (). However, no significant association was observed between β-blocker use and all-cause mortality in the multivariate analysis (). Evaluation of the causes of death revealed that in the non-β-blocker group, six, three, two, and one patients died of infection-unrelated COPD exacerbation, COPD exacerbation, sudden cardiac death, and acute HF, respectively. In the β-blocker group, three, two, two, and two patients died of infection-unrelated COPD exacerbation, COPD exacerbation, sudden cardiac death, and acute HF, respectively. The percentages of each cause of death did not significantly differ between the two treatment groups.
Table 2 HRs for the risk of all-cause mortality
Figure 1 Cumulative incidence of all-cause death.
Abbreviation: COPD, chronic obstructive pulmonary disease.
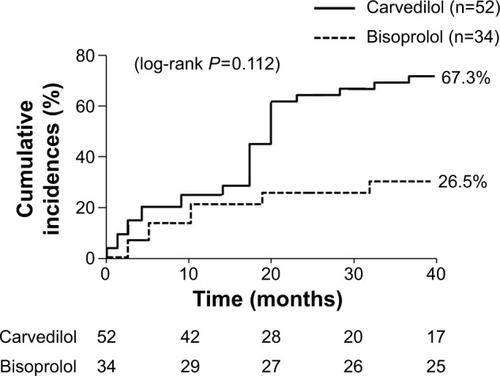
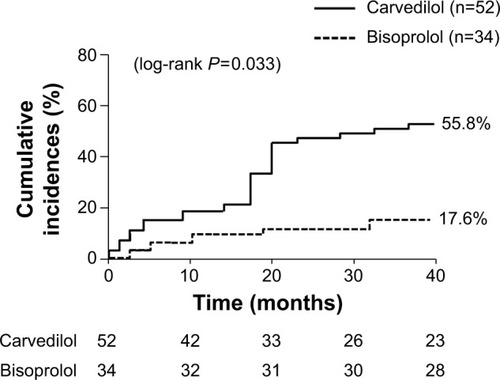
The secondary endpoint events occurred in 35 patients (67.3%) in the carvedilol group and 9 patients (26.5%) in the bisoprolol group (log-rank P=0.112) (). CHF and/or COPD exacerbation occurred in 29 patients (55.8%) in the carvedilol group and 6 patients (17.6%) in the bisoprolol group (log-rank P=0.033) (). The rate of all-cause death did not significantly differ between the two groups (11.5% vs 8.8% in the carvedilol vs bisoprolol groups, data not shown). Univariate Cox regression analysis revealed that bisoprolol significantly reduced the risk of re-hospitalization due to CHF and/or COPD exacerbation (unadjusted HR: 0.38; 95% CI: 0.15–0.98; P=0.046) but carvedilol did not (). In the multivariate analysis, only a past history of COPD exacerbation significantly increased the risk of re-hospitalization due to CHF and/or COPD exacerbation (adjusted HR: 3.11; 95% CI: 1.47–6.61; P=0.003) (). presents the values of SBP, HR, LVEF, TRPG, BNP levels, and estimated GFR at baseline and at the 1-year follow-up. The 1-year changes in SBP, LVEF, TRPG, BNP levels, and estimated GFR were not significantly different between the two groups. However, the decrease in HR was greater in the bisoprolol group than in the carvedilol group.
Table 3 HRs for the risk of COPD or CHF exacerbation
Table 4 Changes of the factors associated with heart failure
Discussion
In the present study, we examined the effects of β-blockers in patients with HF and COPD. Approximately, 70% of the HF patients with COPD had received β-blockers, and these were found to significantly reduce the all-cause mortality. Moreover, the baseline HR and EF before β-blocker administration were found to be higher in patients receiving bisoprolol compared with patients receiving carvedilol, whereas re-hospitalization due to CHF and/or COPD exacerbation occurred less frequently in patients receiving bisoprolol compared to patients receiving carvedilol.
Up to one-third of all deaths in patients with COPD can be attributable to cardiovascular disease, and, for every 10%-decrease in the FEV1, the risk of cardiovascular mortality reportedly increases by 28%.Citation26–Citation28 The mechanistic links between COPD and cardiovascular disease are complex, multifactorial, and not entirely understood. Hyperinflation and increased falls in the intrathoracic pressure might compromise the ventricular preload and afterload, consequently leading to left ventricular dysfunction and acute heart failure. As most of these factors occur concurrently at the moment of acute exacerbation, these episodes are often associated with major cardiovascular events and high mortality.Citation29 Despite increasing evidence that β-blockers are safeCitation30,Citation31 and can be beneficial in patients with COPD, their use continues to be limited in this group. This is a worldwide phenomenonCitation32 and might be related to historical concerns that β-blockers could be harmful in patients with COPD, for example, by inducing bronchospasm. Such concerns, however, have been challenged by recent evidence,Citation33 with several recent studies having advanced arguments in support of the use of β-blockers for COPD patients.Citation34–Citation38 One of these studies showed that β-blockers might reduce the risk of mortality and exacerbations in patients with COPD.Citation34 Similarly, a systematic review and meta-analysis of nine retrospective cohort studies reported a reduction in COPD-related mortality of 31% after β-blocker use,Citation39 and yet another study clearly showed the safety of β-blockers during COPD exacerbations.Citation32 Moreover, the use of β-blockers when started either at the time of hospital admission for myocardial infarction or before a myocardial infarction has been demonstrated to be associated with improved survival after myocardial infarction in patients with COPD.Citation40 In our study, similar to in these previous reports, β-blocker use was found to be associated with lower mortality compared to non-β-blocker use in CHF patients with COPD. Receiving β-blockers decreases the mortality rate through lowering the HR and through their sympatholytic effects,Citation41 and β-blockers might benefit patients with HF and COPD, not only because of their effect on the CHF but also because they can affect the course of the COPD itself.
Regarding the different types of β-blockers, we here compared carvedilol, a typical non-selective β-adrenergic, and α1-AR blocker with bisoprolol, a typical highly selective β1-AR blocker, in order to determine which β-blocker is more appropriate for HF and COPD patients, a question that remains controversial. Bisoprolol is highly β1-selective, providing a wide split between the β1- and β2-adrenocepter blockades.Citation42,Citation43 Further, bisoprolol has recently been shown to be safe in patients with COPD. In a Cochrane database analysis, cardioselective β-blockers were demonstrated to not adversely affect the FEV1, respiratory symptoms, or the response of FEV1 to β2 agonists.Citation44 Conversely, it has been reported that carvedilol may aggravate pulmonary complications through blocking of the β2 receptors, and its effects markedly differ between individuals due to different genetic polymorphisms, as compared with bisoprolol.Citation45–Citation47 Accordingly, switching from carvedilol to bisoprolol may provide beneficial effects with regard to the prognosis and/or reverse remodeling in patients who experience difficulty with the continuation or uptitration of carvedilol due to adverse effects such as dizziness or hypotension.Citation48,Citation49
Several studies have shown that the mortality benefits of carvedilol and other β-blockers are dose dependent,Citation14,Citation18,Citation50 and the dose of carvedilol is moreover considered important for the management of cardiac sympathetic nerve activity.Citation51 Cardioselective β-blockers are also dose dependent,Citation5 and it is also recommended to use β-blockers that are more selective for the β1-AR but without intrinsic sympathomimetic activity at the lowest dose and to titrate them slowly, while paying close attention to lung function and symptoms.Citation52 The doses of the two β-blockers used in our study were lower than the doses commonly used in many large-scale trials. However, trials on Japanese patients with HF have indicated that the BP and HR can be adequately decreased with the use of low doses of carvedilol and bisoprolol.Citation18,Citation53 These differences in the doses administered to Japanese patients and patients of other nationalities may be related to the pharmacological heterogeneity of β-blockers.
Furthermore, bisoprolol is less likely to cause bronchospasm compared to non-selective β-blockers,Citation9 and the risk of bronchospasm can be further reduced by starting the β-blockers at a lower dose and slowly titrating up. In our study, the reduction in HR was more prominent in the bisoprolol group than in the carvedilol group at the 1-year follow-up. This result suggests that bisoprolol has a greater ability to suppress the HR of patients with HF and COPD than carvedilol, as the former is a highly selective β1-AR blocker. In addition, treatment with bisoprolol was also associated with a significantly reduced risk of re-hospitalization due to CHF and/or COPD exacerbation. On the other hand, in the sub-analysis of the OPTIMIZE-HF study, patients receiving non-cardioselective β-blockers had a lower event rate compared to patients receiving cardioselective β-blockers.Citation22 However, it should be noted that, compared to the OPTIMIZE-HF study, the sample size and number of events of this study were relatively small, and re-hospitalization due to COPD exacerbation was also included in the analyzed events, owning to difficulty in distinguishing CHF from COPD exacerbation. Furthermore, only bisoprolol was used as the cardioselective β-blocker in this study, whereas several different selective β-blockers were used in the OPTIMIZE-HF study.
Further, our results suggested that low doses of bisoprolol could also reduce the all-cause mortality in patients with HF and COPD, and it was moreover found to be associated with a significantly reduced risk of re-hospitalization due to CHF and/or COPD exacerbation compared with carvedilol. However, further prospective randomized controlled studies are needed to confirm our results and before initiation of β-blocker therapy to achieve a mortality benefit in COPD patients can be widely recommended.
Study limitations
The main limitations of the present study were the relatively small number of enrolled patients and the retrospective study design. Moreover, the β-blockers were prescribed at discharge for the β-blocker group; at this time, the use of β-blockers was left at the discretion of the treating physicians. Therefore, the beneficial effects of the β-blockers in the present study can only be attributable to the time period of discharge, and we could not exclude the possibility that the addition and selection of the two β-blockers were made on the basis of the clinical status of the patients. The baseline EF was higher in patients receiving bisoprolol compared with patients receiving carvedilol; however, the EF did not significantly correlate with the incidence of CHF or COPD exacerbation in our study. Lastly, regarding the two β-blockers used in this study, different doses were prescribed, given that the equivalent dose of bisoprolol is only one-fifth that of carvedilol.Citation54,Citation55 Although most confounders were included in the multivariate regression analysis, it is nonetheless possible that some potential bias exists, and large-scale, prospective randomized controlled trials are needed in the future to clarify the effects of β-blockers in patients with HF and COPD.
Conclusion
Our findings support the recommendations to use β-blockers in HF patients with COPD. The use of a selective β-blocker reduced the incidence of CHF and/or COPD exacerbation compared with non-selective β-blocker use in the univariate analyses in the present study; however, after controlling for other variables, the effect of β-blockers ceased to be significant. Further studies with longer duration and larger numbers of HF and COPD patients are needed to confirm whether there are any differences between the effects of the two types of β-blockers on HF and COPD improvement.
Disclosure
The authors declare that they have no competing interests.
References
- LainscakMClelandJGLenzenMJFollathFKomajdaMSwedbergKInternational variations in the treatment and co-morbidity of left ventricular systolic dysfunction: data from the EuroHeart Failure SurveyEur J Heart Fail2007929229917023204
- HawkinsNMPetrieMCJhundPSChalmersGWDunnFGMcMurrayJJHeart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiologyEur J Heart Fail20091113013919168510
- SinDDMcAlisterFAThe effects of beta-blockers on morbidity and mortality in a population-based cohort of 11,942 elderly patients with heart failureAm J Med200211365065612505115
- KomajdaMFollathFSwedbergKThe EuroHeart Failure Survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 2: treatmentEur Heart J20032446447412633547
- HawkinsNMPetrieMCMacdonaldMRHeart failure and chronic obstructive pulmonary disease the quandary of Beta-blockers and Beta-agonistsJ Am Coll Cardiol2011572127213821596228
- DicksteinKCohen-SolalAFilippatosGESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM)Eur Heart J2008292388244218799522
- Al-MohammadAMantJThe diagnosis and management of chronic heart failure: review following the publication of the NICE guidelinesHeart20119741141621296784
- GottliebSSMcCarterRJVogelRAEffect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarctionN Engl J Med19983394894979709041
- GalatiusSGustafssonFAtarDHildebrandtPRTolerability of beta-blocker initiation and titration with bisoprolol and carvedilol in congestive heart failure – a randomized comparisonCardiology200410216016515334027
- JabbourAMacdonaldPSKeoghAMDifferences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trialJ Am Coll Cardiol2010551780178720413026
- LainscakMPodbregarMKovacicDRozmanJvon HaehlingSDifferences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trialRespir Med2011105suppl 1S44S4922015086
- NiYShiGWanHUse of cardioselective beta-blockers in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized, placebo-controlled, blinded trialsJ Int Med Res2012402051206523321161
- LothDWBrusselleGGLahousseLHofmanALeufkensHGStrickerBHBeta-Adrenoceptor blockers and pulmonary function in the general population: the Rotterdam StudyBr J Clin Pharmacol20147719020023772842
- BristowMRGilbertEMAbrahamWTCarvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA InvestigatorsCirculation199694280728168941106
- PackerMBristowMRCohnJNThe effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study GroupN Engl J Med1996334134913558614419
- PackerMFowlerMBRoeckerEBCarvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study GroupEffect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) studyCirculation20021062194219912390947
- Poole-WilsonPASwedbergKClelandJGCarvedilol Or Metoprolol European Trial InvestigatorsComparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trialLancet200336271312853193
- HoriMSasayamaSKitabatakeAMUCHA InvestigatorsLow-dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: the Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trialAm Heart J200414732433014760332
- A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and CommitteesCirculation199490176517737923660
- The Cardiac Insufficiency Bisoprolol Study II(CIBIS-II): a randomised trialLancet199935391310023943
- WillenheimerRvan VeldhuisenDJSilkeBCIBIS III InvestigatorsEffect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) IIICirculation20051122426243516143696
- MentzRJWojdylaDFiuzatMChiswellKFonarowGCO’ConnorCMAssociation of beta-blocker use and selectivity with outcomes in patients with heart failure and chronic obstructive pulmonary disease (from OPTIMIZE-HF)Am J Cardiol201311158258723200803
- McKeePACastelliWPMcNamaraPMThe natural history of congestive heart failure: the Framingham studyN Engl J Med1971285144114465122894
- RabeKFHurdSAnzuetoAGlobal Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med200717653255517507545
- LeveyASBoschJPLewisJBGreeneTRogersNRothDA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study GroupAnn Intern Med199913046147010075613
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J20083296296918579551
- ZielinskiJMacNeeWWedzichaJCauses of death in patients with COPD and chronic respiratory failureMonaldi Arch Chest Dis19975243479151520
- AnthonisenNRConnettJEMurrayRPSmoking and lung function of Lung Health Study participants after 11 yearsAm J Respir Crit Care Med200216667567912204864
- HolguinFFolchEReddSCManninoDMComorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001Chest20051282005201116236848
- DransfieldMTRoweSMJohnsonJEBaileyWCGeraldLBUse of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPDThorax20086330130517951276
- ShortPMLipworthSIElderDHEffect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort studyBMJ2011342d254921558357
- StefanMSRothbergMBPriyaAPekowPSAuDHLindenauerPKAssociation between beta-blocker therapy and outcomes in patients hospitalised with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertensionThorax20126797798422941975
- AlbouainiKAndronMAlahmarABeta-blockers use in patients with chronic obstructive pulmonary disease and concomitant cardiovascular conditionsInt J Chron Obstruct Pulmon Dis2007253554018268926
- RuttenFHZuithoffNPHakEGrobbeeDEHoesAWBeta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary diseaseArch Intern Med201017088088720498416
- FarlandMZPetersCJWilliamsJDBielakKMHeidelRERaySMBeta-Blocker use and incidence of chronic obstructive pulmonary disease exacerbationsAnn Pharmacother20134765165623585645
- ZengLHHuYXLiuLZhangMCuiHImpact of beta2-agonists, beta-blockers, and their combination on cardiac function in elderly male patients with chronic obstructive pulmonary diseaseClin Interv Aging201381157116524072964
- Puente-MaestuLCalleMOrtega-GonzálezAGEMEPOC GroupMulticentric study on the beta-blocker use and relation with exacerbations in COPDRespir Med201410873774424635914
- DuQSunYDingNLuLChenYBeta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studiesPLoS One20149e11304825427000
- EtminanMJafariSCarletonBFitzGeraldJMBeta-blocker use and COPD mortality: a systematic review and meta-analysisBMC Pulm Med2012124822947076
- QuintJKHerrettEBhaskaranKEffect of beta blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare recordsBMJ2013347f665024270505
- LechatPHulotJSEscolanoSHeart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II TrialCirculation20011031428143311245648
- DorowPBethgeHTonnesmannUEffects of single oral doses of bisoprolol and atenolol on airway function in nonasthmatic chronic obstructive lung disease and angina pectorisEur J Clin Pharmacol1986311431472879733
- Macquin-MavierIRoudot-ThoravalFClericiCGeorgeCHarfAComparative effects of bisoprolol and acebutolol in smokers with airway obstructionBr J Clin Pharmacol1988262792842902873
- SalpeterSOrmistonTSalpeterECardioselective beta-blockers for chronic obstructive pulmonary diseaseCochrane Database Syst Rev2005CD00356616235327
- NozawaTTaguchiMTaharaKInfluence of CYP2D6 genotype on metoprolol plasma concentration and beta-adrenergic inhibition during long-term treatment: a comparison with bisoprololJ Cardiovasc Pharmacol20054671372016220080
- TaguchiMNozawaTIgawaAPharmacokinetic variability of routinely administered bisoprolol in middle-aged and elderly Japanese patientsBiol Pharm Bull20052887688115863897
- HondaMOguraYToyodaWMultiple regression analysis of pharmacogenetic variability of carvedilol disposition in 54 healthy Japanese volunteersBiol Pharm Bull20062977277816595916
- NulDZambranoCDiazAGrupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en ArgentinaImpact of a standardized titration protocol with carvedilol in heart failure: safety, tolerability, and efficacy-a report from the GESICA registryCardiovasc Drugs Ther20051912513416025231
- TaniguchiTOhtaniTMizoteISwitching from carvedilol to bisoprolol ameliorates adverse effects in heart failure patients with dizziness or hypotensionJ Cardiol20136141742223548374
- SimonTMary-KrauseMFunck-BrentanoCBisoprolol dose-response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II)Eur Heart J20032455255912643888
- NishiyamaKTsutamotoTYamajiMDose-dependent prognostic effect of carvedilol in patients with chronic heart failure – special reference to transcardiac [corrected] gradient of norepinephrineCirc J2009732270227519838002
- MateraMGMartuscelliECazzolaMPharmacological modulation of beta-adrenoceptor function in patients with coexisting chronic obstructive pulmonary disease and chronic heart failurePulm Pharmacol Ther2010231819833222
- KonishiMHaraguchiGKimuraSComparative effects of carvedilol vs bisoprolol for severe congestive heart failureCirc J2010741127113420354334
- DüngenHDApostolovicSInkrotSCIBIS-ELD investigators and Project Multicentre Trials in the Competence Network Heart FailureTitration to target dose of bisoprolol vs carvedilol in elderly patients with heart failure: the CIBIS-ELD trialEur J Heart Fail20111367068021429992
- Di LenardaARemmeWJCharlesworthACOMET InvestorsExchange of beta-blockers in heart failure patients. Experiences from the poststudy phase of COMET (the Carvedilol or Metoprolol European Trial)Eur J Heart Fail2005764064915921806