?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A substantial proportion of patients with chronic obstructive pulmonary disease (COPD) develops various degree of intrathoracic tracheal collapsibility. We studied whether the magnitude of intrathoracic tracheal collapsibility could be different across clinical phenotypes and sex in COPD. Intrathoracic tracheal collapsibility measured at paired inspiratory–expiratory low dose computed tomography (CT) and its correlation with clinical, functional, and CT-densitometric data were investigated in 69 patients with COPD according to their predominant conductive airway or emphysema phenotypes and according to sex. Intrathoracic tracheal collapsibility was higher in patients with predominant conductive airway disease (n=28) and in females (n=27). Women with a predominant conductive airway phenotype (n=10) showed a significantly greater degree of collapsibility than women with predominant emphysema (28.9%±4% versus 11.6%±2%; P<0.001). Intrathoracic tracheal collapsibility was directly correlated with inspiratory–expiratory volume variation at CT and with forced expiratory volume (1 second), and inversely correlated with reduced CT lung density and functional residual capacity. Intrathoracic tracheal collapsibility was not correlated with cough and wheezing; however, intrathoracic tracheal collapsibility and clinical phenotypes of COPD are closely correlated. In patients with a predominant emphysematous phenotype, a reduced collapsibility may reflect the mechanical properties of the stiff hyperinflated emphysematous lung. The high collapsibility in patients with predominant airway disease, mild airway obstruction, and in women with this phenotype may reflect chronic airway inflammation. The lack of relationship with such symptoms as wheezing, cough, and dyspnea could indicate that intrathoracic tracheal collapsibility itself should be considered neither an abnormal feature of COPD nor a relevant clinical finding.
Introduction
Intrathoracic tracheal collapsibility refers to an expiratory collapse of the intrathoracic trachea due to tracheal wall weakness caused by reduction and atrophy of the pars membranacea longitudinal and elastic fibers, with consequent anterior bulging of the trachea posterior wall and/or impaired cartilage integrity.Citation1–Citation5 Various degrees of expiratory tracheal collapse can occur, up to tracheomalacia, when the reduction of intrathoracic tracheal cross-sectional area at computed tomography (CT) is more than 50%.Citation4 As well as some primary congenital syndromes such as polychondritis and Mounier-Kuhn disease, several acquired conditions associated with intrathoracic tracheal collapsibility have been described in adults.Citation1–Citation3
A substantial proportion of patients with chronic obstructive pulmonary disease (COPD) could develop various degrees of airway collapsibilityCitation6 due to chronic inflammation and irritant exposure, instability of the tracheal wall due to the loss of parenchymal elastic fibers, and repeated mechanical stress from coughing.Citation1–Citation3 Definite relationships of intrathoracic tracheal collapsibility have not been established, either with clinical symptoms and pulmonary function testing or with CT quantitative data of lung density and airway wall thickness.Citation6–Citation7 Furthermore, it remains unclear whether tracheal collapsibility should or should not be considered an associated pathological finding of COPD. As a matter of fact, unexpectedly high degrees of intrathoracic tracheal collapsibility have been shown to be present also in normal asymptomatic subjects.Citation8
We studied intrathoracic tracheal collapsibility in a group of patients with COPD and assessed whether its magnitude could be related to clinical features of the disease, to predominant conductive airway disease or predominant emphysema clinical characteristics, to pulmonary function, and to CT densitometric data. Because women with COPD have been reported to display different clinical manifestations of the disease such as more frequent exacerbations, more severe dyspnea, more frequent cough, and increased sputum production than men,Citation9–Citation11 we studied whether the magnitude of tracheal collapsibility in COPD could differ according to sex.
Methods
Patients
Sixty-nine outpatients with COPD (42 males) were consecutively enrolled and completed the study. The study was approved by the hospital ethics committee, and informed written consent was obtained from all patients. We included patients aged 40–85 years, who were smokers or former smokers with a smoking history of at least 10 pack-years. COPD was defined according to a postbronchodilator forced expiratory volume (1 second) (FEV1)/forced vital capacity <0.70 and not reversible airflow obstruction after bronchodilation.Citation12 We excluded subjects who had an exacerbation within 3 months or those who had clinical conditions that could interfere with the assessment of pulmonary function or chest CT quantitative parameters, including asthma, a history of upper airway obstruction, diffuse bronchiectasis, cystic fibrosis, interstitial lung disease, acute heart failure, chemoradiation therapy, lung cancer, lung surgery, known or suspected pregnancy, and metal objects in the chest.
Patients’ evaluation consisted of thorough clinical history, physical examination, whole pulmonary function testing, and inspiratory and expiratory CT scan. Dyspnea severity was assessed by the modified Medical Research Council (mMRC) dyspnea scale. Static and dynamic lung volumes and single breath diffusing capacity for carbon monoxide (DLCO) were measured by a mass-flow sensor and a multigas analyser (V6200 Autobox body plethysmograph; SensorMedics, Yorba Linda, CA, USA) according to American Thoracic Society/European Respiratory Society guidelines and expressed as percentage of the predicted values.Citation12 Each patient was assigned either a predominant airway or a predominant emphysema clinical phenotype according to a multivariate model based on sputum characteristics and lung function variables, which was validated by quantitative CT.Citation13 The variables entered to classify patients according to the predominant mechanism of airflow limitation were linearly combined to determine the relative predominance of conductive airway (negative value) or emphysema phenotype (positive value) in each patient according to the following computation:Citation13
Imaging assessment
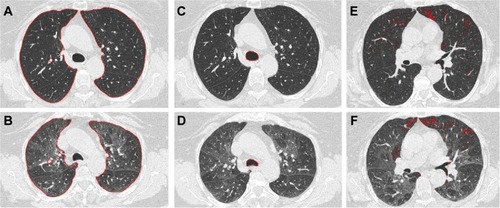
CT examinations were performed on a 16-detector row scanner (Sensation 16, Siemens, Munich, Germany) using a detector configuration of 16×0.75 mm, 140 kVp, 150 effective mAs, tube rotation time of 750 ms, and a pitch of 1.5. Two spiral acquisitions were obtained from the apex to the base during breath-hold at full inspiration and at the end of maximal expiration, respectively. Before undergoing CT scanning, patients were coached by a pulmonologist to hold their breath at full inspiration and full expirationCitation7 and trained to perform inspiratory and expiratory vital capacity maneuvers while lying on the CT examination bed. The acquisition time was 8–12 seconds. For whole lung optimized densitometry, we obtained thick (5 mm) slices with smooth filter (B31S) and 5 mm reconstruction increment.Citation14 For visual qualitative assessment, we used thin (1 mm) slices with sharp filter (B70S) and 1 mm reconstruction increment.Citation14 The system was calibrated daily with air.Citation15 No intravenous contrast material was administered. Inspiratory and expiratory whole lung volumes were automatically measured after lung segmentation () and delta volume was calculated by the following computation:
Figure 1 Application of the proposed algorithms on the CT scans of a COPD patient.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography.
Intrathoracic tracheal collapsibility was assessed by an algorithm for trachea segmentation () entailing the following steps: 1) gray-level thresholding of the CT image; 2) three-dimensional connected component labeling to select lungs, trachea, and main bronchi; and 3) using a region-growing procedure to extract a 5 cm length of trachea starting from 1 cm above the carina. The volume of the selected tracheal segment was calculated for inspiratory (inspTV) and expiratory (expTV) CT scans. Intrathoracic tracheal collapsibility was measured according to the following computation:Citation16
To obtain whole lung densitometric data we utilized a software program that automatically determines the boundaries of each lungCitation17 and the percentage of total lung area with density values below −950 Hounsfield units in the inspiratory scan and below −910 Hounsfield units in the expiratory scan (%LAA−950insp and %LAA−910exp, respectively) (). These density thresholds have been demonstrated to be correlated with the anatomic extent of emphysema in the inspiratory and expiratory chest CT scan.Citation18 Bronchial wall thickness was measured by means of an electronic caliper according to a previously published method.Citation19 As bronchial tree dimensions depend on subjects height and sex, the bronchial wall thickness was normalized in each patient by plotting the square root of the airway wall area against the internal perimeter of each measurable airway. The resulting regression line was used to predict the square root of the wall area for an ideal airway with an internal perimeter of 10 mm and to calculate its average wall thickness (AWTPi10) representing the distal airways compartment in COPD.Citation13
Statistical analysis
Data are presented as means ± standard deviation for continuous variables and as counts and proportions for nominal and ordinal variables. Pearson’s correlation coefficients were used to study the association between quantitative variables; t-test and ANOVA were used to compare the means of continuous variables among different subsets of patients. Fisher’s exact test was used to compare the prevalence of categorical variables among different subsets of patients. Significance was set at P<0.05. A multivariate analysis was performed to estimate tracheal collapsibility (dependent variable) using functional, clinical, and CT data as independent variables. In each of the examined subsets of patients the most significant model with the least number of variables was selected by a forward stepwise process. Parameters were entered or removed from the process according to F-statistics.
Results
Patient features
Anthropometric, smoke exposure, and functional and quantitative CT data of the recruited patients are displayed in and . Among the 69 patients, 28 were classified as being affected by predominant airway disease and 41 by predominant emphysema.Citation13 Patients with predominant emphysema had a significantly higher smoking exposure and lower body mass index (BMI). They also showed a significantly higher degree of airflow obstruction, hyperinflation, and DLCO reduction. AWTPi10 did not significantly differ between the two subgroups.
Table 1 Anthropometric data, smoke exposure, pulmonary function, and presence of purulent sputum in 69 patients with COPD subdivided according to the predominant mechanism of air flow limitation and sex
Table 2 CT quantitative densitometric data, airway wall thickness, inspiratory–expiratory volume variation, and tracheal collapsibility in 69 patients with COPD subdivided according to the predominant mechanism of air flow limitation and sex
Relationship between intrathoracic tracheal collapsibility and clinical, functional, and CT variables
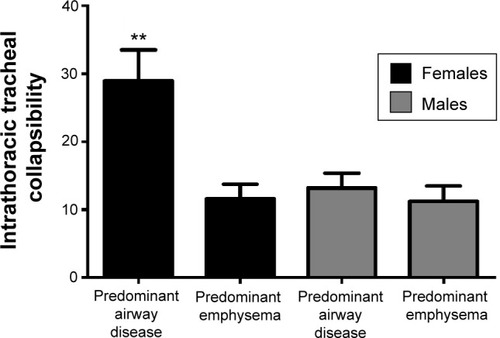
Patients with predominant airway disease had a higher intrathoracic tracheal collapsibility than patients with predominant emphysema ().
The relationships between intrathoracic tracheal collapsibility and lung function parameters are reported in . shows the relationship of intrathoracic tracheal collapsibility and CT expiratory and inspiratory density expressed as %LAA−910exp and %LAA−950insp, respectively. Intrathoracic tracheal collapsibility was positively, although weakly, correlated with FEV1, negatively correlated with functional residual capacity (% of predicted) (FRC%) in the whole set, and more strongly in patients with predominant emphysema. Intrathoracic tracheal collapsibility was negatively correlated with %LAA−910exp and %LAA−950insp in the whole sample and in each subset. shows the positive relationships between changes in lung volume measured at CT from maximal inspiration to maximal expiration and intrathoracic tracheal collapsibility in the whole sample and each subset.
Table 3 Relationships of intrathoracic tracheal collapsibility with functional parameters indicative of airflow obstruction and hyperinflation
Table 4 Relationships of intrathoracic tracheal collapsibility with CT thresholds of lung inspiratory and expiratory density (%LAA−950insp and %LAA−910exp, respectively)
Figure 2 Relationship of intrathoracic tracheal collapsibility with delta volume ([inspiratory CT volume – expiratory CT volume]/inspiratory CT volume) in 69 patients with COPD (A); 28 of whom were classified as being affected by predominant airway disease (B); 41 of whom were classified as being affected by predominant emphysema (C); males (D); and females (E).
![Figure 2 Relationship of intrathoracic tracheal collapsibility with delta volume ([inspiratory CT volume – expiratory CT volume]/inspiratory CT volume) in 69 patients with COPD (A); 28 of whom were classified as being affected by predominant airway disease (B); 41 of whom were classified as being affected by predominant emphysema (C); males (D); and females (E).](/cms/asset/2f0bc97d-2f8f-4747-b28f-48e5457dfe02/dcop_a_80558_f0002_b.jpg)
Relationship between intrathoracic tracheal collapsibility and symptoms
No correlation was found between intrathoracic tracheal collapsibility and the following variables: wheezing in the whole group of patients (mean intrathoracic tracheal collapsibility in patients with wheezing: 11±2; mean intrathoracic tracheal collapsibility in patients without wheezing: 16±2, P=0.16); predominant airway disease (mean intrathoracic tracheal collapsibility in patients with wheezing: 13±3; mean intrathoracic tracheal collapsibility in patients without wheezing: 22±3, P=0.08); predominant emphysema (mean intrathoracic tracheal collapsibility in patients with wheezing: 9±3; mean intrathoracic tracheal collapsibility in patients without wheezing: 12±2, P=0.44); male sex (mean intrathoracic tracheal collapsibility in patients with wheezing: 9±2; mean intrathoracic tracheal collapsibility in patients without wheezing: 14±2, P=0.16); female sex (mean intrathoracic tracheal collapsibility in patients with wheezing: 18±3; mean intrathoracic tracheal collapsibility in patients without wheezing: 17±5, P=0.77); cough in the whole group of patients (mean intrathoracic tracheal collapsibility in patients with cough: 15±2; mean intrathoracic tracheal collapsibility in patients without cough: 11±3, P=0.25); predominant emphysema phenotype (mean intrathoracic tracheal collapsibility in patients with cough: 12±2; mean intrathoracic tracheal collapsibility in patients without cough: 11±3, P=0.81); male sex (mean intrathoracic tracheal collapsibility in patients with cough: 12±1; mean intrathoracic tracheal collapsibility in patients without cough: 12±6, P=0.96); female sex (mean intrathoracic tracheal collapsibility in patients with cough: 21±3; mean intrathoracic tracheal collapsibility in patients without cough: 10±3, P=0.08). All patients with predominant airway disease had productive cough with no different magnitude of intrathoracic tracheal collapsibility in relation to sputum purulence (18±3 in patients with purulent sputum and 20±4 in patients with no purulent sputum, P=0.72). On the contrary, mMRC dyspnea score was significantly higher in patients with an emphysema phenotype of COPD and a lower degree of intrathoracic tracheal collapsibility with respect to patients with predominant airway disease (mean mMRC 2.2±0.2 and 1.2±0.2, respectively, P<0.01).
Relationship between intrathoracic tracheal collapsibility and sex
Females with COPD were less severe, had less severe reduction in %LAA−950insp and %LAA−910exp, lower AWTPi10, and a significantly higher degree of tracheal collapsibility with respect to males ( and ). The proportion of the two COPD phenotypes was the same in males and females (24/18 predominant emphysema/predominant airway disease among males respectively; 17/10 predominant emphysema/predominant airway disease among females; Fisher’s exact test P=0.8, not significant). Women with predominant airway disease showed a significantly greater degree of tracheal collapsibility than women with predominant emphysema phenotype (28.9%±4% versus 11.6%±2%, P<0.001). No difference in intrathoracic tracheal collapsibility was found in the two predominant phenotypes (13.2%±2% in predominant airway disease versus 11.2%±2% in predominant emphysema, P=0.26) in males ().
Multivariate analysis
The results of multivariate analysis in the whole set of patients, in predominant airway and predominant emphysema phenotypes, in males and in females are reported in . Delta volume is the parameter that in all subsets described most of the variance of tracheal collapsibility.
Table 5 Predictive models of intrathoracic tracheal collapsibility by multivariate regression analysis of clinical history data (cough, wheezing, dyspnea), pulmonary function, and CT variables in the whole set of patients with COPD and in the examined subsets
Discussion
We found that intrathoracic tracheal collapsibility was increased in patients with a predominant airway phenotype of COPD, positively related with FEV1, and with changes in CT lung volume from the inspiratory to the expiratory scans, and negatively related with FRC%, with %LAA−950insp and with %LAA−910exp. Intrathoracic tracheal collapsibility was not related with common symptoms of COPD such as cough and wheezing. Intrathoracic tracheal collapsibility was significantly higher in females with respect to males. This finding was more evident in females with a predominant airway disease phenotype of COPD.
Relationships of intrathoracic tracheal collapsibility with conductive airway disease phenotype and with functional and densitometric parameters
The observation of a higher degree of intrathoracic tracheal collapsibility in patients with predominant airway disease confirms previous findings.Citation6 This can been explained by the presence of a common inflammatory process involving upper and lower airways and resulting in tracheal collapsibility and bronchial wall thickening in patients who have clinical and functional characteristics of predominant airway disease. It has been reported that chronic cough, the most frequent symptom in COPD patients with predominant airway disease, could cause repeated mechanical stress with stretching and degeneration of the posterior membranous portion of the trachea.Citation20
Similarly to what has been found by Han et al in the COPDGene study,Citation10 our patients with predominant airway disease were affected by a less severe form of COPD with respect to those affected by emphysema.
That intrathoracic tracheal collapsibility reflects not only the degree of airway obstruction but also lung volume and lung emptying during expiration is shown by its positive correlation with FEV1 and with delta volume, the predictive value of delta volume at multivariate analysis, and the negative relationship with FRC%, %LAA−910exp, and %LAA−950insp. In other words, intrathoracic tracheal volume reduction (collapsibility) and lung volume reduction (delta volume) from maximal inspiration to maximal expiration are proportional, and tracheal collapsibility expresses the capability of the lungs to deflate during expiration. In fact, expiratory lung volume reduction is significantly lower in patients with emphysema and tends to be lower in males with respect to females as reported in .
This confirms previous findings of Yamashiro et alCitation21 and supports the view that hyperinflated patients have small changes in lung volume during the respiratory cycle, and, as a consequence, the intrathoracic pressure and tracheal size at end expiration do not change significantly with respect to inspiration. The overinflated lung of the emphysematous patients could indeed be assimilated to a stiff system, where the altered mechanic and elastic properties do allow neither significant parenchymal inflation and deflation nor tracheal collapsibility during the respiratory cycle. This hypothesis is strongly supported both by the positive relationship between tracheal collapsibility and delta volume and by the negative relationship between tracheal collapsibility with FRC% and %LAA.
In a very recent paper, Boiselle et alCitation22 described a significant association of tracheal collapsibility with obesity in COPD. This finding could be at least in part ascribed to the lung volume changes caused by obesity. In these patients FRC is often near the lower limit of normality or slightly abnormal, and patients often display clinical and functional features of predominant airway phenotype of COPD.
Our results are in contrast with those of Boiselle et alCitation23 and Sverzellati et alCitation6 who did not find any correlation between lung function and the magnitude of tracheal collapsibility measured at the end of forced expiration. In both studies, forced expiratory maneuvers alone could have reduced lung emptyingCitation12 by shortening expiratory time and causing early airways closure. This could be accentuated by both severe obstruction and supine position. Furthermore, during forced inspiratory–expiratory maneuvers, intrathoracic tracheal collapsibility might be influenced by the relative force of the expiratory and inspiratory muscles. We have no direct measurement of the respiratory muscle strength in our patients; however, we observed that BMI was significantly lower in patients with predominant emphysema () and that it was an independent predictor of intrathoracic tracheal collapsibility in this phenotype (). This could imply also that patients with reduced BMI could have reduced muscle strength to perform forced maneuvers. In this connection, the static technique we have used could be appropriate for hyperinflated patients.
Intrathoracic tracheal collapsibility is not related to clinical features of COPD
The lack of relationship between cough, wheezing, and intrathoracic tracheal collapsibility is another important finding of our paper. A significantly higher dyspnea perception measured by mMRC scale has been found in patients with an emphysematous phenotype and a lower degree of intrathoracic tracheal collapsibility. These findings are in keeping with recently published data showing a wide spectrum of tracheal collapse also among healthy volunteers without respiratory symptoms.Citation8 This last result and the direct relationship between intrathoracic tracheal collapsibility and FEV1%, suggest that the magnitude of expiratory tracheal collapsibility is associated with less impaired lung function and could not be linked to a pathologic process.
Intrathoracic tracheal collapsibility is more frequent in women with COPD
In this series of COPD patients, women showed a significantly higher degree of tracheal collapsibility than men. The highest tracheal collapsibility was observed in women with a predominant airway phenotype. Taking into account that the proportion of predominant airway and predominant emphysema phenotype of COPD was the same in women and men, this finding could suggest a sex effect on tracheal collapsibility; on the other hand, we cannot exclude that the high degree of intrathoracic tracheal collapsibility in women could be due to a better preserved lung function, with a significantly lower degree of airway obstruction and of emphysema at CT. This finding was confirmed when comparing tracheal collapsibility and lung function in the whole set of patients, and it is in agreement with the results of another study in which a high degree of tracheal collapsibility was found in healthy young women as compared to men.Citation24 Although the higher level of tracheal collapsibility in women could be included among the different sex-based expressions of COPD, our results cannot be conclusive considering the low number of observation. Other studies have shown that women have both smaller airways’ lumina and thicker bronchial wall and tend to exacerbate more frequently than men.Citation10,Citation11
We found, as already reported by Kim et alCitation25 that airway wall thickness measured by AWTPi10 was lower in women with respect to men. Remarkably, the values of AWTPi10 found in this study for men and women had the same mean value of those found by Kim et al.Citation25 On the other hand, other studies demonstrated that women had higher wall thickness in the subsegmental and subsubsegmental paths, through all Global Initiative for Chronic Obstructive Lung Disease stages.Citation10,Citation11 Thus, in regard to whether airways are thicker in men compared to women, we have to first consider what definition of airway dimension are employed. Anyway, further studies will be needed to clarify these discrepancies and their clinical relevance, and to evaluate which airway parameter measured at CT could be more relevant in clinical setting.
We recognize that this study has some limitations. First, as in most studies on this subject,Citation6,Citation7,Citation23,Citation24 the value of the results obtained is limited by the small sample of COPD patients recruited. Second, we acquired expiratory scans at the end of a maneuver of slow vital capacity, which could be a less sensitive technique,Citation7 underestimating tracheal collapsibility with respect to dynamic expiratory scans.Citation26 Nevertheless, the significant differences we obtained in intrathoracic tracheal collapsibility will be amplified with the more sensitive dynamic acquisition technique. Moreover, the end-expiratory scans we used have been proved to be effective to study patients with COPD.Citation21,Citation27,Citation28 Third, we investigated the degree of intrathoracic tracheal collapsibility in COPD without establishing a threshold value for excessive tracheal collapsibility or tracheomalacia. High threshold values up to 50% or 80% of expiratory reduction in tracheal cross-sectional area have been proposed to define excess of tracheal collapsibility in healthy subjects and patients scanned with dynamic expiration.Citation6,Citation23 Two lower thresholds to define excessive tracheal collapsibility have been proposed by Aquino et alCitation29 who used end expiratory CT scans. In ten patients, they obtained a positive predictive value of 89%–100% for tracheomalacia by using a threshold of more than 18% collapse for the upper trachea and of more than 28% for the mid-trachea. The magnitude of tracheal collapsibility data obtained in the Aquino et alCitation29 study, obtained with a slow vital capacity maneuver, fits well with the range of measurements of intrathoracic tracheal collapsibility found in this study.
Clinical implications
The finding of intrathoracic tracheal collapsibility at CT in patients with COPD is frequent, often incidental, and not correlated with symptoms. This implies that no treatment is required if there are no symptoms. In the presence of bronchospasm, this must be controlled as it could result in large pressure swings in the thorax with increased degree of intrathoracic tracheal collapsibility, increased airway resistance, and work of breathing. A complete functional assessment, a 6-minute walking test, and a quality of life questionnaire administration have been suggestedCitation1 to evaluate objectively the results of interventions. Treatment with noninvasive positive ventilation or with stenting or other surgical procedures should be considered in highly symptomatic patients not responsive to a conservative approach.Citation1
Conclusion
Intrathoracic tracheal collapsibility and clinical phenotypes of COPD are closely correlated. In patients with a predominant emphysematous phenotype, a less collapsible trachea reflects the mechanical properties of the stiff hyperinflated emphysematous lung. The higher degree of tracheal collapsibility found in patients with predominant airway disease, mild airway obstruction, and in women with this phenotype confirms the above mentioned finding. The lack of relationship between intrathoracic tracheal collapsibility and symptoms could indicate that this radiologic finding by itself should not be considered as an abnormal feature, and it should not be necessarily a pathologically relevant finding in patients with COPD.
Acknowledgments
This work was funded by a Research Project of the Italian Ministry of Health (project code RF-2010-2321362).
Disclosure
Doctors Camiciottoli and Diciotti were responsible for the funds allocation of the grant obtained by the Italian Ministry of Health. The other authors report no conflicts of interest in this work.
References
- CardenKABoisellePMWaltzDAErnstATracheomalacia and tracheobronchomalacia in children and adults: an in-depth reviewChest20051273984100515764786
- BoisellePMTracheomalacia: functional imaging of the large airways with multidetector-row CTBoisellePMWhiteCSNew Techniques in Cardiothoracic ImagingNew York, NYInforma2007177185
- FraserRSColmanNMüllerNLParéPDUpper airway obstructionFraserRSColmanNMüllerNLParéPDSynopsis of Diseases of the Chest4th edPhiladelphia, PASaunders199920422046
- LeeKSSunMRErnstAFeller-KopmanDMajidABoisellePMComparison of dynamic expiratory CT with bronchoscopy for diagnosing airway malacia: a pilot evaluationChest2007131375876417356090
- GilkesonRCCiancibelloLMHejalRBMontenegroHDLangePTracheobronchomalacia: dynamic airway evaluation with multidetector CTAJR Am J Roentgenol2001176120521011133567
- SverzellatiNRastelliAChettaAAirway malacia in chronic obstructive pulmonary disease: prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickeningEur Radiol20091971669167819205703
- YamashiroTSan José EstéparRMatsuokaSIntrathoracic tracheal volume and collapsibility on inspiratory and end-expiratory ct scans correlations with lung volume and pulmonary function in 85 smokersAcad Radiol201118329930521215660
- BoisellePMO’DonnellCRBankierAATracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CTRadiology2009252125526219420322
- CamiciottoliGCavigliEGrassiLPrevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trialEur Radiol2009191586618690451
- HanMKKazerooniEALynchDACOPDGene InvestigatorsChronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypesRadiology2011261127428221788524
- GrydelandTBDirksenACoxsonHOQuantitative computed tomography: emphysema and airway wall thickness by sex, age and smokingEur Respir J200934485886519324952
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- CamiciottoliGBigazziFPaolettiMCestelliLLavoriniFPistolesiMPulmonary function and sputum characteristics predict computed tomography phenotype and severity of COPDEur Respir J201342362663523258785
- StoelBCStolkJOptimization and standardization of lung densitometry in the assessment of pulmonary emphysemaInvest Radiol2004391168168815486529
- ParrDGStoelBCStolkJNightingalePGStockleyRAInfluence of calibration on densitometric studies of emphysema progression using computed tomographyAm J Respir Crit Care Med2004170888389015271692
- DiciottiSSverzellatiNKauczorHUDefining the intra-subject variability of whole-lung CT densitometry in two lung cancer screening trialsAcad Radiol201118111403141121971258
- KalenderWAFichteHBautzWSkalejMSemiautomatic evaluation procedures for quantitative CT of the lungJ Comput Assist Tomogr19911522482552002103
- GevenoisPAde MaertelaerVDe VuystPZanenJYernaultJCComparison of computed density and macroscopic morphometry in pulmonary emphysemaAm J Respir Crit Care Med199515226536577633722
- OrlandiIMoroniCCamiciottoliGChronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuationRadiology2005234260461015671010
- LoringSHO’donnellCRFeller-KopmanDJErnstACentral airway mechanics and flow limitation in acquired tracheobronchomalaciaChest200713141118112417426218
- YamashiroTMatsuokaSBartholmaiBJCollapsibility of lung volume by paired inspiratory and expiratory CT scans: correlations with lung function and mean lung densityAcad Radiol201017448949520060751
- BoisellePMLitmanovichDEMichaudGDynamic expiratory tracheal collapse in morbidly obese COPD patientsCOPD201310560461023837455
- BoisellePMMichaudGRobertsDHDynamic expiratory tracheal collapse in COPD: correlation with clinical and physiologic parametersChest201214261539154422722230
- O’DonnellCRLitmanovichDLoringSHBoisellePMAge and sex dependence of forced expiratory central airway collapse in healthy volunteersChest2012142116817422194588
- KimYISchroederJLynchDCopdgene InvestigatorsGender differences of airway dimensions in anatomically matched sites on CT in smokersCOPD20118428529221756032
- O’DonnellCRBankierAAO’DonnellDHLoringSHBoisellePMStatic end-expiratory and dynamic forced expiratory tracheal collapse in COPDClin Radiol201469435736224361144
- AkiraMToyokawaKInoueYAraiTQuantitative CT in chronic obstructive pulmonary disease: inspiratory and expiratory assessmentAJR Am J Roentgenol2009192126727219098209
- MatsuokaSKuriharaYYagihashiKHoshinoMNakajimaYAirway dimensions at inspiratory and expiratory multisection CT in chronic obstructive pulmonary disease: correlation with airflow limitationRadiology200824831042104918710993
- AquinoSLShepardJAGinnsLCAcquired tracheomalacia: detection by expiratory CT scanJ Comput Assist Tomogr200125339439911351189