Abstract
Introduction
COPD is a leading cause of morbidity and mortality. Self-management interventions are considered important in order to limit the progression of the disease. Computer-tailored interventions could be an effective tool to facilitate self-management.
Methods
This randomized controlled trial tested the effectiveness of a web-based, computer-tailored COPD self-management intervention on physical activity and smoking behavior. Participants were recruited from an online panel and through primary care practices. Those at risk for or diagnosed with COPD, between 40 and 70 years of age, proficient in Dutch, with access to the Internet, and with basic computer skills (n=1,325), were randomly assigned to either the intervention group (n=662) or control group (n=663). The intervention group received the web-based self-management application, while the control group received no intervention. Participants were not blinded to group assignment. After 6 months, the effect of the intervention was assessed for the primary outcomes, smoking cessation and physical activity, by self-reported 7-day point prevalence abstinence and the International Physical Activity Questionnaire – Short Form.
Results
Of the 1,325 participants, 1,071 (80.8%) completed the 6-month follow-up questionnaire. No significant treatment effect was found on either outcome. The application however, was used by only 36% of the participants in the experimental group.
Conclusion
A possible explanation for the nonsignificant effect on the primary outcomes, smoking cessation and physical activity, could be the low exposure to the application as engagement with the program has been shown to be crucial for the effectiveness of computer-tailored interventions. (Netherlands Trial Registry number: NTR3421.)
Background
COPD is one of the leading causes of morbidity and mortality worldwide.Citation1 COPD patients suffer from airflow limitation that is typically progressive and not reversible.Citation2 Adequate patient self-management and behavior modification, such as smoking cessation and increasing the level of physical activity, are recommended to decelerate disease progression.Citation3,Citation4
A relatively small number of studies on the effectiveness of COPD self-management interventions have been conducted, and the evidence on effectiveness remains inconclusive.Citation5 Self-management interventions have mainly focused on educating COPD patients using standardized information, but are now increasingly offering personalized information to patients through counseling with a health care provider.Citation5
Another trend in providing health-promoting information is e-Health, which uses information and communication technology.Citation6 Information provided by e-Health interventions has been individualized using computer-tailored technology, often with disease prevention as a main goal.Citation7–Citation9 This intervention method offers computer-generated, personally relevant information by adapting the content of health-promotion messages to users’ characteristics.Citation9 Personalizing and adapting health messages has been found to help attract and keep users’ attention, increase appreciation, and help users process messages more thoroughly.Citation10–Citation12 Computer-tailored interventions have been shown to effectively improve health behaviors, such as smoking cessation and physical activity.Citation13,Citation14 This intervention strategy has also been successful when targeting multiple behaviorsCitation11 and has been found to be more cost-effective than usual care.Citation15 To our knowledge, this promising technique has not yet been tested with the purpose of supporting behavior change in COPD patients.
In the MasterYourBreath (“AdemDeBaas” in Dutch) project, we developed a web-based, computer-tailored self-management application for COPD patients. We evaluated and improved the usability of the prototypeCitation16 and conducted a pilot study.Citation17 In the present paper, we report the effectiveness of this intervention on behavioral (physical activity, smoking cessation, and the intention to be more physically active and to quit smoking) and clinical outcomes (clinical disease control and dyspnea).
Methods
Study design
This randomized controlled trial (RCT) compared an intervention group which received a COPD self-management application to a control group that did not receive the intervention. All participants, whether they were assigned to the control or intervention group, were free to receive usual care or use other resources in order to help them manage their disease or improve their lifestyle.
In the Netherlands, a COPD disease-management approach is widely implemented.Citation18 This approach includes a practice nurse who coaches patients to improve their self-management behavior. We originally planned to integrate the intervention in this disease-management approach, but our pilot study had shown that it was not feasible to recruit enough patients to cover the sample size required for our RCT if the practice nurse recruited the patients.Citation17 Instead, to solve the recruitment issues, we invited patients from five general practices by mail and recruited patients from a Dutch online panel. We also broadened our inclusion criteria to include people at risk for COPD as well as people with known COPD.
The Dutch online panel was assembled by the company Flycatcher Internet Research BV (www.flycatcher.eu) which is an institute for online research certified by the International Organization for Standardization. In total, the online panel consisted of 16,000 Dutch-speaking members, who had an email address and were at least 12 years old. All age groups, education levels, and provinces of the Netherlands were represented in the panel. Flycatcher’s members are recruited by Flycatcher through newsletters, send-to-a-friend promotions, third parties’ contact lists used for research (with the permission of the owner of the contact list and the person on the list), and word-of-mouth advertising. Members receive seven research questionnaires a year on average.
Members from the Dutch online panel and the five general practices were eligible for participation in this study if they were diagnosed with COPD or were at moderate or high risk for COPD, were between 40–70 years of age, were proficient in Dutch, and had access to the Internet and basic computer skills. Dutch proficiency and basic computer literacy were gauged by administration of the first online questionnaire. The Respiratory Health Screening Questionnaire (RHSQ)Citation19 was used to assess the subject’s risk for COPD. This questionnaire contains ten items related to important determinants of COPD for individuals of 40 years and older.Citation19 A scoring system for case-findingCitation20,Citation21 was used to determine if an individual was at low (<16.5 points), moderate (16.5–19.5 points), or high risk (>19.5 points) for COPD (sensitivity =58.7%, specificity =77.0%, for the 16.5 cutoff point).Citation20
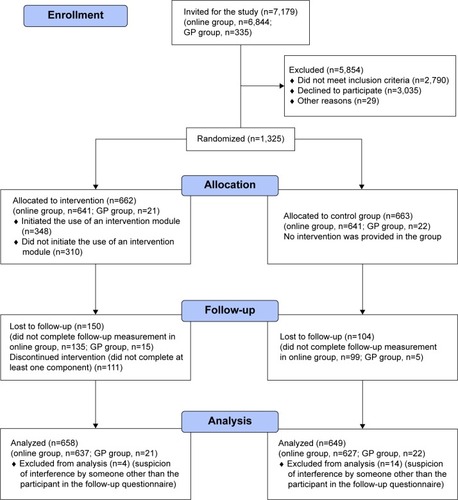
The five general practices were involved in a parallel projectCitation21 in which 40- to 70-year-old patients had already been screened for COPD by their general practitioner using the RHSQ. Potentially eligible members of the online panel were invited for the study by an email from Flycatcher, including a study information letter. Members who decided to participate were screened for eligibility by completing the RHSQ. We implemented a scoring algorithm within the online questionnaire, so eligibility could be determined directly after participants completed the questionnaire. Members received a small incentive equal to €2.55 per completed questionnaire (baseline and follow-up). shows the Consolidated Standards of Reporting Trials (CONSORT) diagram including the two populations.
The study was approved by the Medical Ethical Committee of Maastricht University Medical Center (METC 12-4-033) and registered with the Netherlands Trial Registry (NTR3421). All participants received an online study information letter and completed an online informed consent form before entering the study. A more detailed description of the methods can be found in a study protocol published elsewhere.Citation22
Randomization
A permuted block designCitation23 with a random block size varying from four to 20 was employed to randomize participants stratified by channel of recruitment (online or through general practice). This approach was chosen in order to achieve balanced and evenly distributed samples for both recruitment strategies. A researcher not involved in data collection or analysis of the results performed the randomization using PROC PLAN in SAS software (v 9.1; SAS Institute, Cary, NC, USA). Due to the study design, it was not feasible to blind participants to group assignments.
Intervention
MasterYourBreath application
The intervention, “MasterYourBreath”,Citation22 was designed to change participants’ health behavior by means of a web-based application providing computer-generated tailored feedback. The intervention was based on earlier studies on computer-tailored feedback for lifestyle changes which have been shown to be effective and cost-effectiveCitation11,Citation24–Citation26 and adapted to our target groups. The MasterYourBreath application was built using the online application Tailorbuilder (OverNite Software Europe, Sittard, Netherlands).
Participants assigned to the experimental group were asked by email to access the application with a personalized account and use the application ad libitum for 6 months. Participants in the online group received the email from Flycatcher and those in the general practice group from the research team. Application use was monitored by the research team and email prompts were sent to encourage application use,Citation27 mostly within a 2-week time interval addressing new content on the website, as this could increase the number of follow-up visits.Citation28 The application had a modular design, including two behavior-change modules, smoking cessation and physical activity. Each module was equally divided into six intervention components: (1) health-risk appraisal: feedback on the behavior (smoking or physical activity) based on Dutch guidelines; (2) motivational beliefs: feedback on perceived positive and negative consequences of the behavior; (3) social influence: feedback on the social influences of participants’ partner, family, friends, and coworkers on the behavior; (4) goal setting and action plans: feedback on achievement of goals and on action plans in order to achieve their goal; (5) self-efficacy: feedback on perceived barriers to change the behavior; and (6) maintenance: feedback in order to maintain the healthy behavior. Participants could switch behavior-change modules and choose to enter one or more intervention components according to their preference.Citation10
Feedback was personalized using participants’ names and tailored to participants’ characteristics and key behavior determinants of psychosocial constructs, for which the I-Change model served as theoretical framework.Citation29,Citation30 Examples of the key behavior determinants are: pros and cons of physical activity, perceived social support to quit smoking, action plans to increase physical activity, and self-efficacy to cope with barriers to quit smoking. Participants were asked questions to uncover their personal determinants, using questionnaires that have been tested experimentally among Dutch adults in previous studies in the general public.Citation13,Citation31,Citation32 These questionnaires were adjusted for COPD patients during the usability study.Citation16 The questions were used to generate tailored feedback to the participants about their responses. Participants’ previous responses were also incorporated in the feedback so they could track their own behavior change and goal attainment over the course of the study. The six intervention components per module were available to the participants to be completed over time as they chose. This allowed participants the ability to track their behavior changes by comparing the most current answers with the previous answers.
A more detailed description of the intervention and prompt protocol is described elsewhere.Citation22
Website
The computer-tailored application was embedded in a website. The website contained general information about the MasterYourBreath project, COPD/being at risk for COPD, smoking, and physical activity. Online self-management resources, such as videos with home exercises (seven exercises focusing on strength and balance) and hyperlinks to other informative websites, were also included. The tailored feedback and prompts referred participants to the home exercises and other resources.
Data collection
A web-based questionnaire was administered at baseline between May and November of 2012 with a follow-up questionnaire sent out 6 months later. The frequency of reminders for completing these questionnaires was adapted to the response rate. The online group received one reminder to participate in the study and complete the baseline questionnaire. General practice group participants received two additional reminders if they responded to the invitation but did not complete the baseline questionnaire, since the participation rate in this group was low. The online group received one reminder to complete the follow-up questionnaire, whereas the general practice group received two reminders. Data collection ended in July 2013. All data were captured through these questionnaires, except demographic characteristics in the online group, as these were already documented through an annual update process undertaken by Flycatcher.
Outcome measures
Two primary outcomes were measured, one for each health behavior: smoking cessation and physical activity. Smoking cessation was assessed by one item measuring the 7-day point prevalence abstinence,Citation33,Citation34 and the level of physical activity was assessed by the International Physical Activity Questionnaire – Short Form.Citation35
We created a “behavior-change score” by combining the two behaviors (smoking cessation and physical activity). The behavior-change score was only calculated for participants who smoked at baseline or were below the physical activity norm (defined as being physically active for at least 30 minutes a day on 5 days a week at a moderate or vigorous intensity). The efforts of participants were rated as “successful behavior change” if they achieved smoke-free status for 7 consecutive days prior to the follow-up measurement, or if they achieved the norm level of physical activity at follow-up, reflecting a change from their baseline behavior. The actions of participants who smoked or were below the physical activity norm at baseline and achieved neither smoke-free status nor the norm level of physical activity at follow-up were rated as “unsuccessful behavior change”.
Secondary outcomes included secondary smoking cessation measures, health status, and intention to change behavior. Secondary smoking cessation measures were: number of quit attempts during the past 6 months, 24-hour point prevalence abstinence, tobacco consumption, and continued and prolonged abstinence.Citation33,Citation34 To assess participants’ health status, participants were asked if they experienced any form of breathlessness, and if so, the Medical Research Council (MRC) dyspnea scale was administered in order to measure the level of disability.Citation36 Clinical disease control was measured using the Clinical COPD Questionnaire. This questionnaire assesses both patient guideline goals (health-related quality of life) and clinical guideline goals such as prevention of disease progression.Citation37 Intention to change behavior was measured by separate questions for physical activity and smoking. presents an overview of all outcomes including how and when they were measured.
Table 1 Primary and secondary outcomes
Baseline measurements
There were several additional baseline measures that were not part of the primary and secondary outcome measures, including demographic characteristics: age, sex, marital status, education level, and current employment status. Several additional questions were asked at baseline to capture smoking behavior. Participants were asked if they had ever smoked and about their number of previous quit attempts. The six-item version of the Fagerström Test for Nicotine Dependence (0= not addicted; 10= highly addicted)Citation38 was included in order to assess the addiction level. Health status was further estimated, measuring COPD status and comorbidities, by asking participants whether they were diagnosed with COPD or any other chronic disease, and which other chronic disease(s).
Sample-size calculation
Sample-size calculations assumed 80% power and a significance level of 0.05 for both behaviors and were completed by PS software version 3.0.43,Citation39 according to Fisher’s exact test. Calculations for smoking indicated that 446 participants per group were necessary at the end of the trial to detect a 10% difference in 7-day point prevalence (20% abstinence in the intervention group, compared to 10% in the control groupCitation31). We based this calculation on the assumption that 49.2% of the population with an increased risk for COPD smoked at baseline.Citation40 The number needed for measuring physical activity was smaller and could be obtained following the above sample-size calculation for smoking.Citation22 In another study the standard deviation was 26.63 minutes a day in a Dutch population.Citation41 When including 446 participants per group, a difference of 5 minutes a day, which corresponds to a small standardized effect size (Cohen’s d =0.2), could be detected. In order to allow for a 30% drop-out rate, a baseline total of 1,275 participants was necessary to reach 80% power.
Analyses
Differences at baseline between groups (intervention vs control; and participants who dropped out vs who did not drop out) were compared using chi-square tests for categorical variables and independent-samples t-tests for numerical variables. Data were analyzed according to the intention-to-treat principle. For both primary outcomes, an additional per protocol analysis was conducted. Participants assigned to the experimental group had to have completed at least one of the six intervention components of the specific behavior module to be included in the latter analysis. For the “per protocol” analyses, we conducted two sensitivity analyses with stricter criteria for each primary outcome (smoking cessation and physical activity). For the first analyses, we included only participants who completed at least two intervention components of the specific behavior module. For the second analyses, we included only participants who completed at least three intervention components, since a higher “usage dose” may be necessary to yield a treatment effect.
The uncorrected and corrected effects of the intervention on the primary and secondary outcomes were assessed using logistic regression for categorical outcomes and linear regression for numerical outcomes measured at 6 months. Linear mixed models were used for outcomes measured at baseline and at 6 months to account for the correlation between repeated measurements of the same participant and to include all participants, including those with missing data. As for correction, the models included: the stratification variable – that is, recruitment channel (online or general practices); baseline variables if they showed a statistically significant difference between intervention and control group; and baseline variables that were related to drop out, missing data, and/or related to the outcome at 6 months (P value ≤0.20 in univariable regression analysis) to increase the precision of the intervention effect. To assess potential effect modifiers, the interaction of the treatment variable with age, sex, intention to increase level of physical activity, educational level, dyspnea status, and COPD status were added in the corrected mixed-model analyses for level of physical activity. Primary outcomes were analyzed for subgroups based on age (40–50, 50–60, 60–70), sex, intention to change behavior (those who had no intention or were not sure; those who intended to change), education level (low, middle, high), dyspnea (yes, no), COPD status (diagnosed, at risk).
The robustness of our results of the primary outcome for smoking cessation was tested by conducting a best- and a worst-case scenario, where respondents lost to follow-up were considered to have quit smoking in the best-case scenario and considered to still be smoking in the worst-case scenario. In addition, as a sensitivity analysis, the intervention effect on the behavior-change score was tested using logistic regression analysis.
All statistical analyses were performed using IBM SPSS (v 19). Two-sided P values ≤0.05 were considered statistically significant.
Results
Recruitment
A total of 7,179 individuals were invited for the study of which 3,035 declined to participate, 2,790 did not meet the inclusion criteria, and 29 were excluded because they did not complete the baseline questionnaire (n=24) or provided unreliable responses (n=5). Responses were unreliable if answers were straight lined (ie, the same answer options selected for each set of items) or the questionnaire was completed within 3 minutes (which was considered to be unrealistically fast).
A total of 1,325 participants (1,282 in the online group and 43 in the general practice group) completed the baseline questionnaire and were randomly assigned to the experimental (online group: n=641, general practice group: n=21) or control group (online group: n=641, general practice group: n=22). Of the 1,325 participants, 1,071 (80.8%) completed the 6-month follow-up questionnaire, including 1,048 (81.7%) of the online group and 23 (53.5%) of the general practice group. Eighteen participants of the online group were excluded from further analyses, due to a high level of suspicion of interference by someone other than the participant, (eg, a partner with whom they shared an email address). Participants were excluded when at least two of the following variables did not match their Flycatcher profile on the follow-up questionnaire: sex, day of birth, month of birth, year of birth. If only one variable was inconsistent or day and month were reversed, we suspected a typing error and did not exclude those participants.
In the group of smokers, 447 participants were included of whom 341 completed the follow-up questionnaire. shows the CONSORT diagram of our RCT.
Sample characteristics
shows the baseline characteristics of the overall sample and the experimental and control groups separately. The only significant difference between the groups was the employment status of participants (P=0.039). As for the participants who smoked at baseline, we did not find any significant differences in baseline characteristics between the groups.
Table 2 Baseline characteristics of study participants – overall, experimental and control group
More participants were lost to follow-up in the general practice group (46.5%) than in the online group (18.5%) (P<.001), more in the experimental group (22.8%) than in the control group (16%) (P=.002), more smokers (23.7%) than non-smokers (17.2%) (P=.005), and more female (22.6%) than male participants (15.9%) (P=.002). Participants lost to follow-up were also significantly younger (mean =55.9 years) than completing participants (mean =58.1 years) (P<0.001).
Application use
The application was used by 237 (36%) participants of the experimental group (ie, at least one of the six components of a behavior-change module was completed). The average number of components completed by those participants was 2.1 (standard deviation =2.4, range 1–21). For physical activity, 193 (29.3%) of the participants completed at least one intervention component. For smoking cessation, 51 (21.2%) of the smokers at baseline, and seven (1.7%) of the nonsmokers at baseline completed at least one intervention component (although nonsmokers were not included in the effect analyses for smoking cessation). shows how many participants completed zero, one, two, or three or more intervention components of both modules.
Table 3 Number of participants (%) who completed intervention components of the physical activity and smoking cessation modules
Intervention effect
Before correction for baseline characteristics, no significant treatment effect was found for any primary or secondary outcome, except clinical disease control. After correction, all effects were nonsignificant ().
Table 4 Effects of the web-based COPD self-management intervention on all primary and secondary outcomes
As for sensitivity analyses, similar results were found for the primary outcomes. More specifically, per protocol analyses yielded nonsignificant results for both primary outcomes (). Regarding physical activity, all interaction terms were nonsignificant. Also, when evaluating for smoking cessation, neither the uncorrected nor corrected effects on 7-day point prevalence abstinence were significant for best- or worst-case scenarios. All subgroup analyses for both outcomes were nonsignificant. Besides, uncorrected and corrected analyses on the behavior-change score, combining the two behaviors, yielded nonsignificant results.
Table 5 Corrected effects of the per protocol analyses for primary outcomes
Discussion
This study examined the effects of a computer-tailored COPD self-management intervention. The intervention had no significant impact on the primary outcome measures (physical activity and smoking), or on the secondary outcome measures (intention to change behavior and dyspnea). The only significant effect found was on clinical disease control, but the improvement was too small to have clinical relevanceCitation42 and was not significant after correction for relevant baseline characteristics. Moreover, a borderline significant effect for smoking was found when analyzing the effects among those who completed at least two intervention components. Also, the effect size, for both smoking and physical activity, increased as more intervention components were used. Yet, possibly due to the sample size and thus decreased power of the study, these analyses did not yield a significant effect.
Possible explanations for the lack of effect may be: (a) low exposure to the intervention, (b) that the intervention method was not sufficient for our target population, and (c) inadequate content of the intervention itself. Although it was not significant, the trend was for an increased effect size as the participants completed more of the six intervention components. This helps verify the importance of exposure to the application and correlates with other studies which have shown that this is essential for the effectiveness of such interventions.Citation43,Citation44 To enhance exposure to the intervention, we used different strategies to attract participants to the application: sending email prompts every 2 weeks, of which some referred to new content on the website, as this has been shown to effectively increase application use;Citation28 including multiple feedback moments to evaluate participants’ behavior and track their goal achievement;Citation45,Citation46 prompting revisits to the application and evaluate these previously set goals; and embedding a website that provided regular news updates,Citation45–Citation47 such as behavioral journalism stories,Citation48 which were personal stories addressing how other patients overcame potential barriers to use the application and improve their health behavior. Additionally, since the length of the program was of concern in the pilot study,Citation17 we separated the two modules (smoking cessation and physical activity) into six small components, giving participants the opportunity to decide which components they wanted to complete, and consequently, how much time they wanted to spend working with the application. Unfortunately, only one out of three participants completed one or more of the six intervention components. In hindsight, giving participants an option to choose intervention components based on the results of our pilot study was probably not desirable, since it has recently been shown that less freedom of navigation on a website enhances application use.Citation49,Citation50 The intervention did not include ongoing peer or counselor support (eg, by a practice nurse) which could have improved the exposure to the application.Citation47
Another potential cause for our study results could be that the intervention method was not sufficient for our target population. Although similar computer-tailoring approaches have found significant effects among the general population,Citation11,Citation14,Citation25,Citation26 several other studies reported a relatively low success rate of disease-management programs for COPD patientsCitation51–Citation53 and found that COPD patients are more likely to have characteristics that are associated with a higher resistance to smoking cessation interventionsCitation54 than other target populations. Negative results are not uncommon in COPD self-management studies according to a systematic review by Jonsdottir.Citation5 The author describes the need for a paradigm shift in which a prominent health professional-centered approach should make way for a patient-family-centered approach with emphasis on the relationship with the health care professional. As we were aware of this, the MasterYourBreath intervention was patient-centered, and social influence of family members was addressed in the intervention. However, the application did not enable the active participation of family members. An improvement to future versions could be to integrate the MasterYourBreath intervention with a social media platform to facilitate engagement of the family members in the patient’s self-management process. The relationship with the health care professional was indeed emphasized in the original version of the MasterYourBreath intervention, by integration of the application in primary care, but recruiting the number of participants necessary for an RCT from primary care practices was found to be unfeasible in the pilot study,Citation17 so the original study design was changed.
Another possible cause for the lack of effect could be related to the web-based intervention content itself. We used key behavioral determinants that were experimentally tested in the Dutch population but not validated for COPD patients. The usability study in which we adjusted these determinants for COPD patientsCitation16 is not a replacement for a validation study. Behavioral determinants for this specific group might be different from the general Dutch population. For example, COPD patients are likely to experience different barriers to physical activity due to disease complications. Moreover, the main difference we found comparing our application to programs that were very similar but found positive intervention effectsCitation11,Citation14,Citation25,Citation26,Citation43,Citation55 was that participants in our study were free to choose which intervention components they wanted to complete. These other programs contained similar tailored messages, but directed participants through a specified intervention pathway. As described previously, this strategy might increase exposure, as it limits freedom of navigation. Instead of shortening the intervention content by tailoring the use of components to user’s preference,Citation10 it may have been more effective to offer participants only components adapted to their level of motivation to change their behavior; Stanczyk et alCitation55 found this strategy effective. Peels et alCitation56 tested a web-based basic version of their program and a version in which they added additional environmental information with links to other resources to increase physical activity in adults aged 50 and over. They found that only the latter was not effective. The authors suspected that participants might have been distracted from the intervention pathway by visiting other resources. Likewise, the provision of additional information and self-management resources in the MasterYourBreath application, including home exercises and links to other websites might have decreased a potential intervention effect, as these could have distracted participants from the intervention components, which was the core content of the intervention.
Limitations
Our study had several limitations. First, despite our relatively large sample of participants, compared to many other COPD self-management studies,Citation5 our study still lacked power concerning the primary outcome for smoking cessation. The number of smokers at the start of the study was lower than expected, and loss to follow-up among smokers was higher than among nonsmokers.
Second, we were not able to evaluate selection bias, because we could not collect additional data to differentiate between participants who enrolled versus those who declined participation. The clinical information that would help us address selection bias was not available from the online recruiting company.
Third, the inclusion of people at risk for COPD may make it more difficult to compare our results with self-management studies that include only COPD patients. However, we argue that including individuals at risk for COPD is clinically relevant, since early smoking cessation is pivotally important for a greater health benefit in COPD.Citation57 Including both diagnosed patients and individuals at risk for COPD also poses a methodological challenge, since these groups may benefit from different interventions. However, the approach to care in the MasterYourBreath program has been tailored to varying levels of COPD (risk), and thus it was able to address differing patient needs.
Fourth, while our intentions were to do so, we were not able to integrate the MasterYourBreath intervention into the Dutch COPD disease-management approach,Citation18 due to recruitment issues.Citation17 We know that it is important that COPD self-management interventions can be incorporated in an existing health care structure.Citation58–Citation60 The recruitment strategy used in this RCT (including an online group and individuals at risk for COPD) was certainly beneficial for the sample size of our RCT, but hampered the integration of our intervention in primary care.
Conclusion
MasterYourBreath, a web-based COPD self-management intervention with tailored feedback, did not show statistically significant effects on health-related behavioral or clinical outcomes. Given the structurally low exposure to the application in this study, we believe more research is needed to find effective strategies to increase the use of the web-based applications by COPD patients. To further explain this phenomenon and to generate hypotheses for better strategies, we will reevaluate our RCT and explore in depth the characteristics of the intervention and the participants that may have contributed to the use and appreciation of the application.
Acknowledgments
The authors would like to thank Onno van Schayck, PhD, for his assistance with the study design; Jos Dirven, MD, for his contribution to participant recruitment; and Jean Muris, PhD, for screening feedback messages on content and providing advice as a COPD specialist. The authors also thank the University of Oklahoma, Health Sciences Center, for providing an office; James W Mold, MD, MPH, for providing general support; and E Wickersham, MD, for editing the manuscript. This study was funded by ZonMw, the Netherlands Organization for Health Research and Development.
Disclosure
Hein de Vries is scientific director of Vision2Health, a company that licenses evidence-based innovative computer- tailored health-communication tools. The other authors declare that they have no competing interests in this work.
References
- ViegiGPistelliFSherrillDLMaioSBaldacciSCarrozziLDefinition, epidemiology and natural history of COPDEur Respir J2007305993101317978157
- SiafakasNMVermeirePPrideNBOptimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task ForceEur Respir J199588139814207489808
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- BourbeauJNaultDDang-TanTSelf-management and behaviour modification in COPDPatient Educ Couns200452327127714998597
- JonsdottirHSelf-management programmes for people living with chronic obstructive pulmonary disease: a call for a reconceptualisationJ Clin Nurs2013225–662163723398312
- EversKEeHealth promotion: the use of the Internet for health promotionAm J Health Promot2006204Suppl 17
- LustriaMLCorteseJNoarSMGlueckaufRLComputer-tailored health interventions delivered over the Web: review and analysis of key componentsPatient Educ Couns200974215617318947966
- KrebsPProchaskaJORossiJSA meta-analysis of computer-tailored interventions for health behavior changePrev Med2010513–421422120558196
- de VriesHBrugJComputer-tailored interventions motivating people to adopt health promoting behaviours: introduction to a new approachPatient Educ Couns19993629919510223015
- HawkinsRPKreuterMResnicowKFishbeinMDijkstraAUnderstanding tailoring in communicating about healthHealth Educ Res200823345446618349033
- de VriesHKremersSPSmeetsTBrugJEijmaelKThe effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviorsAm J Health Promot200822641742518677882
- DijkstraAWorking mechanisms of computer-tailored health education: evidence from smoking cessationHealth Educ Res200520552753915701665
- SmeetsTBrugJde VriesHEffects of tailoring health messages on physical activityHealth Educ Res200823340241317032705
- SmitESde VriesHHovingCEffectiveness of a Web-based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokersJ Med Internet Res2012143e8222687887
- SmitESEversSMde VriesHHovingCCost-effectiveness and cost-utility of Internet-based computer tailoring for smoking cessationJ Med Internet Res2013153e5723491820
- Voncken-BrewsterVMoserAvan der WeijdenTNagykaldiZde VriesHTangeHUsability evaluation of an online, tailored self-management intervention for chronic obstructive pulmonary disease patients incorporating behavior change techniquesJMIR Res Protoc201321e323612363
- Voncken-BrewsterVTangeHMoserANagykaldiZde VriesHvan der WeijdenTIntegrating a tailored e-health self-management application for chronic obstructive pulmonary disease patients into primary care: a pilot studyBMC Fam Pract201415424400676
- SteutenLVrijhoefBVan MerodeFWesselingGJSpreeuwenbergCEvaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary diseaseInt J Qual Health Care200618642943617032687
- PriceDBTinkelmanDGHalbertRJSymptom-based questionnaire for identifying COPD in smokersRespiration200673328529516330875
- PriceDBTinkelmanDGNordykeRJIsonakaSHalbertRJCOPD Questionnaire Study GroupScoring system and clinical application of COPD diagnostic questionnairesChest200612961531153916778271
- DirvenJATangeHJMurisJWvan HaarenKMVinkGvan SchayckOCEarly detection of COPD in general practice: patient or practice managed? A randomised controlled trial of two strategies in different socioeconomic environmentsPrim Care Respir J201322333133723966214
- Voncken-BrewsterVTangeHde VriesHNagykaldiZWinkensBvan der WeijdenTA randomised controlled trial testing a web-based, computer-tailored self-management intervention for people with or at risk for chronic obstructive pulmonary disease: a study protocolBMC Public Health20131355723742208
- ZelenMThe randomization and stratification of patients to clinical trialsJ Chron Dis1974277–83653754612056
- SchulzDNSmitESStanczykNEKremersSPde VriesHEversSMEconomic evaluation of a web-based tailored lifestyle intervention for adults: findings regarding cost-effectiveness and cost-utility from a randomized controlled trialJ Med Internet Res2014163e9124650860
- SmeetsTKremersSPJBrugJDe VriesHEffects of tailored feedback on multiple health behaviorsAnn Behav Med200733211712317447863
- SchulzDNKremersSPVandelanotteCEffects of a web-based tailored multiple-lifestyle intervention for adults: a two-year randomized controlled trial comparing sequential and simultaneous delivery modesJ Med Internet Res2014161e2624472854
- FryJPNeffRAPeriodic prompts and reminders in health promotion and health behavior interventions: systematic reviewJ Med Internet Res2009112e1619632970
- SchneiderFde VriesHCandelMvan de KarAvan OschLPeriodic email prompts to re-use an internet-delivered computer-tailored lifestyle program: influence of prompt content and timingJ Med Internet Res2013151e2323363466
- de VriesHMestersIvan de SteegHHoningCThe general public’s information needs and perceptions regarding hereditary cancer: an application of the Integrated Change ModelPatient Educ Couns200556215416515653244
- de VriesHEggersSMBolmanCThe role of action planning and plan enactment for smoking cessationBMC Public Health20131339323622256
- DijkstraADe VriesHRoijackersJLong-term effectiveness of computer-generated tailored feedback in smoking cessationHealth Educ Res199813220721410181019
- DijkstraAde VriesHBakkerMPros and cons of quitting, self-efficacy, and the stages of change in smoking cessationJ Consult Clin Psychol19966447587638803366
- WestRHajekPSteadLStapletonJOutcome criteria in smoking cessation trials: proposal for a common standardAddiction2005100329930315733243
- MuddeANWillemsenMCKremersSde VriesHMeetinstrumenten voor onderzoek naar roken en stoppen met roken [Measurements for research on smoking and smoking cessation]2nd edThe HagueStivoro2006 Dutch
- CraigCLMarshallALSjöströmMInternational physical activity questionnaire: 12-country reliability and validityMed Sci Sports Exerc20033581381139512900694
- BestallJPaulEGarrodRGarnhamRJonesPWedzichaJUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- van der MolenTWillemseBWSchokkerSten HackenNHPostmaDSJuniperEFDevelopment, validity and responsiveness of the Clinical COPD QuestionnaireHealth Qual Life Outcomes200311312773199
- HeathertonTFKozlowskiLTFreckerRCFagerströmKOThe Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance QuestionnaireBr J Addict1991869111911271932883
- DupontWDPlummerWDJrPower and sample size calculations. A review and computer programControl Clin Trials19901121161282161310
- DirvenJAMurisJWvan SchayckCPCOPD screening in general practice using a telephone questionnaireCOPD20107535235920854050
- RüttenAZiemainzHSchenaFUsing different physical activity measurements in eight European countries. Results of the European Physical Activity Surveillance System (EUPASS) time series surveyPublic Health Nutr20036437137612795825
- KocksJWTuinengaMGUilSMvan den BergJWStåhlEvan der MolenTHealth status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaireRespir Res200676216603063
- ElfeddaliIBolmanCCandelMJWiersRWde VriesHPreventing smoking relapse via Web-based computer-tailored feedback: a randomized controlled trialJ Med Internet Res2012144e10922903145
- StrecherVJMcClureJAlexanderGThe role of engagement in a tailored web-based smoking cessation program: randomized controlled trialJ Med Internet Res2008105e3618984557
- BrouwerWOenemaACrutzenRde NooijerJde VriesNBrugJWhat makes people decide to visit and use an internet-delivered behavior-change intervention? A qualitative study among adultsHealth Educ20091096460473
- BrouwerWOenemaACrutzenRde NooijerJde VriesNKBrugJAn exploration of factors related to dissemination of and exposure to internet-delivered behavior change interventions aimed at adults: a Delphi study approachJ Med Internet Res2008102e1018417443
- BrouwerWKroezeWCrutzenRWhich intervention characteristics are related to more exposure to internet-delivered healthy lifestyle promotion interventions? A systematic reviewJ Med Internet Res2011131e221212045
- McAlisterABehavioral journalism: beyond the marketing model for health communicationAm J Health Promot19959641742010172363
- CrutzenRCyrDde VriesNKThe role of user control in adherence to and knowledge gained from a website: randomized comparison between a tunneled version and a freedom-of-choice versionJ Med Internet Res2012142e4522532074
- McClureJBShortreedSMBogartAThe effect of program design on engagement with an internet-based smoking intervention: randomized factorial trialJ Med Internet Res2013153e6923529377
- WeingartenSRHenningJMBadamgaravEInterventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reportsBMJ2002325737092512399340
- OfmanJJBadamgaravEHenningJMDoes disease management improve clinical and economic outcomes in patients with chronic diseases? A systematic reviewAm J Med2004117318219215300966
- MattkeSSeidMMaSEvidence for the effect of disease management: is $1 billion a year a good investment?Am J Manag Care2007131267067618069910
- Jiménez-RuizCAMasaFMiravitllesMSmoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPDChest200111951365137011348940
- StanczykNBolmanCvan AdrichemMCandelMMurisJde VriesHComparison of text and video computer-tailored interventions for smoking cessation: randomized controlled trialJ Med Internet Res2014163e6924589938
- PeelsDAvan StralenMMBolmanCThe differentiated effectiveness of a printed versus a Web-based tailored physical activity intervention among adults aged over 50Health Educ Res201429587088224980023
- FletcherCPetoRThe natural history of chronic airflow obstructionBr Med J19771607716451648871704
- EffingTvan der PalenJFrithPEducation in COPD self-management: only part of the gameRespirology201419215115224372929
- EffingTWBourbeauJVercoulenJSelf-management programmes for COPD: moving forwardChron Respir Dis201291273522308551
- AdamsSGSmithPKAllanPFAnzuetoAPughJACornellJESystematic review of the chronic care model in chronic obstructive pulmonary disease prevention and managementArch Intern Med2007167655156117389286
- WilsonJSElbornJSFitzsimonsDMcCrum-GardnerEDo smokers with chronic obstructive pulmonary disease report their smoking status reliably? A comparison of self-report and bio-chemical validationInt J Nurs Stud201148785686221288520
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory QuestionnaireAm Rev Respir Dis19921456132113271595997
- GuyattGHBermanLBTownsendMPugsleySOChambersLWA measure of quality of life for clinical trials in chronic lung diseaseThorax198742107737783321537
- LincolnNBGladmanJRThe Extended Activities of Daily Living scale: a further validationDisabil Rehabil199214141431586760
- WareJEJrSherbourneCDThe MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selectionMed Care19923064734831593914
- SmitESHovingCSchelleman-OffermansKWestRde VriesHPredictors of successful and unsuccessful quit attempts among smokers motivated to quitAddict Behav20143991318132424837754