Abstract
Objective
The nutritional status of chronic obstructive pulmonary disease (COPD) patients is associated with their exercise capacity. In the present study, we have explored the relationship between nutritional risk and exercise capacity in severe male COPD patients.
Methods
A total of 58 severe COPD male patients were enrolled in this study. The patients were assigned to no nutritional risk group (n=33) and nutritional risk group (n=25) according to the Nutritional Risk Screening (NRS, 2002) criteria. Blood gas analysis, conventional pulmonary function testing, and cardiopulmonary exercise testing were performed on all the patients.
Results
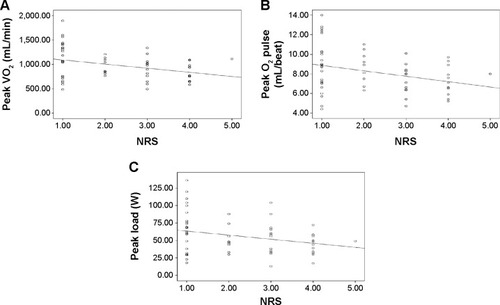
Results showed that the weight and BMI of the patients in the nutritional risk group were significantly lower than in the no nutritional risk group (P<0.05). The pulmonary diffusing capacity for carbon monoxide of the no nutritional risk group was significantly higher than that of the nutritional risk group (P<0.05). Besides, the peak VO2 (peak oxygen uptake), peak O2 pulse (peak oxygen pulse), and peak load of the nutritional risk group were significantly lower than those of the no nutritional risk group (P<0.05) and there were significantly negative correlations between the NRS score and peak VO2, peak O2 pulse, or peak load (r<0, P<0.05).
Conclusion
The association between exercise capacity and nutritional risk based on NRS 2002 in severe COPD male patients is supported by these results of this study.
Introduction
Chronic obstructive pulmonary disease (COPD), as a common problem in the elderly, is a major cause of chronic morbidity and mortality throughout the world.Citation1 It is characterized by chronic obstruction of expiratory flow affecting peripheral airways, and is often associated with chronic bronchitis and emphysema, thereby accelerating the decline in lung function.Citation2,Citation3
Previous studies have found that nutritional status is the prognostic factor that is associated with the mortality in COPD.Citation4–Citation6 Weight loss is the major characteristic of malnutrition in COPD.Citation7 In 2002, “nutritional risk” was defined by the European Society for Parenteral and Enteral Nutrition (ESPEN).Citation8 Recently, nutritional risk was found to be associated with the exacerbation of COPD in the patients who were hospitalized because of COPD.Citation9 Meanwhile, it has been reported that nutritional risk is related to the development of COPD in male smokers.Citation10
In addition, the exercise capacity is also another factor related to mortality in COPD.Citation11 Limitation of the exercise capacity seriously affects the quality of life in COPD patients.Citation12 Therefore, many studies have aimed at strategies for improving the exercise capacity of COPD patients.Citation13,Citation14 However, this effective and feasible strategy still needs further exploration. It has been reported that nutritional status could affect the exercise capacity in COPD patients.Citation15,Citation16 Moreover, nutritional supply seems to contribute to improving the exercise capacity.Citation15,Citation17 In view of these findings, we speculated that there might be some relationship between nutritional risk and exercise capacity. Therefore, we explored this relationship in severe COPD male patients in this study, which could provide evidence for the effect of nutritional supply on exercise capacity.
Materials and methods
Patients
Between January 2012 and December 2013, a total of 58 severe COPD male patients (age range: 47–78 years) who were hospitalized in the Department of Respiratory Medicine, Shanghai Pulmonary Hospital, affiliated to Tongji University, were enrolled in the present study. All the patients were diagnosed with severe COPD (forced expiratory volume in 1 second as percentage of predicted, FEV1%pred <50) according to the new Global initiative for chronic Obstructive Lung Disease (GOLD) classification criteria of 2012.Citation18 The patients were excluded if they had diseases in other organs (such as the cardiovascular, renal, endocrine, digestive, and nervous systems). The study protocol was approved by the Ethics Committee of Tongji University. Patients provided written informed consent before inclusion.
Nutritional risk assessment
Before nutritional risk assessment, the height (m) and weight (kg) of patients were measured. Body mass index (BMI) was calculated according to formula BMI = weight/height squared (kg/m2). Nutritional risk assessment was conducted using the continuous sampling method according to the Nutritional Risk Screening (NRS, 2002) criteria developed by ESPEN.Citation19 The NRS score (0–7) was the sum of the scores in disease severity, nutritional status, and age. Disease severity of the patients was scored based on the diagnosis records at admission. The nutritional status of each patient was scored based on the changes in body weight and BMI in the recent 1–3 months and food intake in the recent 1 week. Besides, one score was assigned to the patients who were more than 70 years old. According to the NRS score, the patients were divided into two groups: nutritional risk group (NRS score ≥3) and no nutritional risk group (NRS score <3).
Blood gas analysis
Blood samples were collected from the right forearm radial artery before conventional pulmonary function testing (PFT) and cardiopulmonary exercise testing (CPET) were performed. Blood gas analysis was performed to confirm whether partial pressure of oxygen in the artery (PaO2) and partial pressure of carbon dioxide in the artery (PaCO2) were in the normal range (PaO2: 80–100 mmHg; PaCO2: 35–45 mmHg) using an ABL 800 FLEX blood gas analyzer (Radiometer Medical A/S, Copenhagen, Denmark). It was used to evaluate whether the patients had respiratory failure. Patients with respiratory failure were excluded from this study.
PFT and CPET
PFT and CPET were performed on the patients in stable condition.
A Master Screen Diffusion system (Jaeger Corp., Hoechberg, Germany) was used for PFT. Strict calibration was required before PFT. Prediction equations for normal lung function, which were developed in 1988, were used in this assessment.Citation20 The following parameters were detected in this testing: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1%pred, FEV1/FVC ratio, residual volume (RV), total lung capacity (TLC), RV/TLC, and pulmonary diffusing capacity for carbon monoxide (DLco). PFT was performed three times for each patient and the best result was chosen for analysis.
CPET was performed using a cardiopulmonary exercise testing system (Mastercreen-CPX; Jaeger Corp.). Before CPET was performed, the flow sensors and gas analyzers were strictly calibrated. In this testing, a symptom-limited incremental exercise test was performed with different loads (10–25 W/min) based on the conditions of the patients.Citation21 During testing, the indicators, consisting of 12-lead electrocardiogram (ECG), blood pressure, pulse oximetry, pulmonary ventilation, gas exchange of oxygen and carbon dioxide (real-time breath-by-breath analysis), and anaerobic threshold (AT, V-slope analysis), were monitored. First, a 3-minute rest was used to stabilize the data of each indicator. Afterward, exercise without load was performed for 3 minutes, and then the load was gradually increased until some symptoms (such as leg pain, exhaustion, or difficulty in breathing) occurred. In addition, the testing would be stopped when one of the following conditions occurred: 1) 2 mm of ST-segment depression with chest pain or 3 mm of ST segment without chest pain; 2) frequent ventricular premature beat; 3) second- or third-degree atrioventricular block; 4) systolic blood pressure ≥250 mmHg or/and diastolic blood pressure ≥120 mmHg; 5) decreased blood pressure ≥30 mmHg compared to the baseline level; or 6) pulse oxygen saturation ≤80%. The prediction value for CEPT was calculated according to the American Thoracic Society/American College of Chest Physicians statement on CPET in 2003.Citation22,Citation23 After testing, the following data were recorded: peak load, peak VO2 (peak oxygen uptake), peak VO2%pred (peak oxygen uptake as percentage of predicted), peak VO2/kg (peak oxygen uptake per kilogram of body mass), peak O2 pulse (peak oxygen pulse).
Statistical analyses
Data are shown as mean ± SD. A two-sample t-test was used for comparison between the groups. The correlations between NRS score and peak VO2, peak O2 pulse, or peak load were determined using Pearson correlation analysis. For all the analysis, P<0.05 was considered statistically significant.
Results
Characteristics of patients
Among the total 58 severe COPD male patients, 25 were assessed as having nutritional risk and assigned to the nutritional risk group. The other 33 patients without nutritional risk were enrolled into the no nutritional risk group. The age (no nutritional risk group, 63.73±9.03 years; nutritional risk group, 61.52±7.13 years; P=0.319) and the height (no nutritional risk group, 166.52±6.10 cm; nutritional risk group, 167.12±5.83 cm; P=0.707) of the patients in the nutritional risk group were similar to those in the no nutritional risk group. However, the weight (no nutritional risk group, 64.58±6.31 kg; nutritional risk group, 51.24±4.68 kg; P<0.0001) and BMI (no nutritional risk group, 23.30±2.02 m/kg; nutritional risk group, 18.34±1.33 m/kg; P<0.0001) of the patients in the nutritional risk group were significantly lower than those in the no nutritional risk group ().
Table 1 Characteristics of COPD patients in each group (mean ± SD)
Results for blood gas analysis
Before exercise, there was no statistically significant difference in PaO2 (no nutritional risk group, 73.30±10.71; nutritional risk group, 73.68±13.00; P=0.903) and PaCO2 (no nutritional risk group, 42.85±6.82; no nutritional risk group, 41.95±7.84; P=0.643) between severe COPD male patients with and without nutritional risk (). All the patients had normal PaCO2 and PaO2. No one was excluded due to respiratory failure.
Table 2 Results of blood gas analysis in each group (mean ± SD)
Pulmonary function comparison between groups
The results for PFT are shown in . There was no significant difference between severe COPD male patients with and without nutritional risk in FVC (no nutritional risk group, 2.26±0.53; nutritional risk group, 2.29±0.67; P=0.850), FEV1 (no nutritional risk group, 0.86±0.25; nutritional risk group, 0.80±0.23; P=0.353), FEV1%pred (no nutritional risk group, 32.40±8.47; nutritional risk group, 29.00±8.97; P=0.146), FEV1/FVC (no nutritional risk group, 37.92±5.34; nutritional risk group, 36.39±8.47; P=0.433), TLC (no nutritional risk group, 7.89±1.39; nutritional risk group, 7.80±1.93; P=0.844), RV (no nutritional risk group, 5.66±1.31; nutritional risk group, 5.66±2.12; P=1.000), and RV/TLC (no nutritional risk group, 71.28±6.53; nutritional risk group, 71.40±11.01; P=0.962). Nevertheless, the DLCO (no nutritional risk group, 11.98±4.02; nutritional risk group, 8.97±4.37; P=0.009) of the patients in the no nutritional risk group was significantly higher than that of the patients in the nutritional risk group.
Table 3 Results for PFT in each group (mean ± SD)
Results for CPET
As shown in , the severe COPD male patients in the no nutritional risk group had significantly higher peak VO2 (no nutritional risk group, 1,068.33±327.38; nutritional risk group, 871.24±219.37; P=0.008), peak VO2%pred (no nutritional risk group, 60.27±17.13; nutritional risk group, 52.04±12.64; P=0.048), and peak O2 pulse (no nutritional risk group, 8.80±2.37; nutritional risk group, 7.32±1.47; P=0.008) than those in the nutritional risk group. But there was no significant difference between the groups in peak load (no nutritional risk group, 61.21±30.04; nutritional risk group, 49.48±20.82; P=0.100) and peak VO2/kg (no nutritional risk group, 16.57±4.61; nutritional risk group, 16.92±3.50; P=0.753).
Table 4 Results for CPET in each group (mean ± SD)
Correlation between NRS score and peak VO2, peak O2 pulse, or peak load
shows the correlation between the NRS score and peak VO2, peak O2 pulse, or peak load. The results show that there are significant negative correlations between the NRS score and peak VO2 (r=−0.353, P<0.01), peak O2 pulse (r=−0.322, P<0.05), or peak load (r=−0.272, P<0.05) in severe COPD male patients.
Discussion
Lack of exercise capacity limits the quality of life of COPD patients.Citation12 Some previous studies have found that there was an association between the nutritional status and exercise capacity in COPD patients.Citation15,Citation16 However, it is still unclear whether a correlation exists between nutritional risk and exercise capacity in COPD patients. Therefore, we investigated the relationship between nutritional risk and exercise capacity in severe COPD male patients.
In this study, we found significantly lower weight and BMI in severe COPD male patients with nutritional risk compared to those of severe COPD male patients without nutritional risk, which are the characteristics of COPD patients with nutritional risk.Citation24–Citation26 Besides, PFT showed that DLCO in severe COPD male patients without nutritional risk was significantly higher than that in severe COPD male patients with nutritional risk. It has been reported that reduced DLCO could impair exercise capacity in patients with heart failure through increased dead space ventilation.Citation27 Meanwhile, a low DLCO could impair oxygen transport by a reduction in maximum oxygen consumption.Citation28 Moreover, it has been reported that weakened exercise capacity is attributed to reduced locomotor muscle oxygen transport.Citation29,Citation30 Normally, oxygen transport would adaptively vary with oxygen demand during exercise,Citation31,Citation32 and oxygen uptake would increase during exercise for biochemical adaptations.Citation33,Citation34 Thus, the exercise capacity can decline through impaired oxygen transport, which cannot adaptively regulate the oxygen uptake during exercise.
As expected, the impaired oxygen transport might limit the oxygen uptake in severe COPD male patients with nutritional risk, as seen in this study. CPET showed that the peak VO2 and peak O2 pulse of the nutritional risk group were significantly lower than those of the no nutritional risk group and there were significant negative correlations between the NRS score and peak VO2 or peak O2 pulse. These results indicate that the oxygen uptake of severe COPD male patients with nutritional risk was significantly lower than that of severe COPD male patients without nutritional risk, which might be due to the impaired oxygen transport in COPD,Citation33,Citation35 thereby affecting the utilization of oxygen.Citation36 It was reported that inadequate oxygen supply during exercise caused accumulation of oxygen deficit, which was responsible for the reduced exercise capacity in patients with COPD.Citation37,Citation38 Meanwhile, decreased muscle oxygen utilization could reduce the exercise capacity in patients with COPD.Citation39 Thus, impaired oxygen transport may be the main reason for reduced exercise capacity in COPD patients with nutritional risk.
In addition, it has been reported that nutritional depletion is related to the respiratory and peripheral skeletal muscle function in outpatients with COPD.Citation40,Citation41 Nutritional supplements have been found to be useful for the prevention and treatment of exercise-induced skeletal muscle injury.Citation42 Moreover, muscle function is associated with the oxygen transport.Citation43–Citation45 Thus, patients with nutritional risk may have decreased exercise capacity due to impaired oxygen transport. Given these facts, there may be a close relationship between nutritional risk and exercise capacity.
Furthermore, blood gas analysis indicated that the PaO2 and PaCO2 of patients were all in normal ranges before exercise and they were similar between groups. However, the PaO2 and PaCO2 were not measured after the exercise. The changes induced by exercise in oxygen and CO2 pressure in arterial blood could not be assessed. This is a limitation of this study. Further studies are required to investigate whether nutritional risk could affect the changes induced by exercise in oxygen and CO2 pressures in arterial blood.
Besides the above results, there are some other limitations of this study. First, we enrolled only male patients, and the sample size was small. Second, we assessed only the total weight but not the fat-free mass, so further studies are required. In addition, not all of the indicators in PFT and CEPT were found to be associated with the nutritional risk. Thus, the relationship between nutritional risk and exercise capacity should be further investigated in female patients and also studies with large sample sizes for verifying the result of this study.
Conclusion
The results of this study supported the association between exercise capacity and nutritional risk according to NRS 2002 in severe COPD male patients. The impairment of oxygen transport may be the main mechanism of this relationship between nutritional risk and exercise capacity. More studies are needed to confirm this relationship in COPD.
Disclosure
The authors declare no conflicts of interest in this work.
References
- NazirSAErblandMLChronic obstructive pulmonary disease: an update on diagnosis and management issues in older adultsDrugs Aging20092681383119761275
- SuzukiMMakitaHOstlingJLower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary diseaseAnn Am Thorac Soc201411101511151925372271
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax20025784785212324669
- HallinRGudmundssonGSuppli UlrikCNutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD)Respir Med20071011954196017532198
- Gray-DonaldKGibbonsLShapiroSHMacklemPTMartinJGNutritional status and mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19961539619668630580
- LandboCPrescottELangePVestboJAlmdalTPPrognostic value of nutritional status in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19991601856186110588597
- SchwartzDBMalnutrition in chronic obstructive pulmonary diseaseRespir Care Clin N Am20061252153117150429
- AnthonyPSNutrition screening tools for hospitalized patientsNutr Clin Pract20082337338218682588
- HallinRKoivisto-HurstiUKLindbergEJansonCNutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD)Respir Med200610056156716019198
- CelikFTopcuFNutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokersClin Nutr20062595596116782241
- OgaTNishimuraKTsukinoMSatoSHajiroTAnalysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health statusAm J Respir Crit Care Med200316754454912446268
- JacobsenRFrølichAGodtfredsenNSImpact of exercise capacity on dyspnea and health-related quality of life in patients with chronic obstructive pulmonary diseaseJ Cardiopulm Rehabil Prev2012329210022193931
- BroekhuizenRWoutersECreutzbergEWeling-ScheepersCScholsAPolyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary diseaseThorax20056037638215860712
- Pascual-GuardiaSWodjaEGorostizaAMejoría de la calidad de vida y capacidad de ejercicio sin cambios en la biología muscular tras entrenamiento general en pacientes con enfermedad pulmonar obstructiva crónica grave. [Improvement in quality of life and exercise capacity without muscular biology changes after general training in patients with severe chronic obstructive pulmonary disease]Med Clin2013140200206 Spanish
- SteinerMCBartonRLSinghSJMorganMDNutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trialThorax20035874575112947128
- SabinoPGSilvaBMBrunettoAFNutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patientsClinics20106559960520613936
- RaoYXingXGuoXEffect of breathing-exercise combined with nutritional support treatment on rehabilitation in patients with chronic obstructive pulmonary diseaseJ North China Coal Med Univ20105004
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new gold classification: a study of the general populationAm J Respir Crit Care Med201218697598122997207
- KondrupJRasmussenHHHambergOStangaZAd Hoc ESPEN Working GroupNutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trialsClin Nutr20032232133612765673
- ZhaoRYZhuLLiLChenQYangYJ1988. 年上海地区成人肺功能 正常预计值公式的适用性检验. [The applicability of the 1988 version of the prediction equations for adult normal lung function in shanghai]Zhonghua Jie He He Hu Xi Za Zhi201134586589 Chinese22168980
- YanRYangWLiuJGaoBGuoKSunDCardiopulmonary exercise capacity and ventilation effectiveness in patients after clinical cure of acute irritant gas poisoningCell Biochem Biophys201571278979425480428
- RossRMATS/ACCP statement on cardiopulmonary exercise testingAm J Respir Crit Care Med20031671451 author reply 145112738602
- American Thoracic Society; American College of Chest PhysiciansATS/ACCP statement on cardiopulmonary exercise testingAm J Respir Crit Care Med200316721127712524257
- HansonAMHarrisonBCYoungMHStodieckLSFergusonVLLongitudinal characterization of functional, morphologic, and biochemical adaptations in mouse skeletal muscle with hindlimb suspensionMuscle Nerve20134839340223893872
- RossiterHBHowlettRAHolcombeHHEntinPLWagnerHEWagnerPDAge is no barrier to muscle structural, biochemical and angiogenic adaptations to training up to 24 months in female ratsJ Physiol2005565993100515845588
- LaudeKCaiHFinkBHemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in miceAm J Physiol Heart Circ Physiol2005288H7H1215471976
- HicksAOhlendieckKGopelSOPetteDEarly functional and biochemical adaptations to low-frequency stimulation of rabbit fast-twitch muscleAm J Physiol1997273C297C3059252468
- McAllisterRMReiterBLAmannJFLaughlinMHSkeletal muscle biochemical adaptations to exercise training in miniature swineJ Appl Physiol199782186218689173951
- WetterTJHarmsCANelsonWBPegelowDFDempseyJAInfluence of respiratory muscle work on VO(2) and leg blood flow during submaximal exerciseJ Appl Physiol19998764365110444624
- HarmsCAWetterTJSt CroixCMPegelowDFDempseyJAEffects of respiratory muscle work on exercise performanceJ Appl Physiol20008913113810904044
- ShawSRZernickeRFVailasACDeLunaDThomasonDBBaldwinKMMechanical, morphological and biochemical adaptations of bone and muscle to hindlimb suspension and exerciseJ Biomech1987202252343584148
- DudleyGAAbrahamWMTerjungRLInfluence of exercise intensity and duration on biochemical adaptations in skeletal muscleJ Appl Physiol Respir Environ Exerc Physiol1982538448506295989
- HolloszyJOBoothFWBiochemical adaptations to endurance exercise in muscleAnnu Rev Physiol197638273291130825
- HolloszyJOBiochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscleJ Biol Chem1967242227822824290225
- TerjungRLKoernerJEBiochemical adaptations in skeletal muscle of trained thyroidectomized ratsAm J Physiol197623011941197179330
- BhanAMalhotraAScheuerJBiochemical adaptations in cardiac muscle: effects of physical training on sulfhydryl groups of myosinJ Mol Cell Cardiol19757435442125798
- YanoTYunokiTOgataHRelationship in simulation between oxygen deficit and oxygen uptake in decrement-load exercise starting from low exercise intensityJ Physiol Anthropol Appl Human Sci20032215
- SperlichBSchifferTHoffmannUStruederHKHollmannWThe spirografic oxygen deficit: its role in cardiopulmonary exercise testingInt J Sports Med201334121074107823670361
- OscaiLBHolloszyJOBiochemical adaptations in muscle. II. Response of mitochondrial adenosine triphosphatase, creatine phosphokinase, and adenylate kinase activities in skeletal muscle to exerciseJ Biol Chem1971246696869724256681
- EngelenMPScholsAMBakenWCWesselingGJWoutersEFNutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPDEur Respir J19947179317977828687
- IzawaKPWatanabeSHiranoYThe relation between geriatric nutritional risk index and muscle mass, muscle strength, and exercise capacity in chronic heart failure patientsInt J Cardiol20141771140114125156851
- BloomerRJThe role of nutritional supplements in the prevention and treatment of resistance exercise-induced skeletal muscle injurySports Med20073751953217503877
- AraiEWatanabeSTakeyasuNOJ-347 relation between muscle function and early dynamics of oxygen uptake at the onset of constant workload exercise (exercise test/cardiac rehabilitation 4 (IHD): OJ42) (oral presentation (Japanese))Circ J200468313
- GregorevicPLynchGSWilliamsDAHyperbaric oxygen improves contractile function of regenerating rat skeletal muscle after myotoxic injuryJ Appl Physiol2000891477148211007585
- HoppelerHWeibelERStructural and functional limits for oxygen supply to muscleActa Physiol Scand200016844545610759581