Abstract
Respiratory infection is a major cause of exacerbation in chronic obstructive pulmonary disease (COPD). Infectious contributions to exacerbations remain incompletely described. We therefore analyzed respiratory tract samples by comprehensive real-time polymerase chain reaction (PCR) in combination with conventional methods. We evaluated multiple risk factors for prolonged hospitalization to manage COPD exacerbations, including infectious agents. Over 19 months, we prospectively studied 46 patients with 50 COPD exacerbations, collecting nasopharyngeal swab and sputum samples from each. We carried out real-time PCR designed to detect six bacterial species and eleven viruses, together with conventional procedures, including sputum culture. Infectious etiologies of COPD exacerbations were identified in 44 of 50 exacerbations (88%). Infections were viral in 17 of 50 exacerbations (34%). COPD exacerbations caused by Gram-negative bacilli, including enteric and nonfermenting organisms, were significantly associated with prolonged hospitalization for COPD exacerbations. Our results support the use of a combination of real-time PCR and conventional methods for determining both infectious etiologies and risk of extended hospitalization.
Introduction
Acute exacerbations are associated with prolonged hospitalization and increased mortality rates for patients with chronic obstructive pulmonary disease (COPD).Citation1 Most of COPD exacerbations are caused by respiratory infections, particularly involving rhinoviruses (RVs), influenza viruses, Haemophilus influenzae, and Streptococcus pneumoniae.Citation2 Recent reports demonstrated a causal role for viral infection in 29%–44% of COPD exacerbations.Citation3 Conventional methods including sputum culture identify mainly bacteria, which account for 30%–50% of COPD exacerbations.Citation4,Citation5 Furthermore, conventional methods have limited ability to detect not only respiratory viruses but also atypical bacteria. Even common bacteria can go undetected in patients who recently have taken antibiotics. Real-time polymerase chain reaction (PCR) can efficiently detect respiratory viruses as well as typical and atypical bacteria.Citation6,Citation7 However, few studies have evaluated usefulness of real-time PCR in identifying the respiratory virus, atypical bacteria, and polymicrobial infections that can underlie COPD exacerbation.Citation8–Citation10 More extensive examination of infectious etiologies of COPD exacerbations by real-time PCR is of great practical importance.
Advanced age is among the most important risk factors for COPD progression. COPD symptoms tend to worsen during respiratory infection, which tends to prolong COPD-related hospitalization. Extended hospitalization for COPD exacerbation tends to reflect physical deterioration and socioeconomic factors.Citation11 Pseudomonas aeruginosa and other Gram-negative bacilli infect patients with advanced COPD, often prolonging COPD-related hospitalizations. However, a relationship between infection and COPD-related hospitalization has not been clearly demonstrated.
Inflammatory indices may relate to clinical severity of COPD exacerbations.Citation12 COPD exacerbations associated with viral infection recently were linked with high serum C-reactive protein (CRP) concentrations.Citation13 However, various viruses and bacteria might show different inflammatory features during COPD exacerbations, and relationships between specific causative microorganisms and characteristics of inflammation remain incompletely understood.
To identify microbial causes of COPD exacerbations, we carried out real-time PCR with respiratory samples, in combination with conventional methods. We then evaluated risk factors for prolonged hospitalization to manage COPD exacerbations, including types of microorganisms and various biologic markers.
Subjects and methods
Study subjects, definition of COPD exacerbation, and respiratory samples
We prospectively studied patients with COPD diagnosed with COPD exacerbation who were at least 20 years old, presented within 7 days of onset of exacerbation symptoms, and provided written informed consent. Participants had sought treatment in the outpatient clinic or emergency department at The Jikei University Hospital, The Jikei University Daisan Hospital, or Toranomon Hospital between August 2012 and March 2014. COPD exacerbation was defined as any sustained increase beyond patients’ baseline condition in any respiratory symptom that required a change of regular medication or necessitated hospital treatment.Citation14
Our research protocol was approved by the review boards of The Jikei University Hospital, The Jikei University Daisan Hospital, and Toranomon Hospital. After obtaining informed consent from each patient, a nasopharyngeal swab (NPS) and a sputum sample were collected by a physician or nurse.
Data collection
Patient age, sex, smoking history, underlying disease, baseline pulmonary function, laboratory test result, and chest radiographic and computed tomographic findings were ascertained from medical records. We also recorded specific reasons for hospitalization, frequency of COPD exacerbations, mortality, duration of hospitalization, need for intensive care unit admission, and need for ventilatory support.
Real-time PCR to detect viruses and bacteria
The sputum sample was homogenized with an enzymatic sputum lysis reagent (Sputazyme, Kyokuto, Tokyo). NPS samples were suspended in 0.5 mL of pleuropneumonia-like organism broth (Becton, Dickinson and Company, Sparks, MD, USA). NPS and lyzed sputum samples were subjected to DNA and RNA extraction as well as inoculation for bacterial culture. Specifics of DNA/RNA extraction, reverse transcription reaction, and real-time PCR were as described previously.Citation15–Citation17 The Cycleave PCR kit (Takara Bio, Shiga, Japan; catalog number CY216) used for virus in the present study could detect the respiratory syncytial virus subgroups A (RSV A) and B (RSV B); parainfluenza virus types 1 (PIV1), 2 (PIV2), and 3 (PIV3); human metapneumovirus; influenza virus types A (FLU A) and B (FLU B); adenovirus; human bocavirus; and RV.Citation16 Sensitivity of PCR for detection of RSV, PIV, and FLU is reported to be approximately 10 plaque-forming units per well.Citation16
Another Cycleave PCR kit (catalog number CY 214) was used to identify six bacterial pathogens: S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Streptococcus pyogenes, and Legionella pneumophila. Sensitivity of this PCR kit has been described as approximately 10 colony-forming units per well.Citation15
PCR results were considered positive for a pathogen if the pathogen was detected in at least one of the two specimens (NPS and/or sputum). Exceptions were S. pneumoniae, H. influenzae, and S. pyogenes where widespread colonization requires in sputum for PCR diagnosis of infection.
Conventional methods of pathogen identification in COPD exacerbations
Conventional methods used for pathogen identification included sputum culture, serologic tests, urine antigen assays for S. pneumoniae and L. pneumophila, and an FLU antigen assay. Sputum cultures for M. pneumoniae and culture-based species identification were performed by methods described in the Manual of Clinical Microbiology.Citation18 A fourfold increase in M. pneumoniae titres at the convalescent phase compared to the acute phase was considered positive for M. pneumoniae infection. Positive results using urinary antigen assays for S. pneumoniae (Binax, Portland, ME) and L. pneumophila (Binax) were indicative of S. pneumoniae and L. pneumophila infection, respectively. Positive results of an FLU antigen test (Tauns Laboratories, Numazu, Shizuoka, Japan) were accepted as evidence of FLU infection.
Statistical analysis
We used Microsoft Excel 2010 for Statistics (SSRI, Tokyo) and Stat View 5.0 (Tokyo) software programs to perform statistical analysis. Categorical data are presented as frequencies and percentages. As inflammatory biomarkers, we compared white blood cell (WBC) counts and CRP values between five pathogen groups: the S. pneumoniae group, including both pure and mixed S. pneumoniae infection, n=8; the Gram-negative bacilli group, which included enteric and nonfermenting Gram-negative bacilli, n=11; other bacteria, including H. influenzae (3), Staphylococcus aureus (2), and M. pneumonia (2), n=8; mixed viral and bacterial infection, n=6; viruses, n=11; and the “no pathogen detected” group, n=6. Categorical data concerning WBC and CRP, presented as medians with interquartile range, were compared using the Kruskal–Wallis test and then analyzed post hoc by the Bonferroni test. We also analyzed risk factors for extended hospitalizations to treat COPD exacerbations. We divided durations of hospitalization into short-term, less than 14 days, and extended, 14 days or more. Hospitalizations of patients who died were considered extended, and patients not hospitalized were classified as short term. Proportions were compared using the chi-squared test and Fisher’s exact test as appropriate. Age, sex, albumin, and WBC were submitted to univariate analysis. Then, variables associated with a P-value <0.1 were submitted to multivariate analysis. Variables included in the multivariate analysis were age, male sex, albumin, WBC, home oxygen therapy, and enteric or nonfermenting Gram-negative bacilli. Odds ratios were calculated, with 95% intervals and P-values derived from the likelihood ratio.
Results
Baseline characteristics
Between August 2012 and March 2014, we evaluated 50 exacerbations of COPD in 46 patients who met the inclusion criteria. Forty-two patients had a single episode, while four had two episodes during the study period. Characteristics of the 46 patients are summarized in . Most of the 50 exacerbations occurred in man (43 patients, 86%). Twenty-one patients (45.7%) were receiving home oxygen therapy. Of the 46 patients (60.9%), 28 were diagnosed as stage III or IV of COPD according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease.Citation14
Table 1 Baseline characteristics of patients (n=46)
Clinical status of patients at COPD exacerbation
Forty-three patients (86%) were admitted to the hospital. The median hospital stay was 16 days (range 5–60 days). Four patients (8%) required ICU admission; subsequently three patients (6%) required mechanical ventilatory support. Radiologic evaluation detected pneumonia in 10 patients (20%). Detected causative agents of pneumonia were S. pneumoniae (four patients), H. influenzae (one patient), infection with both FLU and S. pneumoniae (one patient), mixed bacterial infection with Klebsiella pneumoniae and H. influenzae (one patient), and mixed infection with P. aeruginosa and S. aureus (one patient). Three patients died of severe respiratory failure, attributed to pneumonia caused by S. aureus, S. pneumoniae, or mixed infection with RSV, S. pneumoniae, and H. influenzae, one patient each.
Monthly distribution of frequencies and causative pathogens of COPD exacerbation
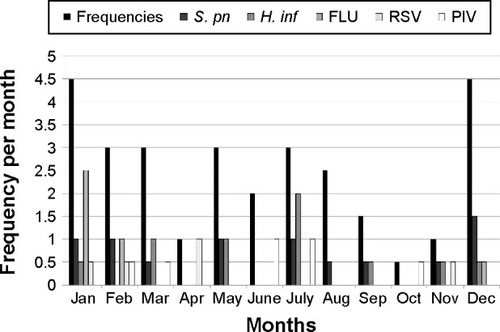
Monthly distributions of frequencies and most common causative pathogens for COPD exacerbation are shown in . COPD exacerbations followed a clear seasonal pattern, with the highest monthly rates occurring in winter (December through February), when 24 instances of exacerbation were observed (48%). Mean monthly exacerbation rates during the winter (4.0 exacerbations per month) were 1.5 times the mean rate for a typical year (2.5 exacerbations rates per month). Increases in COPD exacerbations coincided with the epidemic period for FLU and S. pneumonia infection.
Figure 1 Monthly distribution of frequencies of COPD exacerbations for time of year and for pathogens.
Pathogens identified in COPD exacerbations
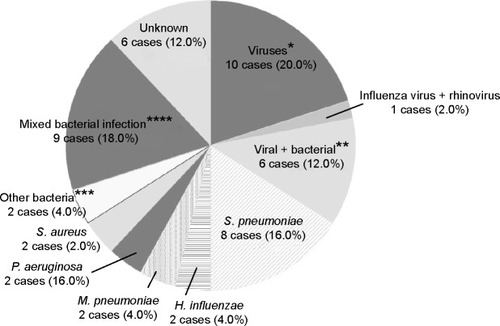
Pathogens identified in respiratory samples by comprehensive real-time PCR and conventional methods are listed in ; percentages of pathogens identified are shown in . Conventional methods identified the causative pathogens in only 26 (52%) of the 50 exacerbations, while comprehensive real-time PCR together with conventional methods made the identification in 44 (88%) of the 50 exacerbations. Twenty-eight (56%) were attributed to a single-microbe infection, while 16 (32%) were considered polymicrobial infections. The most commonly identified microorganisms were S. pneumoniae, H. influenzae, and FLU (26%, 18%, and 16% of exacerbations, respectively). Among the 8 of 13 S. pneumoniae-related exacerbations that were culture positive, most strains were covered by pneumococcal polysaccharide vaccine (types 3 (2), 11A (2), 15B (1), 16F (1), 33B (1), and 33F (1)) and were not penicillin resistant. However, most strains detected were macrolide resistant (75%).
Table 2 Identity of pathogens in patients with COPD exacerbation
Figure 2 Percentages of pathogens underlying COPD exacerbations.
Abbreviations: COPD, chronic obstructive pulmonary disease; RSV, respiratory syncytial virus.
Comparison of WBC, CRP levels among five groups in COPD exacerbation
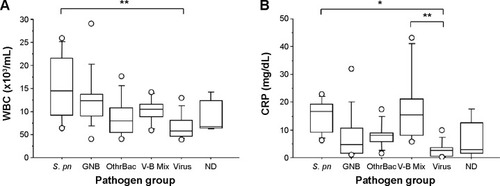
COPD exacerbations were grouped by causative pathogen (). Median WBC and CRP values differed significantly between groups (P<0.01 and P<0.005, respectively). WBC was significantly higher in “the S. pneumoniae group” than “the viral group” (P=0.0005), although WBC in “the S. pneumoniae group” did not differ from findings in any other groups. CRP was significantly higher in “the S. pneumoniae group” and “the mixed viral and bacterial infection group” than “the viral group” (P=0.0012 and P<0.0002, respectively).
Figure 3 Box-and-whisker plots showing (A) white blood cell (WBC) count and (B) C-reactive protein (CRP) comparing the Streptococcus pneumoniae (S. pn) group, including isolated only S. pneumoniae infection (n=8) with Gram-negative bacilli (GNB) group, including enteric and nonfermenting GNB (n=11); the other bacteria (OthrBac) group, including Haemophilus influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus (n=8); the V-B Mix group, including mixed viral and bacterial infection (n=6); the viral group (n=11); and the not determined (ND) group, in which no pathogen was detected (n=6). Data are presented as median (interquartile range).
Risk factors for prolonged hospitalization for COPD exacerbation
We analyzed risk factors for prolonged hospitalization. Univariate and subsequent multivariate analyses were carried out for age, sex, albumin, WBC, and each microorganism ( and ). Respiratory viruses, S. pneumoniae, and H. influenzae were not independent variables related to prolonged hospitalization. However, COPD exacerbation related to Gram-negative bacilli (including enteric and nonfermenting organisms), as well as prior treatment with home oxygen therapy were independent variables related to prolonged hospitalization (OR=5.25, P=0.042; and OR=6.23, P=0.012, respectively).
Table 3 Risk factors for prolonged hospitalization associated with COPD exacerbation
Table 4 Multivariate analysis of risk factors for prolonged hospitalization associated with COPD exacerbation
Discussion
Our study of comprehensive real-time PCR in patients with COPD exacerbations yielded four major findings. First, combining real-time PCR with conventional methods improved ability to detect infectious etiologies of COPD exacerbations including both bacteria and respiratory viruses. Second, a clear seasonal distribution of COPD exacerbations was observed. Third, COPD exacerbations caused by S. pneumoniae showed higher WBC and CRP values than those caused by viruses. Fourth, COPD exacerbations caused by Gram-negative bacilli were associated significantly with prolonged hospitalization.
A previous report concluded that use of real-time PCR in combination with conventional methods could effectively identify the infectious etiology of COPD exacerbation, particularly improving detection of viruses.Citation9,Citation10 In the present study, we extended application of real-time PCR together with conventional methods to include not only viruses and atypical bacteria but also typical respiratory bacterial pathogens. This increased the infectious etiology detection rate for COPD exacerbation to 88% (44 of 50 exacerbations), while conventional methods could determine the etiology in only 52% (26 of 50 exacerbations). We believe that real-time PCR methods can compensate for shortcomings of conventional methods, such as by extending the range of microorganisms covered, specifically to include respiratory viruses and atypical bacteria. Additionally, in the samples obtained from patients recently taking antibiotics, bacteria still could be detected.Citation19 While cell-culture and serologic methods indicated that viral infections caused approximately 20% of COPD exacerbations,Citation9,Citation20–Citation22 real-time PCR identified respiratory viruses in 16 of the 50 exacerbations that we studied (32%). Viruses identified included FLU, RSV, PIV, and RV, as result consistent with previous studies ().Citation9 Earlier investigation found M. pneumoniae and C. pneumoniae to explain up to 14% and 5% of COPD exacerbation, respectively.Citation23,Citation24 Although real-time PCR has an advantage in detecting atypical bacteria, we observed relatively low percentage of such atypical pathogens in COPD exacerbations (). We believe that during the study period only low-level endemic infections prevailed in our locality. Six bacteria, including S. pneumoniae and H. influenza among others, could be detected by real-time PCR in our present study. Among these, H. influenzae was detected more frequently by real-time PCR than by conventional sputum culture, possibly because prior antibiotics use in some patients decreased bacterial numbers. In contrast, S. aureus, as well as P. aeruginosa and other Gram-negative bacilli, were detected frequently by conventional sputum culture but were not covered by available materials for our real-time PCR. Thus, the methods complemented on another. Another advantage of simultaneously using both methods was ability to detect polymicrobial exacerbations, which we could characterize in 16 instances (32%).
Relatively few reports have dealt with seasonal patterns of COPD exacerbations.Citation25 A recent European study demonstrated peak incidence of COPD exacerbations in winter, with a nadir during summer.Citation25 We believe that the present study may be the first to identify seasonal patterns of CODP exacerbations in Japan, which proved similar to the European pattern. The peak of COPD exacerbations in winter were associated with a rise in both bacterial and viral infection, including S. pneumoniae and FLU, suggesting the potential for effective prevention by immunization against these microorganisms.
A recent study found that COPD exacerbations caused by viral infection were significantly associated with high CRP, suggesting a link between infectious etiology of COPD exacerbation and inflammatory biomarkers.Citation12,Citation13 In contrast, our S. pneumoniae group showed significantly higher WBC and CRP than our viral group. In our study, S. pneumoniae infection was in two of three fatal cases. Additional study is needed to determine how closely highly elevated inflammatory biomarkers in COPD exacerbation caused by S. pneumoniae are associated with disease severity and mortality.
Prolonged hospitalization to treat COPD exacerbation often contributes to physical deterioration and medical economic burdens.Citation11 The present study indicated that the COPD exacerbations caused by enteric or nonfermenting Gram-negative bacilli were significantly associated with prolonged hospitalization. The association of Gram-negative bacilli causing COPD exacerbation with prolongation of hospitalization might result from development of selective pressure in patients with COPD from their frequent use of antibiotics, favoring infection by antibiotic-resistant Gram-negative bacilli. Further, airway clearance mechanisms are impaired in COPD, allowing ordinarily nonvirulent bacteria to colonize the lower respiratory mucosa. Such colonization could cause a chronic inflammatory response and progressive deterioration in airway defenses, increasing severity of the exacerbation to prolong hospitalization.Citation26–Citation28
We acknowledge limitations of the present study. First, a relatively small number of samples were evaluated. Second, the limited number of patients precluded meaningful comparison between pneumonic and nonpneumonic exacerbations of COPD. Third, since the study period was relatively short, results were influenced by annual variation in incidence of specific infections. Accordingly, a larger population examined over a longer period should be analyzed.
In conclusion, this study supports the usefulness of combining comprehensive real-time PCR with conventional methods to determine infectious etiologies of COPD exacerbations. We also identified Gram-negative bacilli and requirement of home oxygen therapy as risk factors for prolonged hospitalization to treat COPD exacerbations.
Author contributions
K Shimizu and YY involved in conception and design of research; K Shimizu, YY, NS, MM, NC, and KU performed experiments; K Shimizu, YY, MM, NC, KU, and KN analyzed data; K Shimizu and YY interpreted results of experiments; K Shimizu prepared figure; K Shimizu and YY drafted manuscript; K Shimizu, YY, MM, NC, KU, HU, SH, NS, TK, HW, SI, NT, SM, JK, TN, HH, MK, K Saito, JA, YK, KN, K Kishi, and K Kuwano approved final version of manuscript; K Shimizu and YY drafted manuscript; K Shimizu, YY, MM, NC, KU, HU, SH, NS, TK, HW, SI, NT, SM, JK, TN, HH, MK, K Saito, JA, YK, KN, K Kishi, and K Kuwano edited and revised manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We wish to thank all the hospital staff at The Jikei University Hospital, The Jikei University Daisan Hospital, and Toranomon Hospital, Tokyo, Japan. This work was supported financially by Meiji Seika Pharma and Daiichi-Sankyo.
Disclosure
The authors report no conflicts of interest in this work.
References
- NiewoehnerDEThe impact of sever exacerbation on quality of life and the clinical course of chronic obstructive pulmonary diseaseAm J Med2006119384516996898
- SapeyEStockleyRACOPD exacerbations 2: aetiologyThorax20066125025816517585
- SethiSSethiREschbergerKAirway bacterial concentrations and exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200717635636117478618
- MohabAChandraSAgawalDPrevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic reviewRespirology20101553654220415983
- HirschmannJVDo bacteria cause exacerbations of COPD?Chest200011819320310893379
- TempletonKEScheltingaSAvan den EedenWCImproved diagnosis of the etiology of community-acquired pneumonia in adultsClin Infect Dis20054134535116007532
- LiebermanDShimoniAShemer-AvniYRespiratory viruses in adults with community-acquired pneumoniaChest201013881181620363845
- De SerresGLampronNLa ForgeJImportance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbationsJ Clin Virol20094612913319665425
- HutchinsonAFGhimireAKThompsonMAA community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPDResp Med200710124722481
- PerotinJMDurySRenoisFDetection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective studyJ Med Virol20138586687323447038
- MiravitillesMMurioCGuerreroTGisbertRDAFNE Study GroupPharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPDChest20021211449145512006427
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med20061731114112116484677
- ClarkTWMedinaMJBathamSC-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPDEur Respir J2015451768625186260
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung DiseaseNHLBI/WHO Workshop ReportClaude Lenfant, MDNational Heart, Lung, and Blood Institute2011
- MorozumiMNakayamaEIwataSSimultaneous detection of pathogens in clinical samples from patients with community-acquired pneumonia by real-time PCR with pathogen-specific molecular beacon probesJ Infect Chemother20064414401446
- Hamano-HasegawaKMorozumiMNakayamaEComprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumoniaJ Infect Chemother20081442443219089556
- OkadaTMorozumiMSakataHA practical approach estimating etiologic agents using real-time PCR in pediatric inpatients with community-acquired pneumoniaJ Infect Chemother20121883284022569795
- MurrayPRBaronEJJorgensenJHManual of Clinical Microbiology9th edWashington, DCAmerican Society of Microbiology2007
- TheerthakaraiREl-HaleesWIsmailMNonvalue of the initial microbiological studies in the management of nonsevere community-acquired pneumoniaChest200111918118411157602
- SeemungalTAHarper-OwenRBhowmikARespiratory viruses, symptoms and inflammatory markers in acute exacerbations and stable chronic obstructive diseaseAm J Respir Crit Care Med20011641618162311719299
- GlezenWPGreenbergSBAtmarRLPiedraPACouchRBImpact of respiratory virus infections on persons with chronic underlying conditionsJAMA2000283449950510659876
- GreenbergSBCadenaALinJRespiratory viral infections in adults with and without chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200016216717310903237
- LiebermanDLiebermanDYaakovBMInfectious etiologies in acute exacerbations of COPDDiagn Microbiol Infect Dis2001409510211502375
- BeatyCDGraystonJTWangSPChlamydia pneumoniae, strain TWAR, infections in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis199116140814101741558
- KlausFRLeonardoMFVogelmeierCSeasonal distribution of COPD exacerbations in the Prevention of Exacerbations with Tiotropium in COPD trialChest201314371171923188489
- WilsonRA vicious circle hypothesis operating during infective exacerbations of chronic bronchitisMonaldi Arch Chest Dis1994491591648049702
- MiravitllesMCalleMSoler-CataluñaJJClinical phenotypes of COPD: identification, definition and implications for guidelinesArch Broncopneumol2012488698
- SethiSMalliaPJohnstonSLNew paradigms in the pathogenesis of chronic obstructive pulmonary disease IIProc Am Thorac Soc2009653253419741263
