Abstract
Background
Although chronic obstructive pulmonary disease (COPD) is closely associated with gastroesophageal reflux disease (GERD), the clinical significance of laryngopharyngeal reflux (LPR) is not fully understood in COPD.
Methods
Prospective cohorts were established among 118 patients with COPD from March 2013 to July 2014. Thirty-two age-matched and sex-matched normal controls, who had routine health check-ups during the study period, were included. Laryngopharyngeal reflux finding scores (RFS) and reflux symptom index (RSI) for LPR were subjected to association analysis with severity and acute exacerbation of COPD during the 1-year follow-up.
Results
The mean age of patients enrolled in the study was 69.2±8.8 years, with 93.2% being male. Positive RFS (>7) and RSI (>13) were observed in 51 (42.5%) and six patients (5.0%), respectively. RFS and RSI were significantly higher in patients with COPD than in normal, healthy patients (P<0.001). RFS was significantly correlated with residual volume/total lung capacity (%, P=0.048). Scores for diffuse laryngeal edema, erythema, and hyperemia were significantly higher in the high-risk group (Global Initiative for Chronic Obstructive Lung Disease classification C and D; P=0.025 and P=0.049, respectively), while RSI was significantly higher in the more symptomatic group (Global Initiative for Chronic Obstructive Lung Disease classification B and D; P=0.047). RSI and RFS were significant predictors for severe acute exacerbation of COPD (P=0.03 and P=0.047, respectively), while only RSI was associated with severity of dyspnea.
Conclusion
Laryngeal examination and evaluation of laryngeal reflux symptom could be a surrogate clinical indicator related to severe acute exacerbation of COPD. Further studies of LPR in COPD patients should be considered.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive and irreversible expiratory flow limitation and dyspnea. In patients with COPD, frequent exacerbations are the most prominent and distressing symptom.Citation1 Patients with COPD may be particularly vulnerable to reflux and gastroesophageal reflux disease (GERD), which is common in advanced COPD. Although little is known about the prevalence and role of GERD in COPD, exaggerated intrathoracic pressure shifts, increased frequency of coughing, diaphragmatic flattening, and use of β2-agonists are thought to exacerbate reflux.Citation2,Citation3 However, patients with respiratory disease are often asymptomatic for reflux, and show isolated abnormal proximal reflux, resulting in symptoms that lack sufficient sensitivity and specificity to reliably diagnose or exclude GERD.Citation4,Citation5
Laryngopharyngeal reflux (LPR) is considered a different disease from classic reflux or GERD. It is believed that the primary defect in LPR might be upper esophageal sphincter dysfunction in less than half of LPR patients with GERD.Citation6 Most patients with LPR have throat symptoms like dysphonia, chronic cough, globus pharyngeus, and chronic throat clearing.Citation7 A diagnosis of LPR may be established by interviewing patients and questioning about specific symptoms, videolaryngoscopic evaluation of the larynx, or double probe pH monitoring.Citation8–Citation10 Ambulatory 24-hour double probe (pharyngeal and esophageal) pH monitoring is highly sensitive and specific for the diagnosis of LPR.Citation11 While pH monitoring is not widely available in clinical practice due to its inconvenience and cost, videolaryngoscopic examination is more easily accessible. Therefore, if a clinical diagnosis of LPR in patients with COPD is identified, physicians may have a valuable tool in the management of reflux-related disease in COPD.
Considering the fact that COPD may exacerbate gastroesophageal reflux, LPR can be aggravated by similar mechanisms. However, limited studies have investigated the association between LPR and COPD or the clinical significance of LPR in patients with COPD. In addition, previous studies have focused on the effect of LPR treatment on COPD, and shown that treatment of LPR resulted in significant improvement in symptoms of COPD.Citation12 In contrast, a study of LPR and asthma showed that reflux symptom score and LPR disease index are not reliable for diagnosing LPR in patients with asthma.Citation13 In this present study, we aimed to assess the prevalence of LPR in COPD patients, stage the LPR severity, and correlate findings/symptoms with indices of COPD.
Materials and methods
Study population
All of the patients enrolled in the study were recruited from March 2013 to July 2014 at routine follow-up visits to the pulmonary clinic, located at an academic hospital in South Korea. The inclusion criteria required participants to be aged 40 years or older, diagnosed with COPD based on guidelines,Citation1 have a smoking history of over ten packs/year, and be able to provide written informed consent. Exclusion criteria included previous diagnosis of LPR or GERD, recent history of exposure to proton pump inhibitors within 2 months, airflow limitation resulting from parenchymal destruction with previous infection (eg, pulmonary tuberculosis, diffuse bronchiectasis), a recent history of alcohol abuse, known esophageal disease including cancer, achalasia and peptic ulcer disease, and any clinically significant concurrent disease. COPD patients with exacerbation within 1 month of enrollment were also excluded. The institutional review board (approval 06-2011-72) of Seoul National University Boramae Medical Center approved all of the protocols and study design, and all patients gave written informed consent. Data on demographic, physiologic, and clinical characteristics, including age, sex, height, body weight, smoking status, spirometric data, and use of respiratory medications, were obtained from each patient.
Study protocol
This cross-sectional study evaluated the prevalence of LPR in COPD patients, and prospectively observed the patients for 1 year after enrollment to measure the frequency of acute exacerbations of COPD (AECOPD). On enrolment, patients were surveyed with the modified Medical Research Council dyspnea scale and laryngeal symptom index (RSI).Citation8,Citation14 Spirometric data were also collected. Patients were regularly followed at 2, 6, and 12 months after initial registration. The number of acute moderate or severe exacerbations were evaluated at each follow-up. The severity of AECOPD was determined by the requirement for antibiotics or systemic steroids (moderate) and admission to hospital due to exacerbation (severe).
Laryngoscopy was performed by an otolaryngologist (YHJ) and interpreted by two independent otolaryngologists (YHJ and DYL). Reflux finding scores (RFS) were summated by calculating the score of each of the findings, ie, subglottic edema (0/2), vocal fold edema (0/1/2/3/4), ventricular obliteration (0/2/4), diffuse laryngeal edema (0/1/2/3/4), erythema/hyperemia (0/2/4), posterior commissure hypertrophy (0/1/2/3/4), thick mucus (0/2), and granuloma (0/2). LPR was defined as an RFS over 7 and an RSI over 13.Citation8,Citation10
To compare RFS and RSI of non-COPD controls, 32 age-matched and sex-matched normal controls who had routine health check-ups during the study period were included. The controls also did not have a previous history of GERD or LPR, and any pulmonary disease was ruled out by chest X-ray and pulmonary function testing.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation, and categorical variables as frequencies and group percentages. Continuous variables were compared according to the severity of airflow limitation (Global Initiative for Chronic Obstructive Lung Disease grade) and acute exacerbation of COPD by independent samples t-test or analysis of variance test. Categorical variables were compared using chi-square analysis or a Cochran-Armitage trend test. RFS and RSI were considered both continuous and categorical variables (RFS positive >7 and negative ≤7, RSI positive >13 and negative ≤13). The established risk factors for exacerbations were included in a bivariate logistic regression analysis model. The odds ratios and 95% confidence intervals were then calculated. P<0.05 was considered to be statistically significant. The statistical analysis was performed using Statistical Package for the Social Sciences version 20.0 software (SPSS Inc, Chicago, IL, USA).
Results
Characteristics of study population
The detailed characteristics of the study population are summarized in . A total of 118 patients with COPD were enrolled. The mean age was 69.2±8.8 years and the male to female ratio was 110:8. The mean follow-up duration was 14.3±5.4 months. According to the Global Initiative for Chronic Obstructive Lung Disease classification, patients were categorized into four groups: A, low risk with less symptoms (n=27, 22.9%); B, low risk with more symptoms (n=45, 38.1%); C, high risk with less symptoms (n=13, 11.0%); and D, high risk with more symptoms (n=33, 28.0%). The four groups showed no significant difference in age, sex ratio, or body mass index. The follow-up duration was significantly shorter in group A (P=0.008; ). In the entire cohort, moderate and severe AECOPD was observed in 23 (19.5%) and 15 patients (12.7%), respectively. Both moderate and severe AECOPD were more frequent in groups C and D than in groups A and B (P<0.001; ). The mean RFS and RSI were 6.86±2.60 and 4.57±4.71, respectively. Positive RFS (>7) and RSI (>13) were observed in 51 (42.5%), and six (5.0%) patients, respectively. Only five patients (4.2%) were diagnosed with LPR by RFS and RSI criteria. Comparing the area under receiver operating curve for RSI, 8.5 points of RSI coincided with the cut-off value of RSI (7 points) for LPR, which was lower than the usual cut-off value of RSI (13 points) for LPR.
Table 1 Characteristics of COPD patients (n=118)
Table 2 Demographic and spirometric characteristics according to GOLD classification
Association between LPR parameters, AECOPD, and dyspnea severity
When comparing the low-risk groups (A and B) and the high-risk groups (C and D), the total RFS and RSI were not significantly different (P=0.344 and P=0.252, respectively), although positive correlations in trends were suggested in each parameter for LPR (). With regard to the RFS parameter, diffuse laryngeal edema and erythema/hyperemia showed significantly higher scores in the high-risk groups than in the low-risk groups (P=0.025 and P=0.049, respectively). Patients with RSI >3 were more prevalent in the high-risk groups (P=0.032). When comparing the groups with less symptoms (groups A and C) with the groups with more symptoms (groups B and D), RFS and each of the parameters showed no significant difference (). However, total RSI was significantly higher in the groups with more symptoms (P=0.047), and there were more patients with RSI >8.5 in the groups with more symptoms (P=0.006).
Table 3 RFS and RSI according to risk and symptoms
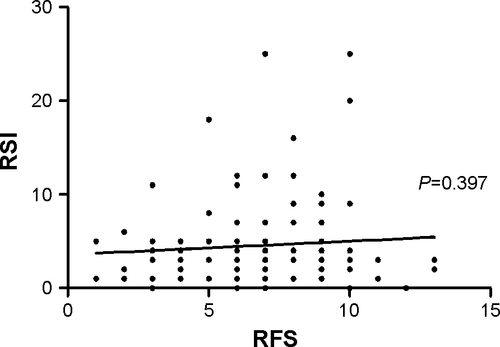
Multivariate analysis revealed that no parameter was significantly associated with acute moderate exacerbation. However, both RSI (continuous or dichotomous) and RFS (continuous) were significantly associated with acute severe exacerbation (P=0.03, P=0.01, and P=0.047, respectively; ). There was a significant negative correlation between RFS and residual volume/total lung capacity (%) in simple correlation analysis (P=0.048), while correlation of RFS and RSI with forced expiratory volume in 1 second (FEV1; %), residual volume/total lung capacity (%), and diffusing capacity of the lungs for carbon monoxide (%) showed no significant correlation (). With regard to the severity of dyspnea measured with the modified Medical Research Council scale, RSI was a significant predictor for severe dyspnea after adjusting for age, sex, body mass index, and FEV1% predicted ().
Table 4 Multivariate analysis for association between indices of reflux and severe acute exacerbation/mMRCTable Footnote*
Figure 1 Correlation of reflux symptom index and finding score with post FEV1 (%) (A, B), RV/TLC (%) (C, D), and DLCO (%) (E, F) in patients with COPD.
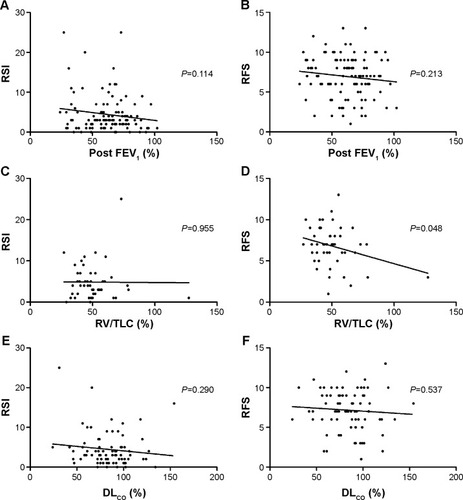
Comparison between COPD patients and normal controls
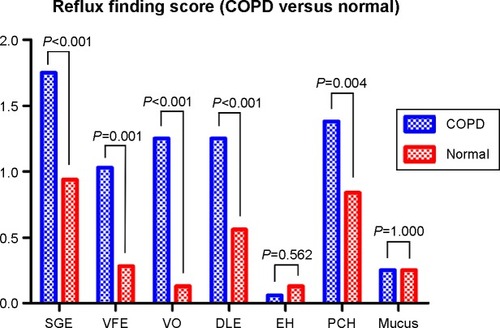
Normal controls showed mean RFS and RSI scores of 3.13±2.83 and 3.25±3.57, respectively. RFS and RSI were significantly higher in patients with COPD (P<0.001; ). RFS findings indicated that, subglottic edema, vocal fold edema, ventricular obliteration, diffuse laryngeal edema, and posterior commissure hypertrophy were significantly prevalent or more severe in patients with COPD than in normal controls (, ).
Table 5 Comparison of RFS between patients with COPD and normal controls matched for age and sex
Figure 2 RSI (A) and RFS (B) of patients with COPD and a normal control.
Abbreviations: COPD, chronic obstructive pulmonary disease; RFS, reflux finding score; RSI, reflux symptom index.
Figure 3 Reflux finding score of patients with COPD and normal controls.
Discussion
GERD is a well-known comorbidity of COPD,Citation12,Citation15 and is negatively associated with symptoms, quality of life, and exacerbation of COPD.Citation16–Citation18 However, the association between COPD and LPR, a laryngeal manifestation and airway effect of GERD, has not been extensively studied. To the best of our knowledge, this is the first report of an association between LPR and AECOPD. In the course of this study, we identified features of LPR in patients with COPD.
The prevalence of GERG reported has been varied in studies, according to differences in study population and diagnostic definition. Self-reported GERD was found in 29% of patients in the COPDGene study cohort,Citation18 and 28% of COPD patients reported GERD in a nationwide health insurance data survey.Citation15 We excluded patients with alleged gastric disorders, and RFS-based LPR was found in 42.5% of patients, whereas RSI-defined LPR was identified in only 5.0% of patients with COPD in this study. Although the prevalence of LPR in COPD has rarely been reported in large populations, RFS-defined LPR was higher than expected considering that LPR may be a part of GERD. Nevertheless, it has been reported that in severe COPD, pathologic reflux, as defined by pH monitoring, was increased in up to 62% of subjects. Consistent with our results, 58% of these patients did not report any reflux symptoms.Citation18,Citation19 In other words, symptom-defined prevalence may be lower than objectively-defined pathologic findings. The correlation between RFS and RSI was low in our study group, even considering the small number of subjects (Figure S1). There are two hypotheses: First, the common respiratory symptoms of COPD can be presented with those in RSI such as dysphonia, throat clearing, excess throat mucus, and cough. As a result, any symptoms they may be experiencing could be ignored or attributed to more common daily symptoms rather than LPR. Supporting our hypothesis, the number of patients with an RSI >13 (the cut-off value for LPR in the general population) was very small in the main analysis and additive analysis when compared with normal controls, although the mean difference in RSI was significantly different (). In contrast, the number of patients with RFS greater than 7 (the cutoff value for LPR in the general population) was more prevalent in COPD patients (). Second, there is a possibility of overdiagnosis of RFS. However, the second explanation may be less likely considering that the prevalence of symptomatic GERD was lower than that measured by ambulatory pH monitoring,Citation19 and a higher prevalence and more severe grade of RFS was identified in patients with COPD () when compared with age-matched and sex-matched normal subjects. In this respect, LPR findings in COPD could be an indicator of acid reflux rather than reflux symptoms in patients with COPD.
We found that RFS and RSI were associated with severe AECOPD independent of age, sex, FEV1, or body mass index. In addition, the prevalence and grade of RFS and severity of RSI increased according to the increasing COPD severity; the failure of this association to reach statistical significance could be attributable to the small number of subjects.
AECOPD is a major treatment target in COPD,Citation20 and many contributing factors have been reported, including GERD.Citation21,Citation22 Our study is the first to examine the possible relationship between LPR and AECOPD. Dysfunction of the upper esophageal sphincter (UES) is considered a critical pathogenic step in LPR, and is also associated with dysfunction of the UES as well as the lower esophageal sphincter, as shown in an esophageal motility study.Citation23 The role of the UES is more emphasized during coughing, and is important in preventing esophagopharyngeal reflux during cough, even in elderly people.Citation24 Hyperinflation-induced poor exercise capacity, higher intrathoracic pressure, and flattening of the diaphragm are expected to contribute to the pathogenesis of GERD in COPD.Citation2,Citation3 If a similar mechanism occurred in patients with LPR, the increased residual volume (145.6% of predicted value) in our study population can be explained by the proposed mechanism. Moreover, our findings demonstrate that the residual volume/total lung capacity (%) was significantly correlated with RFS. This supports the previous theory concerning the correlation between LPR and COPD, along with the association of COPD with reflux findings rather than reflux symptoms as mentioned previously. Among the RFS, diffuse laryngeal edema and erythema/hyperemia showed significantly higher scores in the high-risk group. Subglottic edema and posterior commissure hypertrophy were typical and frequent findings in LPR patients without COPD (data not shown). We consider that these findings could be unique for COPD, especially for the high-risk groups.
The present study had some limitations. First, as mentioned earlier, it had weak statistical power due to the small number of patients and had a lack of generalizability due to the preponderance of male subjects. We tried to exclude patients with alleged reflux-related gastrointestinal disease and those who had used proton pump inhibitors. A high prevalence of GERD and prevalent use of proton pump inhibitors led us to restrict the enrolled numbers of study subjects. As a result, statistical power was not achieved for some parameters. Second, a therapeutic trial with proton pump inhibitors and follow-up laryngeal examination were not performed in this study. Thus, the clinical significance of each RFS finding cannot be discussed. Third, RSI and RFS could have limited sensitivity and specificity for diagnosing the LPR. The innate weakness in diagnosing LPR is that there is no diagnostic method of gold standard yet. Although double probe pH monitoring could be used for suspicious patients, poor cooperation of the patients from discomfort during the test and lack of strong correlation with the LPR symptom limit the wide appliance of the double probe pH monitoring. In this study, diagnostic definition of LPR was based on RSI and RFS, because those parameters were validated in terms of the correlation and response to treatment.
Conclusion
In summary, laryngeal reflux findings were prevalent in patients with COPD, and were found in 42.5% of subjects in this study. RFS and RSI for LPR indicated significant risk factors for AECOPD. Laryngeal examination may be clinically indicated during AECOPD, and further studies of LPR in COPD patients should be considered.
Acknowledgments
This study was supported by a clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (03-2011-10).
Supplementary material
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- CrowellMDZayatENLacyBESchettler-DuncanALiuMCThe effects of an inhaled beta(2)-adrenergic agonist on lower esophageal function: a dose-response studyChest200112041184118911591558
- MokhlesiBMorrisALHuangCFCurcioAJBarrettTAKampDWIncreased prevalence of gastroesophageal reflux symptoms in patients with COPDChest200111941043104811296167
- KempainenRRSavikKWhelanTPDunitzJMHerringtonCSBillingsJLHigh prevalence of proximal and distal gastroesophageal reflux disease in advanced COPDChest200713161666167117400682
- RaghuGFreudenbergerTDYangSHigh prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosisEur Respir J200627113614216387946
- KoufmanJAThe otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injuryLaryngoscope19911014 Pt 2 Suppl 531781895864
- KoufmanJALaryngopharyngeal reflux is different from classic gastroesophageal reflux diseaseEar Nose Throat J2002819 Suppl 27912353431
- BelafskyPCPostmaGNKoufmanJAValidity and reliability of the reflux symptom index (RSI)J Voice200216227427712150380
- BeaverMEStasneyCRWeitzelEDiagnosis of laryngopharyngeal reflux disease with digital imagingOtolaryngol Head Neck Surg2003128110310812574767
- BelafskyPCPostmaGNKoufmanJAThe validity and reliability of the reflux finding score (RFS)Laryngoscope200111181313131711568561
- PostmaGNBelafskyPCAvivJEKoufmanJALaryngopharyngeal reflux testingEar Nose Throat J2002819 Suppl 2141812353426
- EryukselEDoganMOlgunSKocakICelikelTIncidence and treatment results of laryngopharyngeal reflux in chronic obstructive pulmonary diseaseEur Arch Otorhinolaryngol200926681267127119221778
- KilicMOzturkFKirmemisOImpact of laryngopharyngeal and gastroesophageal reflux on asthma control in childrenInt J Pediatr Otorhinolaryngol201377334134523277300
- JungYHChangDYJangJHReflux symptom index (RSI) and reflux finding score (RFS) of persons taking health checkup and their relationship with gastrofiberscopic findingsKorean J Otolaryngol2007505431437 Korean
- Pacheco-GalvanAHartSPMoriceAHRelationship between gastro-oesophageal reflux and airway diseases: the airway reflux paradigmArch Bronconeumol201147419520321459504
- KimJLeeJHKimYAssociation between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort studyBMC Pulm Med2013135123927016
- LiangBWangMYiQFengYAssociation of gastroesophageal reflux disease risk with exacerbations of chronic obstructive pulmonary diseaseDis Esophagus201326655756023301861
- MartinezCHOkajimaYMurraySImpact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohortRespir Res2014156224894541
- CasanovaCBaudetJSdel Valle VelascoMIncreased gastro-oesophageal reflux disease in patients with severe COPDEur Respir J200423684184515218995
- HwangYIParkYBOhYMComparison of Korean COPD guideline and GOLD initiative report in term of acute exacerbation: a validation study for Korean COPD guidelineJ Korean Med Sci20142981108111225120321
- Rascon-AguilarIEPamerMWludykaPRole of gastroesophageal reflux symptoms in exacerbations of COPDChest200613041096110117035443
- SakaeTMPizzichiniMMTeixeiraPJExacerbations of COPD and symptoms of gastroesophageal reflux: a systematic review and meta-analysisJ Bras Pneumol201339325927123857694
- GadelAAMostafaMYounisAEsophageal motility pattern and gastro-esophageal reflux in chronic obstructive pulmonary diseaseHepatogastroenterology2012591202498250223178615
- AmarisMDuaKSNainiSRCharacterization of the upper esophageal sphincter response during coughChest201214251229123622797662