Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with abnormal systemic inflammation, and apoptosis is one of the pathogenic mechanisms of COPD. Several studies have suggested that tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and its receptors were not only involved in diseases associated with apoptosis but also in inflammatory diseases. However, limited data about the possible relationship between COPD and TRAIL/TRAIL-receptors are available.
Objective
To evaluate the potential relationship between TRAIL/TRAIL-receptors and COPD.
Methods
Serum levels of TRAIL, decoy receptor 5 (DR5), C-reactive protein, and tumor necrosis factor-α were analyzed using multiplex enzyme-linked immunosorbent assay kits. Then, serum levels of TRAIL and DR5 in 57 COPD patients with 35 healthy controls were compared and correlated with lung function and systemic inflammation.
Results
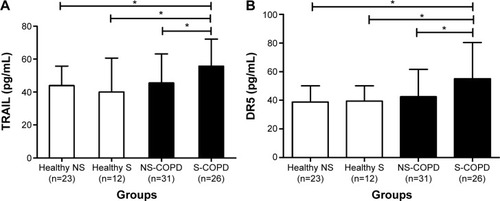
Mean levels of serum TRAIL and DR5 were significantly higher in COPD patients than those in controls (50.17±17.70 versus 42.09±15.49 pg/mL, P=0.029; 48.15±22.88 versus 38.94±10.95 pg/mL, P=0.032, respectively). Serum levels of TRAIL and DR5 correlated inversely with forced expiratory volume in 1 second % predicted, an index of lung function in COPD (r=-0.354, P=0.007 for TRAIL; r=−0.394, P=0.002 for DR5) in all participants (r=-0.291, P=0.005 for TRAIL; r=−0.315, P=0.002 for DR5), while DR5 correlated positively with C-reactive protein (r=0.240, P=0.021 for total subjects) and TRAIL correlated positively with tumor necrosis factor-α (r=0.371, P=0.005 for COPD; r=0.349, P=0.001 for total subjects).
Conclusion
Our results suggested that circulating TRAIL and DR5 increased in COPD patients and were associated with lung function and systemic inflammation in COPD. Future studies are needed to verify whether and how TRAIL and its receptors play roles in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD), a kind of chronic airway inflammatory disease, has been considered as the third most life-threatening disease, with high morbidity and mortality.Citation1 The pathogenesis and pathology of COPD are complicated, but abnormal airway inflammation is the most important hypothesis, combined with imbalance between protease/antiprotease system, oxidative stress, and immunological mechanisms.Citation2 In addition, it has been treated not only as a respiratory disease, but also as a systemic process, which could cause target organ injury, leading to various comorbidities of COPD patients.Citation3,Citation4 Over the past few years, a growing number of studies have reported that the apoptotic alveolar epithelial and endothelial cells were significantly increased in the lungs of COPD patients, which suggested that apoptosis might be involved in the pathogenesis of COPD.Citation5–Citation7 However, the functional relationship between apoptosis and systemic inflammation in COPD pathogenesis remains unclear.Citation8
Tumor necrosis factor (TNF) plays an important role in the pathogenesis of COPD. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a member of the TNF family and mainly expressed by CD11c+ dendritic cells, airway epithelial cells, human peripheral blood T-lymphocytes, natural killer cells, eosinophils, neutrophils, and monocytes can be activated by cytokine or antigen exposure.Citation9–Citation13 Decoy receptor 5 (DR5) is one of the most important receptor of TRAIL, the cytoplasmic death domains of which can activate nuclear factor kappa B cells (NF-κB), inducing apoptotic cell death.Citation14,Citation15 Moreover, DR5 has been confirmed to be the highest affinity receptor for TRAIL, and it plays a vital role in TRAIL-induced apoptosis.Citation16
Previous studies mainly focused on the function of TRAIL in tumors, since it could activate the extrinsic apoptotic pathway in immortalized cell lines and primary tumor cells.Citation17–Citation19 In recent years, some studies have found that TRAIL and its receptors played important roles in certain inflammatory responses.Citation20–Citation22 Furthermore, the underlying roles of the TRAIL/TRAIL-receptor system in the conditions of chronic pulmonary diseases, such as asthma,Citation11,Citation23 lung fibrosis,Citation24 and pulmonary hypertension,Citation25 have also been investigated. It has been shown that the TRAIL/TRAIL-receptors system was one of various mechanisms implicated in alveolar apoptosis of emphysematous lung.Citation26 Morissette et alCitation27 previously reported that DR5 was upregulated in the lung tissues of patients with emphysema when compared with healthy controls (smokers and nonsmokers). They also discovered that cigarette smoking and oxidative stress could sensitize alveolar epithelial cell to TRAIL-mediated apoptosis, and TRAIL-mediated apoptosis was abnormally increased in the lung of patients with emphysema.Citation28 Despite these findings, studies concerning the association between TRAIL/TRAIL-receptors system and COPD are still limited. Therefore, the main objectives of this study were to measure the circulating TRAIL and DR5 in stable COPD patients and analyze their correlations with pulmonary function and systemic inflammatory markers of COPD.
Methods
Subjects
Between January 2013 and October 2014, 57 patients with COPD were recruited from the outpatient department of West China Hospital. They all underwent a chest X-ray and a standard pulmonary function test using a Spirotel® spirometer purchased from Mir Medical International Research Srl (Rome, Italy). COPD was diagnosed and classified for this research in accordance with Global Initiative for Chronic Obstructive Lung Disease criteria.Citation1 Patients with COPD had to have a forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) less than 70%, and a less than 12% increase in FEV1 after inspiring bronchodilator, and they should not have had an exacerbation within the last 3 months prior to the study. None of the patients had ever received inhaled corticosteroids or antibiotics. They were divided into nonsmokers with COPD and smokers with COPD. Thirty-five smoking history, sex, and age-matched subjects with normal pulmonary function were recruited as controls, and they also underwent a chest X-ray and a standard pulmonary function test as the COPD patients did. We collected all the subjects’ basic health information through questionnaires and physical examinations. Patients with kidney disease, heart disease, liver disease, diabetes mellitus, cancer, and autoimmune disease were excluded. To avoid the bias caused by a long time of research, we recruited a limited number of subjects and could not divide subjects into multigroups in more diverse ways. The Institutional Review Board for Human Studies of West China Hospital of Sichuan University, People’s Republic of China, approved the study, and all the subjects provided written consent.
Measurement of TRAIL, DR5, TNF-α, and CRP
Peripheral blood samples were obtained from all subjects, and serum was separated immediately and stored at −80°C until processing. Levels of TRAIL, DR5, TNF-α, and C-reactive protein (CRP) were analyzed using Human Magnetic Luminex Screening Assay (LXSAHM; R&D Systems, Inc., Minneapolis, MN, USA) on Bio-Plex 200 platform (Bio-Rad, Irvine, CA, USA). Manufacturer-specified limits of detection were 9.65 pg/mL for TRAIL, 2.2 pg/mL for DR5, 1 pg/mL for TNF-α, and 116 pg/mL for CRP. Technicians performing tests were blinded to the clinical details of the subjects.
Statistical analysis
Data were expressed as mean ± standard deviation. After normal distribution of these data was been confirmed, differences between groups were statistically analyzed using an unpaired Student’s t-test and, for multiple comparisons, one-way analysis of variance was used. The nonnormal data were transformed to be normally distributed through log transformation. Correlations between variables were analyzed using Pearson’s correlation analysis. The indication of statistically significant difference was P<0.05. Data were analyzed using SPSS 19.0 for Windows (IBM, Chicago, IL, USA). Pictures were created using Graphpad Prism5.0 (GraphPad Software, San Diego, CA, USA).
Results
Clinical characteristics of subjects
Demographic and clinical characteristics (including smoking pack-years, body mass index [BMI], and serum levels of TRAIL and DR5) of patients with COPD and healthy controls are summarized in and . The age, sex, BMI, blood oxygen saturation, proportion of smoking, and smoking pack-years of both groups were similar. There were significant reductions in FEV1, FEV1% predicted, and FEV1/FVC in the COPD group compared with those in the control group, suggesting that the COPD group had airflow limitation. In multigroup analysis, age, BMI, blood oxygen saturation of all the groups were similar. Even though the sex composition and smoking pack-years were different between COPD smokers with COPD nonsmokers and healthy non-smokers, they were similar between smoking COPD group and healthy smokers.
Table 1 Characteristics of patients with COPD and healthy subjects
Table 2 Characteristics of multigroups
Serum levels of TRAIL and DR5 increase in COPD patients
The circulating TRAIL levels in COPD patients were significantly upregulated in comparison with healthy volunteers (50.17±17.70 versus 42.09±15.49 pg/mL, P=0.029, ). Similar result was found in DR5 (48.15±22.88 versus 38.94±10.95 pg/mL, P=0.032, ). In addition, in multigroup analysis, we found that TRAIL and DR5 expressions were higher in smokers with COPD than those in nonsmokers with COPD (55.68±16.44 versus 45.55±17.65 pg/mL, P=0.024; 55.06±25.26 versus 42.35±19.22 pg/mL, P=0.008, respectively), healthy smokers (41.03±21.00 pg/mL, P=0.013; 39.38±10.64 pg/mL, P=0.021, respectively), and healthy nonsmokers (42.65±12.22 pg/mL, P=0.007; 38.71±11.34 pg/mL, P=0.002, respectively). However, there was no difference observed among the healthy nonsmokers, healthy smokers, and nonsmokers with COPD ().
Figure 1 Levels of TraIl (A) and DR5 (B) in plasma from the non-COPD group and COPD group.
Notes: Circulating TRAIL and DR5 concentrations were measured using multiplex enzyme-linked immunosorbent assay. COPD patients had significantly elevated TRAIL and DR5 levels when compared with those in healthy controls (P=0.029 and P=0.032, respectively). DR5 was log transformed in the statistical analyses. The expression of TRAIL and DR5 are presented as mean (error bar) and compared by unpaired Student’s t-test (*P<0.05).
Abbreviations: COPD, chronic obstructive pulmonary disease; DR5, decoy receptor 5; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
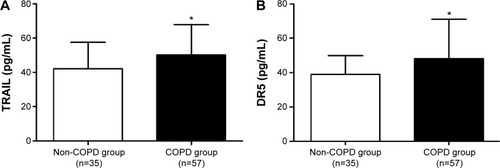
Figure 2 Multigroup analysis: TRAIL and DR5 in the plasma from the healthy nonsmokers group, healthy smokers group, COPD nonsmokers group, and COPD smokers group.
Abbreviations: COPD, chronic obstructive pulmonary disease; DR5, decoy receptor 5; NS, nonsmokers; S, smokers; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
TRAIL and DR5 are correlated with lung function, TNF-α, and CRP
We analyzed the correlations of TRAIL and DR5 with FEV1% predicted, FEV1/FVC, CRP, and TNF-α in COPD patients and in all subjects, and found that TRAIL was inversely correlated with FEV1% predicted (r=−0.354, P=0.007, ) and FEV1/FVC (r=−0.32, P=0.013, ) in COPD. Similar results were also found in all participants (r=−0.291, P=0.005; r=−0321, P=0.002; respectively, ). We also observed that DR5 had a negative correlation with FEV1% predicted (r=−0.394, P=0.002 for COPD; r=−0.315, P=0.002 for total subjects, ) and FEV1/FVC (r=−0.316, P=0.017 for COPD; r=−0.358, P=0.000 for subjects, ). More importantly, we still found a positive relationship between TRAIL and TNF-α in COPD and in all subjects (r=0.371, P=0.005; r=0.349, P=0.001, respectively; ) and a positive correlation between DR5 and CRP in subjects (r=0.240, P=0.021, ). All these correlations are shown in the and .
Table 3 Correlations between TRAIL, lung function, and TNF-α
Table 4 Correlations between DR5, lung function, and CRP
Figure 3 TraIl and DR5 were correlated with pulmonary function.
Notes: TraIl in the serum was inversely correlated with (A) FEV1% predicted (r=−0.354, P=0.007 for COPD; r=−0.291, P=0.005 for all participants) and (B) FEV1/FVC (r=−0.32, P=0.013 for COPD; r=−0.321, P=0.002 for all participants). DR5 was inversely correlated with (C) FEV1% predicted (r=−0.394, P=0.002 for COPD; r=−0.315, P=0.002 for total subjects) and (D) FEV1/FVC (r=−0.316, P=0.017 for COPD; r=−0.358, P=0.000 for total subjects). DR5 was log transformed in the statistical analyses. The orange solid line denotes the line of best fit in COPD, the blue line represents the line of best fit in all subjects, and Pearson’s correlation coefficient is presented as an r-value.
Abbreviations: COPD, chronic obstructive pulmonary disease; DR5, decoy receptor 5; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
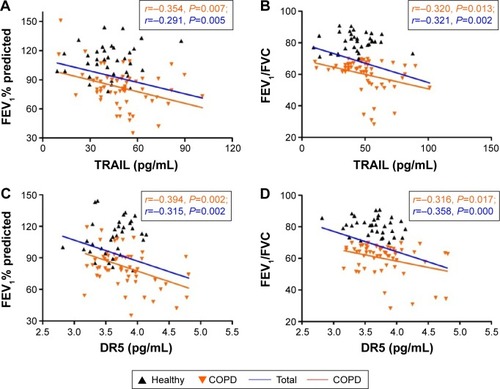
Figure 4 Correlation analyses between TRAIL/DR5 levels and established COPD biomarkers.
Abbreviations: COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DR5, decoy receptor 5; TNF-α, tumor necrosis factor-α; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Discussion
In this study, we explored the serum levels of TRAIL and DR5 in 57 COPD cases and 35 controls using multiplex enzyme-linked immunosorbent assay (Luminex platform). We found that serum TRAIL and DR5 levels were significantly higher in patients with COPD than those in healthy controls. Moreover, in COPD patients, circulating TRAIL and DR5 had relationships with smoking history. Further analyses indicated that both TRAIL and DR5 concentrations correlated negatively with lung function, while TRAIL and DR5 correlated positively with TNF-α and CRP, respectively. To the best of our knowledge, this is the first study to assess the association between TRAIL/DR5 and COPD.
It has been proven that circulating TRAIL and DR5 play important roles in many diseases, such as cancer,Citation29–Citation31 asthma,Citation32 allergic rhinoconjunctivitis,Citation33 scleroderma,Citation34 and chronic hepatitis B.Citation35 Recent studies have also connected TRAIL and its receptors to COPD. It has been reported that alveolar epithelial cell was sensitive to TRAIL-mediated apoptosis due to the presence of cigarette smoke extract and H2O2, while recombinant TRAIL could activate caspase-3 and caspase-8 of emphysematous lung but decrease those apoptosis-related proteins in the nonemphysematous lung,Citation28 which might be a new mechanism for persistent alveolar destruction after smoking cessation. Moreover, TRAIL could induce the proliferation of smooth muscle cells through activating NF-κB and inducing insulin-like growth factor-1 receptor, which may play a role in vascular remodeling in COPD.Citation36,Citation37 Besides, it could also activate proinflammatory signaling pathways, like the MAPK and phosphoinositoide 3-kinase pathways, which are classic signal pathways in COPD.Citation38–Citation40 Similarly, a previous investigation has shown that DR5 levels were higher in the lung of subjects with emphysema than normal smokers and nonsmokers, regardless of smoking cessation, and that oxidative stress increased DR5 expression in A549 cells.Citation27 DR5 expression in alveolar epithelial cells was upregulated in idiopathic pulmonary fibrosis with overexpression of proapoptotic marker p53.Citation24 All of the accumulated evidences suggested that TRAIL and DR5 are also expressed in the cells of lung tissue and had strong relationships with chronic pulmonary inflammatory disease, including COPD.
Therefore, our study took a further step toward exploring the involvements of TRAIL and DR5 in COPD. In this study, we found higher serum TRAIL and DR5 concentrations in COPD patients compared with those in healthy subjects, which was in accordance with previously published results. Meanwhile, in the analysis of stratification, both TRAIL and DR5 levels were higher in smoking COPD than those in nonsmoking COPD patients, healthy smokers, and healthy nonsmokers, indicating that circulating TRAIL/DR5 levels may have relations with smoking history and that different mechanisms may exist between smokers COPD and nonsmokers COPD, although the exact reason remains unclear. The differences between COPD smokers and COPD nonsmokers or healthy nonsmokers might attribute to the differences in either the sex or pack-years. However, there were no differences between smoking COPD group and healthy smokers regarding the sex composition and smoking pack-years. It can be deduced that TRAIL and DR5 may only contribute to the COPD induced by smoking cigarette, as cigarette smoking is responsible for the apoptosis of alveolar structural cells. More interestingly, the apoptosis progress would not stop after smoking cessation, indicating that other mechanisms perpetuate apoptosis in COPD. Whether the TRAIL/TRAIL-receptors play any role in these mechanisms remains an open question and deserves further research.
More importantly, we observed that either TRAIL or DR5 had a negative relationship with pulmonary function (FEV1% predicted) in COPD patients, suggesting that TRAIL and DR5 may be related to the severity of airway obstruction. Last but not least, we found that serum CRP and TNF-α levels in COPD patients were elevated relative to controls, and TRAIL and DR5 correlated positively with TNF-α and CRP, respectively. It is well established that circulating TNF-α and CRP levels were elevated in COPD, thus considered as appropriate biomarkers of systemic inflammation in COPD,Citation41–Citation43 and were associated with the presence of airflow obstruction.Citation44,Citation45 Therefore, our results suggested that TRAIL and DR5 might function as biomarkers of the severity of systemic inflammation in COPD. However, the underlying mechanisms could not be clarified in the present study. It is well known that TRAIL and its receptors are apoptosis-related proteins. As mentioned earlier, several disease mechanisms contribute to the development of COPD such as inflammation, proteinase/antiproteinase imbalance, oxidative stress, etc. There are a series of complicated interactions between apoptosis and all other pathways.Citation7 Our findings might be further evidence of the relationship between apoptosis and inflammation in COPD. Nevertheless, further experiments should be performed in order to clarify how TRAIL and DR5 are induced in COPD and which signaling pathways they involve.
Our research also had some limitations. First, we recruited relatively small number of samples, which might lead to an inexact assessment of serum TRAIL and DR5 concentration. Second, most of our patients had relatively mild COPD, so this result might not be applicable for patients with more severe airway obstruction. Furthermore, we measured the serum levels of TRAIL and DR5 only once and chose the stable COPD patients, so it was unlikely to assess their dynamic levels during the progression of COPD and their expressions in the acute exacerbation of COPD. Finally, only two systemic inflammatory indicators (CRP and TNF-α) were analyzed, thus we could not gain more detailed insights into whether and how TRAIL/DR5 contribute to the disease.
In conclusion, this is the first study to research the correlations of serum TRAIL and DR5 with lung function and systemic inflammation in COPD. The results indicate that circulating TRAIL and DR5 may serve as blood-based markers for COPD severity and systemic inflammation and further confirm the roles of TRAIL and its receptors in COPD. However, more fundamental researches to demonstrate how TRAIL/TRAIL-receptors contribute to the pathogenesis of COPD should be made.
Author contributions
All authors contributed toward data collection, statistical analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81230001, 81300032, 81470236, and 31171103), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China during the “12th Five-Year Plan” (2012BAI05B02), and projects in the Science and Technology Pillar Program from the Department of Science and Technology of Sichuan province (2015SZ0151). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- VijayanVKChronic obstructive pulmonary diseaseIndian J Med Res2013137225126923563369
- WangZLEvolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary diseaseChin Med J (Engl)2010123233467347822166533
- MacNeeWSystemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary diseaseAnn Med201345329130023110517
- AoshibaKYokohoriNNagaiAAlveolar wall apoptosis causes lung destruction and emphysematous changesAm J Respir Cell Mol Biol200328555556212707011
- MoudedMEgeaEEBrownMJEpithelial cell apoptosis causes acute lung injury masquerading as emphysemaAm J Respir Cell Mol Biol200941440741419188661
- DemedtsIKDemoorTBrackeKRJoosGFBrusselleGGRole of apoptosis in the pathogenesis of COPD and pulmonary emphysemaRespir Res2006715316571143
- ParkJWRyterSWChoiAMFunctional significance of apoptosis in chronic obstructive pulmonary diseaseCOPD20074434735318027162
- RobertsonNMRosemillerMLindemeyerRGSteplewskiAZangrilliJGLitwackGTRAIL in the airwaysVitam Horm2004672014916715110176
- KayagakiNYamaguchiNNakayamaMExpression and function of TNF-related apoptosis-inducing ligand on murine activated NK cellsJ Immunol199916341906191310438925
- WeckmannMCollisonASimpsonJLCritical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway diseaseNat Med200713111308131517934471
- AlmasanAAshkenaziAApo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapyCytokine Growth Factor Rev2003143–433734812787570
- DaigleISimonHUAlternative functions for TRAIL receptors in eosinophils and neutrophilsSwiss Med Wkly200113117–1823123711420819
- SchneiderPThomeMBurnsKTRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaBImmunity1997768318369430228
- KischkelFCLawrenceDAChuntharapaiASchowPKimKJAshkenaziAApo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5Immunity200012661162010894161
- TrunehASharmaSSilvermanCTemperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptorJ Biol Chem200027530233192332510770955
- JohnstoneRWFrewAJSmythMJThe TRAIL apoptotic pathway in cancer onset, progression and therapyNat Rev Cancer200881078279818813321
- BilskiAPasz-WalczakGKubiakRTRAIL protein expression in breast cancer cells correlates with nuclear gradeArch Med Sci20106454555122371798
- YildizYYaylim-EraltanIArikanSErgenHAKucucukSIsbirTIs there any correlation between TNF-related apoptosis-inducing ligand (TRAIL) genetic variants and breast cancer?Arch Med Sci20106693293622427769
- McGrathEEMarriottHMLawrieATNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammationJ Leukoc Biol201190585586521562052
- BemRABosAPWosten-van AsperenRMPotential role of soluble TRAIL in epithelial injury in children with severe RSV infectionAm J Respir Cell Mol Biol201042669770519635930
- CollisonAFosterPSMattesJEmerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responsesClin Exp Pharmacol Physiol200936111049105319656161
- TisatoVGarrovoCBiffiSIntranasal administration of recombinant TRAIL down-regulates CXCL-1/KC in an ovalbumin-induced airway inflammation murine modelPLoS One2014912e11538725506835
- AkramKMLomasNJForsythNRSpiteriMAAlveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53Int J Clin Exp Pathol20147255256424551275
- HameedAGArnoldNDChamberlainJInhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertensionJ Exp Med2012209111919193523071256
- MorissetteMCParentJMilotJAlveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to knowInt J COPD200941931
- MorissetteMCVachon-BeaudoinGParentJChakirJMilotJIncreased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysemaAm J Respir Crit Care Med2008178324024718511705
- MorissetteMCParentJMilotJThe emphysematous lung is abnormally sensitive to TRAIL-mediated apoptosisRespir Res20111210521824395
- GantenTMSykoraJKoschnyRPrognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancerJ Mol Med (Berl)20098710995100719680616
- ToiyamaDTakahaNShinnohMSignificance of serum tumor necrosis factor-related apoptosis-inducing ligand as a prognostic biomarker for renal cell carcinomaMol Clin Oncol201311697424649125
- BisginAKargiAYalcinADIncreased serum sTRAIL levels were correlated with survival in bevacizumab-treated metastatic colon cancerBMC Cancer2012125822313795
- YalcinADBisginAKargiAGorczynskiRMAkdisCASerum-soluble TRAIL levels in patients with severe persistent allergic asthma: its relation to omalizumab treatmentMed Sci Monit20121831115
- YalcinADBisginASoluble trail as a marker of efficacy of allergen-specific immunotherapy in patients with allergic rhinoconjunctivitisMed Sci Monit20121810617621
- AzabNARadyHMMarzoukSAElevated serum TRAIL levels in scleroderma patients and its possible association with pulmonary involvementClin Rheumatol20123191359136422729471
- DuJWangLHanJGaoLMaCLiangXSerum soluble death receptor 5 concentration in patients with chronic hepatitis B is associated with liver damage and viral antigen levelClin Biochem20124510–1184584722537456
- KavurmaMMSchoppetMBobryshevYVKhachigianLMBennettMRTRAIL stimulates proliferation of vascular smooth muscle cells via activation of NF-kappaB and induction of insulin-like growth factor-1 receptorJ Biol Chem2008283127754776218178561
- SecchieroPZerbinatiCRimondiETRAIL promotes the survival, migration and proliferation of vascular smooth muscle cellsCell Mol Life Sci200461151965197415289937
- StaggHWBowenKASawantDARodriguezMTharakanBChildsEWTumor necrosis factor-related apoptosis-inducing ligand promotes microvascular endothelial cell hyperpermeability through phosphatidylinositol 3-kinase pathwayAm J Surg2013205441942523375756
- Degli-EspostiMTo die or not to die – the quest of the TRAIL receptorsJ Leukoc Biol199965553554210331480
- LiuJQuXXuLPhosphoinositide 3-kinase/Akt and nuclear factor kappaB pathways are involved in tumor necrosis factor-related apoptosis-inducing ligand resistance in human gastric cancer cellsMol Med Rep20103349149621472268
- HigashimotoYIwataTOkadaMSatohHFukudaKTohdaYSerum biomarkers as predictors of lung function decline in chronic obstructive pulmonary diseaseRespir Med200910381231123819249197
- TanniSEPelegrinoNRAngeleliAYCorreaCGodoyISmoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patientsJ Inflamm (Lond)201072920534161
- KaradagFKirdarSKarulABCeylanEThe value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary diseaseEur J Intern Med200819210410818249305
- ChiangCHChuangCHLiuSLTransforming growth factor-beta1 and tumor necrosis factor-alpha are associated with clinical severity and airflow limitation of COPD in an additive mannerLung201419219510224153451
- Pinto-PlataVMMullerovaHTosoJFC-reactive protein in patients with COPD, control smokers and non-smokersThorax2006611232816143583
