Abstract
Purpose
The aim of this study was to formulate stable film-coated montelukast sodium (MS) tablets using Opadry® yellow 20A82938 (Montikast® tablets) and to evaluate their in vitro and in vivo release profile.
Methods
MS core tablets were manufactured using a direct compression method. Opadry yellow 20A82938 aqueous coating dispersion was used as the film-coating material. Dissolution of the film-coated tablets was tested in 900 mL of 0.5% sodium lauryl sulfate solution and the bioequivalence of the tablets was tested by comparing them with a reference formulation – Singulair® tablets. In vitro–in vivo correlation was evaluated. The stability of the obtained film-coated tablets was evaluated according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines.
Results
The efficiency of the film coating was determined by subjecting the coated tablets to gastric pH and drug release was analyzed using high-performance liquid chromatography. The coated tablets had no obvious defects. MS release met the study criterion of not less than 80% dissolved after 30 minutes in 0.5% sodium lauryl sulfate solution. Statistical comparison of the main pharmacokinetic parameters clearly indicated no significant difference between test and reference in any of the calculated pharmacokinetic parameters. Level A correlation between in vitro drug release and in vivo absorption was found to be satisfactory.
Conclusion
These findings suggest that aqueous film coating with Opadry yellow 20A82938 is an easy, reproducible, and economical approach for preparing stable MS film-coated tablets without affecting the drug-release characteristics.
Introduction
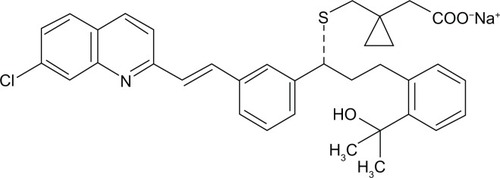
Montelukast sodium (MS) (R,E)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl) phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propylthio)methyl)cyclopropyl)acetic acid, monosodium salt () is a potent selective cysteinyl leukotriene receptor (cys-LT1) antagonist that is used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies.Citation1
It is administered orally and has been shown to block LTD4-induced bronchoconstriction in patients with mild asthma,Citation2 prevent exercise-induced asthma, and to improve baseline pulmonary function in patients with chronic asthma.Citation3–Citation5 The oral bioavailability of MS administered as a 10 mg film-coated tablet in adults is around 58%–66%.Citation6 Presence of food in the gastrointestinal tract does not affect bioavailability when the tablet is administered with a standard meal in the morning. Biliary excretion has been found to be the predominant pathway for elimination of MS and its metabolites.Citation7 MS in solution is unstable when exposed to light and the major photoproduct that results is its cis-isomer. A 20% reduction in potency has been shown after exposure of its solid state to daylight for a week. The unpacked chewable tablets showed a 10% reduction of the formulation upon exposure to daylight for 2 weeks resulting in montelukast S-oxide as a major photo-oxidation product. Film-coated tablets either unpacked or in their immediate pack showed excellent stability under the same conditions.Citation8 Thus, tablets containing MS should be film coated to protect the drug from light. Film-coated tablets should maintain the immediate release dissolution profile when stored under standard International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) stability conditions.Citation9
Opadry® yellow 20A82938 (Colorcon, West Point, PA, USA and Dartford, UK), an immediate release, pigmented, film-coating system, has been identified to protect MS from the effect of light for an extended period without interfering with the immediate release profile of MS.Citation10 It is a fully formulated, aqueous film-coating system comprised of polymer (based on hydroxypropylmethyl cellulose, a plasticizer, and pigment), which meets compendial requirements of the United States Pharmacopeia/European Pharmacopoeia/Japanese Pharmacopoeia (USP/EP/JPE) for pharmaceutical applications.Citation11 It possesses the excellent strength and modulus of elasticity typical of other commercial Opadry film-coating systems. It is a ready-to-use formulation, is simple to prepare, requires only a short time for preparation and application, and uses standard coating equipment that is easily cleaned. It provides consistent, reproducible immediate release profiles that ensure excellent product performance.
The aim of this study was to formulate stable film-coated MS tablets (Montikast®; Pharmacare, Ramallah, Palestine) using Opadry yellow 20A82938 and evaluate their in vitro and in vivo release profile.
Materials and methods
Materials
Acetonitrile (ACN) was purchased from (BDH, UK), methanol was obtained from Merck KGaA (Darmstadt, Germany), ammonium dihydrogen orthophosphate was purchased from Riedel-de Haën (Seelze, Germany), and double-distilled high-purity water was used. Human plasma was harvested from donors. The high-performance liquid chromatography (HPLC)-grade solvents ACN and methanol were used as received. All other reagents were of analytical grade. MS (batch number QC/MRV/WS001/06, manufacturing date August 2006) and mefenamic acid (batch number CAS 1938, internal standard) were supplied by Pharmacare. Opadry yellow 20A82938 was bought from Colorcon. The produced test tablets, Montikast film-coated tablets (10 mg MS/tablet), had the batch number RD-10H07 and expiry date of August 2009. Singulair® tablets (10 mg MS/tablet) were obtained from Merck Sharp and Dohme (Haarlem, Netherlands; batch number NE 27350; expiry date July 2009) to use as the reference formulation.
Chromatographic analysis
A validated HPLC assay method for the determination of MS in the study samples was performed. A summary of the chromatographic conditions is presented in .
Table 1 Summary of chromatographic conditions
Preparation of calibration standards
A volume of 25 μL was taken from each working solution and from the internal standard solution and transferred into a set of test tubes. After evaporation of the methanolic solution, 0.25 mL of blank plasma was added to each tube to form a set of calibration standards with concentrations of 2, 1, 0.5, 0.2, 0.1, 0.05, 0.025 μg/mL. The retention times of MS and the internal standard (mefenamic acid) were 5.0 ± 0.5 and 2.9 ± 0.5 minutes, respectively.
Preparation of samples for HPLC determination
From each study sample, 25 μL was transferred into a clean test tube. The study samples were dealt with in the same manner as the calibration standards after addition of mefenamic acid.
Validation procedures
To assess linearity, drug-free plasma was spiked with known amounts of the drug to achieve serial concentrations in the range of 0.025–2.0 μg/mL. The performance of the assay during analysis of the study samples was evaluated by analysis of the quality control (QC) samples. The QC samples were prepared to have three MS concentrations (0.05, 0.25, and 1 μg/mL).
Formulations and evaluation of tablets
Formulations and evaluation of core tablets
Several trials were undertaken to develop suitable MS core tablets with the desired hardness, friability, weight, content uniformity, and appearance (). All ingredients were accurately weighed and sieved through a 24-mesh sieve. The core tablets contained lactose anhydrous and mannitol as fillers and magnesium stearate as a lubricant. Avicel® PH 102 (FMC Corporation, Philadelphia, PA, USA) was used as a filler and as a disintegrant along with Ac-Di-Sol® (FMC Corporation). After mixing the first three materials, the final mixture was lubricated with magnesium stearate (Magnesia GmbH, Lüneburg, Germany), and colloidal silicon dioxide (Aerosil®; Evonik Industries AG, Hanau-Wolfgang, Germany) was added as a glidant. The obtained flowable granules were directly compressed using a Manesty compression machine (type D3B; Bosch Packaging Technology Ltd, Knowsley, UK). All manufacturing steps were carried out in a dark room to minimize exposing the MS to light. These core tablets were assessed for physical appearance, thickness, hardness, friability, weight variation, identification, disintegration, and dissolution, and assayed.Citation12 Weight uniformity was assessed using an electronic balance (Precisa 205 ASCS, Precisa Gravimetrics, Dietikon, Switzerland). Thickness was evaluated using a vernier caliper. Hardness was determined using the PTB311E “3-in-1” Tester (Pharma Test, Hainburg, Germany), while friability was evaluated using a TA-100 Friabilator (ERWEKA, Heusenstamm, Germany). Assay and identification were carried out according to the reported HPLC validated method using a Merck Hitachi apparatus (pump L7100, diode array detector, L7450, auto sampler L7200, interface D7000, column oven L7300; Tokyo, Japan). Disintegration testing was carried out using an ERWEKA ZT 221 disintegration tester. Dissolution was also carried out according to US Food and Drug Administration (FDA) guidelines using an ERWEKA Dissolution Tester DT 70 apparatus in 900 mL 0.5% sodium lauryl sulfate (SLS) solution maintained at 37°C with the paddle apparatus operated at 50 rpm for 30 minutes.Citation13 After judging that the tablets were of satisfactory quality according to the above tests, the decision was made to coat the core MS tablets.
Table 2 Composition of formulations F1 to F6 (in grams)
Film coating of MS (10 mg/tablet)
Preparation of Opadry yellow 20A82938 dispersion for film coating
Opadry yellow 20A82938 (4.2 kg) was mixed with 30.0 kg of purified water inside a mixing tank for approximately 45 minutes. Following this, the aqueous dispersion was passed through a 250-micron sieve prior to the coating process. The dispersion was continuously stirred during the coating process.
Coating methodology
Tablet coating was performed in an Accela-Cota® coating pan of 150 kg capacity (Thomas Engineering, Hoffman Estates, IL, USA) using one spray gun. The coating pan had been previously cleaned using water and alcohol 95%. The core tablets (120 kg) were loaded into the coating pan. The tablet cores were pre-heated to about 40°C using a dryer and high-pressure air spray gun. Warm air was introduced into the coating pan (temperature 45°C–55°C) during the entire coating process. The spray gun was filled with the Opadry yellow aqueous dispersion and operated with a flow rate according to manufacturer’s instructions. The pan was set into motion and the seal-coating dispersion was sprayed onto the tumbling cores under a suitable air pressure (1.7 bar). The air heater was switched off and tablets air-dried for 20–25 minutes in the coating pan. Following the coating process (conditions shown in ), the weight of the core tablets was found to have increased by about 3%. The obtained coated tablets were packaged in double-aluminum blisters using an Uhlmann 200 packing machine (Laupheim, Germany). The produced tablets were coded “Montikast” (10 mg MS/tablet).
Table 3 Coating conditions
Characterization of MS film-coated tablets
The properties of the film-coated tablets, including physical appearance, thickness, weight uniformity, hardness, disintegration, dissolution, related substances, were determined and assayed. Weight uniformity of coated tablets was evaluated according to the United States Pharmacopeia, 30th edition (USP 30),Citation12 method and the average weight obtained was 186% ± 7.5% mg, which means that the average weight gain of 3% resulted from the film coat. The diameter and thickness of ten tablets were determined using a vernier caliper. Hardness of the coated tablets was also examined according to USP 30 procedures.Citation12 The dissolution of coated tablets was determined according to the recommended dissolution method suggested by the FDA.Citation13 Assay to determine the content of the MS tablets was performed according to the validated assay method to evaluate content uniformity.Citation12 A random selection of 20 tablets from each of three study batches was tested at different time intervals during the study.
Stability of film-coated tablets
Samples of MS-coated tablets were blister packed in aluminum. These samples were then subjected to stability studies according to ICH guidelines.Citation9 The samples were designated as time 0, 3, 6, 9, and 12 months. Samples designed for room temperature (RT) storage were kept at 25°C ±2 °C and 60% ± 5% relative humidity. Additional samples were subjected to accelerated stability study and stored at 40°C ±2 °C and 75% ± 5% relative humidity in a humidity chamber (type 3734009900310, #980233; BINDER, Tuttlingen, Germany). The samples were tested at initially, then at 3- and 6-month intervals. Samples were evaluated for their physical appearance, disintegration, dissolution, hardness, friability, and related substances, such as any product of degradation, and assayed using the previously described procedures to evaluate the stability of the coated tablets.
In vitro release of MS from film-coated tablets
The in vitro release of MS from Montikast 10 mg film-coated tablets and Singulair 10 mg coated tablets was studied. The tablets were placed in the vessels of the dissolution apparatus containing 900 mL of 0.5% w/v SLS in water, using paddles at a speed of 50 rpm.Citation13 The temperature was maintained at 37°C during the 30 minutes of dissolution. Samples (5 mL) were taken at 1, 2, 3, 4, 5, 10, 15, 20, and 30 minutes. Fresh dissolution medium (5 mL) was added to the vessels after each sample was taken. A standard was prepared of 0.011 mg/mL in 0.5% SLS solution. The samples (study and standard) were filtered through a 0.45 μm Millipore filter. A sample of 0.25 μL was injected into the HPLC apparatus.
In vivo study of MS film-coated tablets
Volunteers and clinical protocol
The study protocol and informed consent forms were approved by the Ethical Committee of Tanta University Hospital (Tanta, Egypt). The whole study, which met the requirements of the Declaration of Helsinki, was conducted in accordance with the current ICH Guideline for Good Clinical Practice and Guideline for Good Laboratory Practice. Twenty-four young adult male volunteers, nonsmokers, aged 18–30 years old and weighing 62–80 kg, participated in the present study. The volunteers were not taking any concomitant medications and were free from significant diseases, as determined 4 weeks prior to the beginning of the study by way of medical histories and physical examinations. The health condition of each volunteer was determined through physical examination, laboratory tests, and medical history. The physician reviewed all preclinical laboratory tests for each volunteer to determine if the volunteers were fit to participate in the study.
All volunteers were given a written informed consent form to sign, which explained the nature of the study. All subjects were informed to abstain from taking drugs and alcohol for at least 3 days prior to the study and throughout the study period. On the night before starting the study, the volunteers were asked to fast for at least 10 hours before drug administration.
The study had an open-label randomized two-period crossover design with a 1-week washout period between doses. Subjects were arbitrarily divided into two equal groups of 12 volunteers. A blank blood sample was withdrawn from each volunteer on the morning of the experiments. Each of the 24 volunteers were then given either two Montikast 10 mg tablets or two Singulair 10 mg tablets followed by 200 mL of water. Blood samples for drug-plasma quantitation were collected. Samples were obtained at 0.0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 hours after drug administration and placed in heparinized tubes. The separation of plasma from each sample was carried out by centrifugation at 3000 rpm for 10 minutes, after which they were stored at −20°C until assayed.
Four hours after drug administration, the subjects were allowed to eat a standard breakfast of bread, jam, potatoes, low-fat white cheese, and water (150 mL). They were then allowed controlled access to water and other nonalcoholic beverages. The volunteers had their second meal (a standard lunch of grilled chicken, rice, and vegetables) 4 hours later. After a washout period of 1 week, subjects received the alternate formulation.
Pharmacokinetics and statistical analysis
The statistical analysis was performed using Minitab Statistical Package (v 13; Minitab Inc, State College, PA, USA) to detect any significant differences (P < 0.05) between both products.
The maximum MS plasma concentration (Cmax) and the corresponding time to peak plasma concentration (Tmax) were taken directly from the slope of the semi-logarithmic plot of the terminal phase of the plasma concentration–time curve calculated by linear regression. The elimination half-life (T1/2) was derived by dividing natural logarithm of 2 (ln2) by the elimination rate constant (Ke). The areas under the MS plasma concentration–time curves from 0 to 24 hours (AUC0–24) and 0 hours to infinity (AUC0–∞) were calculated using the linear trapezoidal method. Extrapolation to infinity was done by adding the value Ct/Ke to the calculated AUC0–24 (where “Ct” is the last detectable concentration of MS). For the purpose of bioequivalence analysis, one way analysis of variance was used to assess the effect of formulations, periods, sequences, and subjects on AUC0–24, AUC0–∞, and Cmax.
In vitro–in vivo correlation (IVIVC)
Level A IVIVC was elaborated at different time intervals to assess whether a correlation existed between the in vitro drug release and the in vivo absorbed drug using the Wagner–Nelson equation.Citation14
Results
The analytical method used was demonstrated to be a validated analytical method for the analysis and quantitation of MS. In fact, the correlation coefficient was always greater than 0.99 during the course of the validation. The within-day coefficient of variation ranged from 0.099% to 13.59%, while the between-day coefficient of variation ranged from 1.498% to 12.50%, indicating that the method is precise. The accuracy of the method was proven since the between-day accuracy was in the range of 1.498% to 12.50% and the within-day accuracy was in the range of 0.099%–13.59% during the entire range of the calibration curves.
The stability of the drug in plasma at RT was determined by preparing three different plasma samples for each drug concentration and then injecting the samples immediately into the HPLC system. The samples were kept at RT and were injected again after 24 hours. The difference in the drug concentrations between the two analyses was always less than 10%, indicating the stability of MS in the plasma samples at RT. The stability of the drug in frozen plasma was also investigated. There was no trend in the change in the drug concentration in frozen plasma stored at −20°C during the study period, indicating the stability of MS in frozen plasma. The limit of quantification of MS in this assay was 0.025 μg/mL, which gave a coefficient of variance of less than 15%. The retention times of the drug in the standard and the study samples were identical. There were no peaks for endogenous compounds that appeared at the same retention time of the drug or the internal standard. Three QC samples were incorporated with each analysis run and were treated as unknown samples. The analysis run was accepted, since the three QC samples were within 20% of nominal concentration.Citation15
With respect to the manufacturing of core tablets, the excipients utilized in the formulation of the core tablets were selected in order to obtain the optimum formulation. Lactose anhydrous was chosen as a filler so as to obtain a formula more stable to moisture. Mannitol, also used as a filler, is water-soluble and has a low hygroscopicity and gives a pleasant mouth-feel and is sweet to the taste. Avicel PH 102 was used as a filler and disintegrant, in addition to Ac-Di-Sol.
The obtained tablets had good mechanical strength and an average hardness of 9 kP. Their friability was less than 1%. Physical appearance, weight variation, and drug content were found to be satisfactory under pharmacopeial standards of tablet evaluation (USP 30).Citation12 The tablets were then coated, without creating any visual defects such as roughness, orange peel appearance, chipping, cracking, or other undesired effects. Although traditional aqueous coating formulations may require many component mixing steps before the coating process is begun, this new formulation was dispersed in the minimum amount of time and produced acceptable weight gains. The coated tablets were found to be robust and had no obvious defects or signs of peeling or chipping and all their specifications were within the acceptable limits. The average weight of the final core tablets was about 180 mg. The immediate release characteristics of the obtained film coat were determined by subjecting the coated tablets to gastric pH, and drug release was analyzed using the HPLC system. The tablets also showed sufficient MS release. In fact, the release of MS met the criterion outlined in this study of not less than 80% dissolved after 30 minutes in 0.5% SLS solution. Results of the long-term and accelerated stability studies were satisfactory, showing no significant variation in terms of physical characteristics, color, assay, dissolution, friability, hardness, or disintegration time of the coated tablet, as reported in and .
Table 4 Long-term stability study of film-coated Montikast ® 10 mg/tablet (Pharmacare PLC, Ramallah, Palestine)
Table 5 Accelerated stability study of film-coated Montikast ® 10 mg/tablet (Pharmacare PLC, Ramallah, Palestine)
In vitro drug-release studies were carried out since these are considered the best tool for assessing in vivo drug behavior. The in vitro release of MS from the Montikast tablets was within the acceptable limits. The release of MS from both Singulair and Montikast tablets was more than 85% after 15 minutes, as reported in , meaning that there was no need to use the similarity factor (f1) and difference factor (f2) to compare their profiles. The obtained coated tablets were stable, indicating that MS is highly compatible with the coating material. All data indicated that the film coat was essentially unchanged and provided the desirable protection. Three batches of coated MS were prepared, and the previously mentioned parameters were tested under the same conditions. The results obtained from the three batches showed no significant differences between the batch samples, indicating that this manufacturing process is reliable and reproducible.
Figure 2 In vitro dissolution profile of Montikast® (Pharmacare PLC, Ramallah, Palestine) and Singulair® (Merck Sharp and Dohme, Haarlem, Netherlands) (10 mg montelukast sodium [MS]/tablet).
![Figure 2 In vitro dissolution profile of Montikast® (Pharmacare PLC, Ramallah, Palestine) and Singulair® (Merck Sharp and Dohme, Haarlem, Netherlands) (10 mg montelukast sodium [MS]/tablet).](/cms/asset/c9e400eb-1985-40a2-8190-93aaf4e19991/dddt_a_37369_f0002_c.jpg)
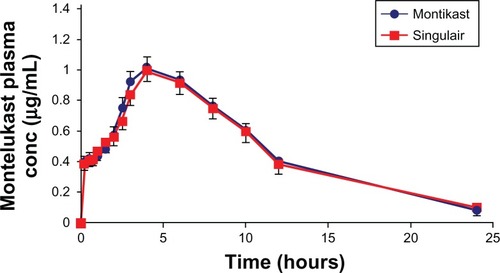
As for the in vivo study results, the Cmax following the administration of two Montikast tablets ranged from 0.443 to 1.652 μg/mL, with a mean value of 1.104 ± 0.332 μg/mL whereas the Cmax following the administration of two Singulair tablets ranged from 0.365 to 1.693 μg/mL, with a mean value of 1.114 ± 0.368 μg/mL. The mean Tmax after administration of the two Montikast tablets was 4.250 ± 1.359 hours, whereas the mean time to reach the mean Tmax after administration of the two Singulair tablets was 4.770 ± 1.601 hours. The AUC0–∞ after administration of the two Montikast tablets ranged from 2.741 to 18.99 μg · hr/mL (mean 12.21 ± 4.270 μg · hr/mL) and after administration of the two Singulair tablets ranged from 3.131 to 18.40 μg · hr/mL (mean 12.17 ±4.169 μg · hr/mL).
The relative bioavailability of the MS from the two Montikast tablets compared with the two Singulair tablets was found to be 100.32%, as determined from the ratio of the AUC0–∞ of both products ( and ). The 90% confidence limit of the difference between the Log transformed mean values of Cmax and AUC0–∞ of MS fell between 96.9% and 106.5% of the reference mean for the two Montikast tablets and between 96.4% and 106.2% of the reference mean for two Singulair tablets. These confidence limits fall within the FDA-specified bioequivalent limit (80%–125%), indicating that the two products are bioequivalent, as summarized in . High level A IVIVC was found between the amount of absorbed drug and the amount released at the used time intervals. The correlation coefficient between the dissolved and absorbed drug was 0.988 and the P value was 0.002.
Table 6 Pharmacokinetic parameters calculated for montelukast sodium after a single oral dose administration of two tablets (each containing 10 mg montelukast sodium) to 24 healthy male volunteers
Figure 3 Mean plasma concentration of montelukast sodium (MS) (±standard deviation) versus time for 24 volunteers after a single oral dose administration of two Montikast® (10 mg MS/tablet; Pharmacare PLC, Ramallah, Palestine) or two Singulair® (10 mg MS/tablet; Merck Sharp and Dohme, Haarlem, Netherlands) tablets.
Discussion
Aqueous-based film-coating materials for oral solid dosage forms, which contain mainly water-soluble polymers and other additives, aim to improve the quality and performance of the film coat. The most commonly used water-soluble film-coating polymers are cellulose derivatives such as hypromellose and hyprolose (HPC).Citation16 These products are present in most of Opadry film-coating formulations.Citation10,Citation11 The commercially produced Singulair tablet coating contains hypromellose and HPC as film-coating polymers. The Opadry used in our formulation does not contain HPC, which may result in the final product costing less.Citation17 Further, the commercially produced Singulair tablets also have a final top coat of carnauba wax. As the tablets produced in this study waived this last step and the sub-coating step, they were quicker and less expensive to produce than the commercially produced branded tablets, yet the MS formulation was just as stable and therapeutically effective. To summarize, the results of this study show that the coated tablets produced were highly stable without a sub-coating layer, meaning significant savings in terms of both time and material cost and the coating obtained maintained its properties.
Tablet coating was carried out using an Accela-Cota coating pan. During coating, certain parameters, such as temperature of the coating pan and the spray rate of the coating dispersion, were maintained carefully in order to obtain the desired smoothness and uniformity of the film coat. Some process parameters, including the two just mentioned, have been investigated by other researchers.Citation18–Citation20 According to these studies, the temperature of the coating pan had no effect on the smoothness of the obtained coat when a low spray rate was used. Conversely, smoother films were obtained at higher spray rates and higher temperatures. In another study, pan speed and coating duration were identified as variables that significantly affect content uniformity.Citation21 It was shown that high inlet-air temperature and low spray rate would result in the coated tablets potentially having lower drug-release rates. Further, increasing the temperature for certain times to remove water and solvent affects the properties of the final product.Citation22
The obtained Montikast tablets were considered high quality, since, when compared with the Singulair tablets, the in vitro dissolution profile and in vivo drug absorption showed that both formulations were well tolerated by all subjects at the administered dose. Unexpected incidents that could have influenced the outcome of the study did not occur and all volunteers were discharged from the hospital in good health. Statistical comparison of the main pharmacokinetic parameters – AUC0–24, AUC0–∞, Cmax, and Tmax – clearly indicates no significant difference between the test and reference 10 mg tablets. The high level A of IVIVC indicates that the dissolution method of MS tablets can be used as an important tool to save time and money in any future production of MS tablet batches. The obtained values were in good agreement with the FDA requirements for bioequivalence of generic drugs.Citation23,Citation24 Since the AUC0–∞ and Cmax mean ratios were within the 80%–125% interval, it was concluded that the tested Montikast tablets were bioequivalent in terms of both extent and rate of absorption to the Singulair tablets.
Conclusion
The validated HPLC method employed here proved to be simple, fast, reliable, selective, and sensitive enough to be used in clinical pharmacokinetic studies of MS use in humans. Aqueous film coating was successfully conducted on an industrial scale and the produced tablets were acceptable in terms of appearance and drug availability. Statistical analysis of the results, performed on AUC0–48, AUC0–∞, and Cmax using the analysis of variance method showed that both the test tablets, Montikast, and the reference tablets, Singulair, are bioequivalent, since they deliver equivalent amounts of MS to the systemic circulation at equivalent rates for both AUC0–24 and Cmax ratios within the 80%–125% interval proposed by the FDA. These results demonstrate that this new generic tablet is well formulated, an essential condition not only for achieving good therapeutic benefits but also to avoid any potential problems that may arise due to poor formulation.
Acknowledgemnts
We would like to extend our special thanks to the Pharmaceutical Services Center, University of Tanta, College of Pharmacy, Egypt, who made this work possible through their extensive efforts.
Disclosure
The authors report no conflicts of interest in this work.
References
- Labelle M Belley M Gareau Y Discovery of MK-0476, a potent and orally active leukotriene D4 receptor antagonist devoid of peroxisomal enzyme induction Bioorg Med Chem Lett 1995 5 3 283 288
- De Lepeleire I Reiss TF Rochette F Montelukast causes prolonged, potent leukotriene D4-receptor antagonism in the airways of patients with asthma Clin Pharmacol Ther 1997 61 1 83 92 9024176
- Blake KV Montelukast: data from clinical trials in the management of asthma Ann Pharmacother 1999 33 12 1299 1314 10630831
- Sorkness CA Reiss TF Zhang J Bronchodilation with a selective and potent leukotriene D4 (LTD4) antagonist (MK-0476) in patients with asthma Am J Respir Crit Care Med 1994 149 A216
- Reiss TF Altman LC Chervinsky P Effects of montelukast (MK-0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma J Allergy Clin Immunol 1996 98 3 528 534 8828530
- Cheng H Leff JA Amin R Pharmacokinetics, bioavailability, and safety of montelukast sodium (MK-0476) in healthy males and females Pharm Res 1996 13 3 445 448 8692739
- Balani SK Xu X Pratha V Metabolic profiles of montelukast sodium (Singulair), a potent cysteinyl leukotriene1 receptor antagonist, in human plasma and bile Drug Metab Dispos 1997 25 11 1282 1287 9351905
- Al Omari MM Zoubi RM Hasan EI Khader TZ Badwan AA Effect of light and heat on the stability of montelukast in solution and in its solid state J Pharm Biomed Anal 2007 45 3 465 471 17719198
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Stability Testing of New Drug Substances and Products: Q1A(R2) ICH Harmonised Tripartite Guideline. Current Step 4 version. Geneva ICH 2003 Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2_Guideline.pdf. Accessed December 17, 2012.
- Colorcon. Opadry® fx™ special effects film coating system [web page on the Internet]. West Point, PA and Dartford: Coloron; nd. Available from: http://www.colorcon.com/products/coatings/immediate-release/opadry-fx. Accessed December 17, 2012.
- Gulian S Friend B Taste Masking Performance and Stability of Opadry®tm, a Taste Mask Film Coating System West Point, PA and Dartford Colorcon 2004 Available from: http://www.colorcon.com/literature/marketing/fc/Opadry%20tm/poster_tastemask_opadrytm.pdf. Accessed February 19, 2012.
- US Pharmacopeial Convention United States Pharmacopeia 30th edition Rockville, MD US Pharmacopeial Convention 2007 1923 1927
- US Food and Drug Administration (FDA) Dissolution methods [webpage on the Internet] Silver Spring, MD FDA nd. Available from: http://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults_Dissolutions.cfm?PrintAll=1. Accessed December 17, 2012.
- Shargel L Wu-Pong S Yu AB Applied Biopharmaceutics and Pharmacokinetics 5th ed New York, NY McGraw-Hill 2005 123 131
- Shah VP Medha KK Dighe SV Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report Eur J Drug Metab Pharmacokinet 1991 16 4 249 255 1823867
- Wheatley TA Water soluble cellulose acetate: a versatile polymer for film coating Drug Dev Ind Pharm 2007 33 3 281 290 17454061
- Merck Sharp and Dohme Ltd Singulair 10 mg tablets [summary of product characteristics]. eMC; updated February 7, 2012. Available from: http://www.medicines.org.uk/emc/medicine/17718/SPC. Accessed December 17, 2012.
- Sauer D Zheng W Coots LB McGinity JW Influence of processing parameters and formulation factors on the drug release from tablets powder-coated with Eudragit L 100-55 Eur J Pharm Biopharm 2007 67 2 464 475 17451929
- Parikh NH Porter SC Rohera BD Aqueous ethylcellulose dispersion of ethylcellulose. I. Evaluation of coating process variables Pharm Res 1993 10 4 525 534 8483834
- Krogars K Heinämäki J Vesalahti J Marvola M Antikainen O Yliruusi J Extrusion-spheronization of pH-sensitive polymeric matrix pellets for possible colonic drug delivery Int J Pharm 2000 199 2 187 194 10802412
- Rege PR Garmise RJ Block LH Spray-dried chitinosans. Part II: in vitro drug release from tablets made from spray-dried chitinosans Int J Pharm 2003 252 1–2 53 59 12550780
- Frisbee SE Mehta KA Mcginity JW Processing factors that influence the in vitro and in vivo performance of film-coated drug delivery systems Drug Deliv. 2002 2 72 76
- FDA Guidance for Industry: Statistical Approaches to Establishing Bioequivalence Silver Spring, MD FDA 2001 Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070244.pdf. Accessed December 17, 2012.
- European Medicines Agency (EMEA) Committee for Proprietary Medicinal Products Note for Guidance on the Investigation of Bioavailability and Bioequivalence CPMP/EWP/QWP/1401/98. London EMEA 2001 Available from: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500003519. Accessed December 17, 2012.