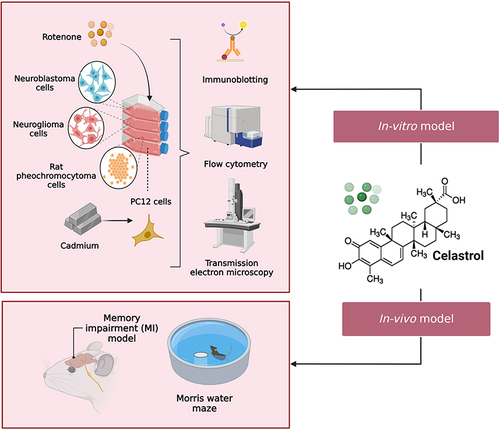
Abstract
Celastrol is a naturally occurring chemical isolated from Tripterygium wilfordii Hook. f., root extracts widely known for their neuroprotective properties. In this review, we focus on the efficacy of celastrol in mitigating memory impairment (MI) in both in vivo and in vitro models. Scopus, PubMed and Web of Science databases were utilised to locate pertinent literatures that explore the effects of celastrol in the brain, including its pharmacokinetics, bioavailability, behavioral effects and some of the putative mechanisms of action on memory in many MI models. To date, preclinical studies strongly suggest that celastrol is highly effective in enhancing the cognitive performance of MI animal models, particularly in the memory domain, including spatial, recognition, retention and reference memories, via reduction in oxidative stress and attenuation of neuro-inflammation, among others. This review also emphasised the challenges and potential associated enhancement of medication delivery for MI treatment. Additionally, the potential structural alterations and derivatives of celastrol in enhancing its physicochemical and drug-likeness qualities are examined. The current review demonstrated that celastrol can improve cognitive performance and mitigate MI in several preclinical investigations, highlighting its potential as a natural lead molecule for the design and development of a novel neuroprotective medication.
Introduction
Memory is the process of acquiring, storing and retrieving information. It encompasses all knowledge acquired over one’s life, including 1) truths that have been learned 2) events that have been recalled and 3) skills that have been developed over time. Declarative and non-declarative memories are the two basic categories of memory; the former includes daily recollections, while the latter mostly includes memories retrieved reflexively. Memory problems, also referred to as memory impairment (MI), are important diagnostic markers for a number of syndrome-related aetiologies including Alzheimer’s, Parkinson’s, Huntington’s, Korsakoff’s and Creutzfeldt-Jakob illnesses ().Citation1–4 As with amnesia and dementia, MI primarily impairs declarative memory, although it is not necessarily the case with dementia which is defined as the decline in two or more cognitive domains. In other words, dementia affects different aspects of memory in addition to declarative memory disorder.Citation5
Figure 1 An illustration of a degenerative disease of the brain as a result of gradual neuronal cell damage. Created with BioRender.com.
Tripterygium wilfordii Hook. f. (Thunder God Vine), Celastrus orbiculatus Thunb., Celastrus aculeatus Merr., Celastrus scandens L., and other members of the Celastraceae family all contain a bioactive compound known as celastrol. The extracts of the root, bark and stem of several of these plants have long been used in China and other Asian nations in the treatment of a variety of chronic inflammatory diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and allergies.Citation6 Celastrol modulates molecular and cellular processes in chronic inflammatory, autoimmune, neurodegenerative and metabolic disorders. In this article, we focus on the role of celastrol in treating neurodegenerative disorders (NDD).Citation7
According to preclinical lines of evidence, celastrol also appears to have positive benefits in a variety of pathological disorders characterised by an excess production of reactive oxygen species (ROS). In this context, it has been widely established that ROS play a critical role in the aetiology of NDD as they serve as important mediators in brain injury. A new mechanism of action, ie, the suppression of the functional interaction between the cytosolic subunits and the membrane flavocytochrome has been identified where celastrol has been shown to directly inhibit the ROS generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, NAPDH oxidase (NOX) enzymes with higher potencies seen against two isoforms (NOX1 and NOX2). To date, NOX enzymes have been identified as a significant ROS generator in NDD and have been directly linked to the emergence of its pathogenic symptoms. Consequently, there is a growing interest in the potential of celastrol in ameliorating NDD.Citation8
Celastrol revealed potential effects against MI based on the review of the literature. Therefore, the purpose of the current review was to give extensive information about celastrol’s effectiveness against MI and to address the challenges and opportunities to overcome this potential candidate to become a drug molecule.
Study Design
Animals and Cell Cultures
In the studies we included in our review, rats were employed in one study and mice in three in vivo studies. All experimental protocols have been approved by the relevant institution’s animal welfare committees and were conducted in accordance with the guidelines for the use and care of laboratory animals. For in vitro studies, two studies opted the use of human cell lines (neuroblastoma and neuroglioma cells) while the remaining single study utilized rat’s pheochromocytoma cell line. The cell lines were cultured properly in Dulbecco’s modified Eagle’s medium (DMEM) that have been supplemented by a 10% bovine serum.
MI Models
There are numerous studies on various MI models that have been previously described in the literature (Lum et al 2021). However, relatively few models have been used in the research on Celastrol’s effectiveness against MI, which is discussed below. For in vivo studies, Kiaei et alCitation9 scrutinized on memory impairment occurring as a result of amyotrophic lateral sclerosisCitation9 while Cleren et al focused on the 3-nitropropionic acid (3-NP) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) memory deficits.Citation10 The other two in vivo studies, Paris et al and Zhang et al employed β-amyloid and transient global cerebral ischemia reperfusion (tGCI/R) MI models respectively.Citation11,Citation12 For in vitro studies, Chen et alCitation13 and Deng et alCitation14 focused, respectively, on cadmium and rotenone-induced neuronal damage,Citation13,Citation14 while Zhao et alCitation15 employed lipopolysaccharides-induced cognitive impairment.Citation15
Dose of Celastrol
During the course of celastrol treatment, all of the reviewed studies utilised purchased celastrol (MW 360.4) with purity greater than 98%. For the in vivo studies, the majority of the investigations utilised celastrol (1–4 mg/kg) with 1 mg/kg as the most common dose. In terms administration route, many in vivo studies utilized oral (P.O.), intraperitoneal (i.p.) and subcutaneous (s.c.) with i.p. being the most common route. For in vitro studies, all studies have utilised celastrol at the dosage range of 0.01–10.00 µM (10–10,000 nM).
Toxicity Profile of Celastrol
In an in vivo study conducted by Sousa,Citation16 the levels of certain toxicological parameters; creatine kinase (CK), lactate dehydrogenase (LDH), urea, alanine transaminase (ALT) and pro-atrial natriuretic peptide (pro-ANP) were measured in celastrol-treated (2.5 µL/g) rats using enzyme linked immunosorbent assay (ELISA). Each parameter represents the following tissue damage:
CK and LDH (systemic tissue damage)
Urea (nephrotic damage)
ALT (hepatic damage)
Pro-ANP (cardiac damage)
It was reported by SousaCitation16 that the celastrol-treated rat group showed no signs of toxicological effect because since there were no significant differences in the levels of the toxicological parameters between celastrol-treated and healthy rat groups. Following toxicological testing, a histological analysis of the celastrol-treated internal organs was performed by a clinical pathologist whom the initial data has not been shown to him to prevent any bias. The outcome of the histological analysis also supports the fact that there were no signs of toxicological effects following celastrol (2.5 µL/g) administration.
Memory Testing Procedure
Three basic types of animal models that cause cognitive dysfunctions: 1) pharmacological models, 2) toxicological models, and 3) genetically engineered modelsCitation17 have been reported. Animal models of cognitive impairment that are critically important were used to identify the neural underpinnings of learning, memory and attention. The neurotransmitter-receptor system’s role in cognitive processes including learning, memory and attention is best understood via pharmacological models, which are the most frequently employed in research on cognitive disorders.Citation17
Critical neuronal roles in cognitive activities are played by the cholinergic system (muscarinic and nicotinic) and glutamate receptors, particularly the N-methyl-D-aspartate (NMDA) receptors. Choline acetyltransferase is an enzyme that converts dietary choline and acetyl coenzyme-A into acetylcholine. On the other hand, acetylcholinesterase aids in the metabolism of acetylcholine at neural synapses. Some cholinesterase inhibitors such as donepezil, rivastigmine and galantamine have been designed to improve memory impairment.Citation18
To date, antimuscarinic drugs such scopolamine, atropine, pirenzepine, trihexyphenidyl, benztropine, biperidine and dicyclomine have been used to cause memory impairment in animal models of the cholinergic system.Citation19 Nicotinic receptor antagonists have been used to induce cognitive defects in animal models, including mecamylamine (a non-competitive non-selective nicotinic receptor antagonist), chlorisondamine and d-tubocurarine (non-specific nicotinic antagonists), dihydro-erythroidine hydrobromide (DhE) and methyl-lycaconitine (MLA), a specific receptor-7 antagonist.Citation20 Similarly, as their activation is linked to a long-term potentiation (LTP) that strengthens the signal transmission between neurons, NMDA receptors also play a crucial role in cognitive functioning. Therefore, many scientists have chosen to use NMDA receptor antagonists such as MK-801, ketamine and phencyclidine (PCP), to induce cognitive impairment in animal models via the glutamate-receptor system.Citation21
Animal models have been successfully used to investigate the neurological toxicology’s application to investigate cognitive impairment. Since the cognitive deficits have been well modelled in monkeys and rats, neurotoxicants including lead, mercury and polychlorinated biphenyls (PCBs) are used to cause neurotoxicity in animal models.Citation17 Heavy metals such as lead can cause oxidative stress by making cells more susceptible to ROS and decreasing antioxidants like catalase (CAT) as well as superoxide dismutase (SOD). Protein kinase C (PKC) and nuclear factor-activated B cells (NF-κB), which can be increased by lead exposure, are the main sources of ROS. Additionally, lead can also cause the apoptosis of neuronal cells by imitating calcium ions and attaching to voltage-gated calcium ion channels. The overall effect causes alteration in the balance of neurotransmitters in the hippocampus, leading to apoptosis and autophagy. Finally, through activation of NF-κB, lead can potentially cause neuro-inflammation.Citation22
Since they may imitate some impairments, including as Alzheimer’s disease (AD), β-amyloid deposition, amyloid precursor protein (APP) and cholinergic-receptor deletion, genetically engineered animals are more frequently utilised in cognitive impairment research.Citation17 However, a variety of experimental techniques are used to access memory, including 1) rotarod 2) histological analysis 3) high performance liquid chromatography (HPLC) 4) enzyme-linked immunosorbent assay (ELISA) 5) Morris water maze (MWM) 6) estimation of malondialdehyde (MDA) glutathione (GSH), SOD and CAT levels 7) Western blot 8) immunofluorescent staining 9) Nissl and Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End Labelling (TUNEL) 10) 4’,6-diaminido-2-phenylindole (DAPI) staining 11) apoptosis analysis 12) measurement of ROS and mitochondria membrane potential (MMP) 13) immunoblotting and 14) electron microscopy. Among these, histological analysis, Western blot, ELISA and immunofluorescent staining are the most commonly used procedures.
Rotarod
In the study by Kiaei et al,Citation9 mice (G93A) at 60 days old were trained to use a rotarod device over the course of two to three days. First, the mice were put on a rotating rod [12 rpm]. Subsequently, the duration that the mice remained on the rod before falling off was recorded to assess the competency of their motor function. The best outcome of the three trials was then reported. They were assessed twice weekly until they were unable to complete the task. Then, the mice were evaluated using a rotarod at 15 rpm for 10 min to determine the development of symptoms using the motor function as an index and the best outcome of the three trials was reported after the first trial.Citation9
Histological Analysis
The researchers have utilised the following techniques to examine the histology of celastrol. In accordance with the past investigations,Citation9–11 fresh brains of either mice or rats were perfused with paraformaldehyde (4%) in phosphate buffered saline (PBS). The brains were cryoprotected overnight using either glucose or sucrose solutions. Serial coronal sections were cut through either the substantia nigra for mice or striatum for rats using a cryostat. For stereological analysis, the collected sections were immunostained with different antibodies to determine the presence of antigens tested inside the cells following celastrol treatment.Citation9–11
High Performance Liquid Chromatography (HPLC)
According to the study by Cleren et al,Citation10 striata were dissected before being placed on dry ice immediately, followed by preservation at −80°C for the purpose of measuring dopamine (DA) and its metabolites. Then, the tissues were sonicated in cold 0.1 M perchloric acid (about 100 µL/mg tissue), before being centrifuged. DA and its metabolites (homovanillic and 3,4-dihydroxyphenylacetic acids) were measured in the supernatant using an HPLC. Briefly, a mobile phase containing 75 mM of NaH2PO4, 1.5 mM octanesulfonic acid (OSA) and 5% acetonitrile (pH 3) was used to isocratically elute 10 µL of supernatant fluid, which was then measured using a two-channel Coulochem II electrochemical detector at 1 mL/min. DA concentrations and its metabolites were expressed in nanograms per milligramme of protein. Utilizing the protein analysis procedure and the HTS7000+ plate reader, protein concentrations of tissue homogenates were then determined.Citation10
Enzyme-Linked Immunosorbent Assay (ELISA)
Cleren et alCitation10 conducted a study on rat striata and dissected mouse substantia nigras were immediately placed on dry ice and kept at −80°C. The expression of heat shock protein (HSP) 70 was measured using a StressXpress HSP70 ELISA kit based on the manufacturer’s instructions with some slight modifications. Frozen tissue was placed in a 1 mL Dounce homogenizer with 250 µL (for the substantia nigra) or 500 µL (for the striata) of extraction reagent. Then, they were manually homogenised with 15 strokes of the pestle. The samples were incubated for 3 hours without any dilution.Citation10
Zhang et alCitation12 utilised ELISA to measure some inflammatory cytokines including tumor necrosis factor alpha (TNF-α) and interleukins inside the hippocampus as well as the serum. Samples from one side of the hippocampus along with uncoagulated blood were carefully aspirated and centrifuged before being added to specific 96-well ELISA plates. Finally, the absorbance was measured by using a spectrophotometer.Citation12
Morris Water Maze
In the study by Zhang et al,Citation12 seven days after transient global cerebral ischemia reperfusion (tGCI/R), Morris water maze (MWM) tests were administered for five continuous days to evaluate hippocampus-dependent spatial learning and memory. The water maze was an open, 60-centimeter-high, 150-centimeter-diameter swimming tank painted dark and was kept in a dimly lit space. An escape platform (10 cm diameter) was positioned in a fixed location in the target quadrant (SW) of the tank. The target quadrant was divided into four other equal quadrants and was labelled as east (E), west (W), south (S) and north (N). The tank was partially filled with warm water in each of the other three quadrants. In order to prevent rodents from seeing the submerged platform, non-toxic black ink was added into the water. The walls of the room were decals with geometric symbols (square, triangle, circle, or star) that could be seen from the tank. Five days of training trials (the place navigation test, or PNT) were followed by a probe test (the spatial probe test, or SPT), which lacked an escape platform. Throughout the test, the researcher who was unaware of the experimental setup was stationed outside the tank silently.
Prior to the initial trial, the rats were trained to remain on the escape platform for 30 seconds. The rats were allowed to search for the submerged escape platform for a maximum of 60 seconds, four times daily, with a 15-minute gap between trials. Rats that took longer than 60 seconds to locate the platform were manually taken to the location and were given another 15 seconds to understand their latest location. The rats’ learning capacity was assessed by calculating the 1) daily average escape latency or 2) average escape path length based on ten rats. The average time or distance to discover the hidden platform (escape latency or path length) were recorded using a video tracking system. Short-term memory was tested using SPT without an escape platform two hours following the last training trial. The duration spent in the target quadrant and the number of crossings over the initial platform location were recorded.Citation12
Estimation of Malondialdehyde (MDA), Glutathione (GSH), Superoxide Dismutase (SOD) and Catalase (CAT) Levels
According to Zhang et al,Citation12 three days following tGCI/R, rat hippocampi were extracted to analyse for MDA, GSH, SOD and CAT activities using commercially available assay kits in accordance with the manufacturer’s instructions. Briefly, the hippocampal samples were promptly extracted and homogenised in icy normal saline. Supernatants were obtained using an Eppendorf micropipette after being centrifuged at 4000 rpm for 10 min at 4°C. Thiobarbituric acid was used to measure the MDA level (a sign of lipid peroxidation) at 532 nm. A microplate approach was used to measure GSH concentrations (a marker of GPx activity) as well as the absorbance at 420 nm. A hydroxylamine technique was used to assess SOD activity followed by determination of absorbance at 550 nm. Utilizing an ultraviolet technique, the absorbance at 240 nm was measured for evaluation of CAT activity. Finally, the hippocampal samples’ protein concentrations were calculated.Citation12
Western Blot
Chen et al,Citation13 conducted a study in a poly-D lysine (PDL)-coated 6-well plate, PC12 cells and/or primary neurons were planted at a density of 2×106 cells/well. Following pre-incubation with/without celastrol (0.1, 0.5 and 1.0 µM) for 1 h, the cells were treated with/without Cd (10 and 20 µM) for 12 hours. Alternatively, the cells were treated with/without Cd (10 µM) for 12 hours following pre-incubation with/without celastrol (1 µM). Then, PC12 cells were pre-treated with celastrol (1 µM) for 1 h before being exposed to Cd (10 µM) for 12 h following infection with Ad-PTEN, Ad-dn-c-Jun, or Ad-GFP. Western blotting was then completed. NIH Image J software was used to semi-quantify the blots for the proteins detected. β-tubulin was used as an internal control to standardise the results.Citation13
Immunofluorescent Staining
According to the protocol reported by Zhang et al,Citation12 rat brains were freshly removed three days (72 h) after tGCI/R, cut in half with a sharp knife on ice where one half was used for immunofluorescent examination. The process of immunofluorescent staining was used. The entire half-rat brain was wrapped in an ideal cutting temperature compound and was submerged for two min in isopentane at 80°C before being cryosectioned (12 m) and transferred to superfrost glass slides. At room temperature (23±2°C), brain slices were blocked with 0.3% Triton X-100/10% normal goat serum after being fixed for 10 min with freshly made 4% paraformaldehyde. Next, the slices were exposed to the primary antibodies for an overnight incubation (4°C). Following a 60-minute incubation period at room temperature using proper secondary antibodies, the slices were counterstained with DAPI for 10 min to highlight the nuclei. After being cleaned with PBS (0.1 M) and mounted with a mounting solution (50% glycerol aldehyde), the slides were stored with coverslips. The immune-stained sections were examined using a fluorescent microscopy. Then, six randomly chosen microscopic fields from the hippocampal CA1 area were chosen for further analysis.Citation12
Nissl and Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End Labelling (TUNEL)
Zhang et alCitation12 tested several alcohol gradients (70, 95 and 100%) to dehydrate the brain sections for 5 min before the addition of xylene. The brain sections were stained with 0.3% cresyl violet acetate (Sigma) for 30 min at room temperature. The sections were viewed under a light microscope after being cover-slipped with neutral balsam media. A researcher who was blinded to the study group measured the number of living neurons using Image-Pro Plus software (version 6.0, Media Cybernetics, United States). Only fully developed neurons with discernible nuclei were counted in the CA1 pyramidal cell layer. A TUNEL reaction buffer was first incubated on the brain slices for 1 hour at 37°C in a humid environment. Apoptotic neurons were next stained with diaminobenzidine substrate solution, and all nuclei were counterstained with hematoxylin after the slides were washed and treated with converter-POD for 30 min at room temperature. The sections were cleaned, dehydrated in ethanol with varying ethanol concentrations (70, 95 and 100%), transparented with xylene, cover-slipped with neutral balsam media and were examined under a light microscope.Citation12
Stain Using 4’, 6-Diaminido-2-Phenylindole (DAPI)
In the study by Chen et al.Citation13 In a six-well plate with a glass coverslip coated with poly-D-lysine (PDL), the cells were seeded at a density of 5×105 per well. The following day, after pre-incubation with/without celastrol (0.1, 0.5 and 1.0 µM) for 1 h, the cells were exposed to Cd (10 and 20 µM). The cells with fractured and contracted nuclei were further identified using a DAPI staining following exposure to Cd for 24 hours. A digital camera-equipped fluorescence microscope was used to capture some pictures.Citation13
Apoptosis Analysis
Detection of apoptotic cells was done by Deng et alCitation14 with the aid of an Annexin V-Fluorescein isothiocyanate (FITC)/PI double-labelling kit. Briefly, the cells were subjected to various testing agents before being cleaned in a cold phosphate buffered saline (PBS), centrifuged twice for five min at 1500 rpm and then suspended in a binding buffer (500 µL). FITC-labeled Annexin V (5 µL) and propidium iodide (PI, 5 µL) were added and incubated with the cells at room temperature for 15 min, according to the manufacturer’s instructions. A FACScan flow cytometer was used to determine the number of apoptotic cells. Early apoptotic cells were defined as annexin V-positive, PI-negative cells. Late apoptotic cells were positive for both Annexin V and PI.Citation14
Measurement of Reactive Oxygen Species (ROS) and Mitochondria Membrane Potential (MMP)
An in-vitro study was conducted by Deng et al,Citation14 ROS was detected using an ROS test kit with 2′,7′-dichlorofluorescein diacetate (DCFH-DA), while MMP was detected using a mitochondrial membrane potential assay kit. Following the manufacturer’s instructions, the cells were collected, suspended in PBS and promptly stained with DCFH-DA or fluorescent JC-1. The samples were examined by a flow cytometer after being washed twice with ice-cold PBS.Citation14
Immunoblotting
According to the research conducted by Deng et al,Citation14 first, cold PBS was used to wash the human neuroblastoma (SH-SY5Y) cells. Then, radio-immunoprecipitation assay buffer (RIPA) (200 µL) containing a protease inhibitor cocktail was used to lyse the cells. Protein (100 µg) from each sample was put onto a polyacrylamide gel with sodium dodecyl sulphate (12%) to measure protein using a bicinchoninic acid assay (BCA) kit. Proteins were moved from the electrophoresis tank onto polyvinylidene fluoride (PVDF) membranes. Non-fat dry milk (5%) dissolved in PBS for two hours inhibited non-specific protein binding. The membranes were treated with secondary antibodies conjugated with HRP at room temperature for 1 hour after being washed with phosphate buffered saline containing 0.05% Tween-20 (PBST). An improved chemiluminescence detection kit was used to detect the signal. Proteins having immunoreactive bands were scanned using the Chemidoc XRS. The digitised data was then measured as integrated optical density (IOD) using the Image J program. Subsequently, cytosolic fraction was extracted from the cell pellets using a cell mitochondria isolation kit to measure the release of cytochrome C from damaged mitochondria. Finally, the cytosolic fraction was extracted and was subjected to an immunoblotting experiment in accordance with the manufacturer’s instructions.Citation14
Electron Microscopy
In accordance with the research done by Deng et al,Citation14 Celastrol-treated SH-SY5Y cells were fixed with glutaraldehyde (2%) for 2 hours, washed with PBS and then post-fixed with 1% osmium tetroxide (OsO4) for 1.5 hours at 48°C to confirm the autophagosome structures following autophagy. The samples were then cleaned and dried with varying degrees of alcohol. Then, the samples were dehydrated before being injected and implanted in 618 epoxy resin. Using a transmission electron microscope H7650, several ultrathin sections were cut, stained with both uranyl acetate and lead citrate before being analysed.Citation14
Effectiveness of Celastrol Against Memory Impairment
Amyotrophic Lateral Sclerosis
In a study by Kiaei et al,Citation9 G93A mice treated with celastrol (2 mg/kg/day) at 30 days of age experienced a 15-day delay in disease onset. The onset for G93A mice treated with celastrol was 109.5 days, compared to 95 days for G93A mice treated with vehicle. Treatment with celastrol (2 mg/kg/day) significantly increased G93A mice life from 128 days to 140 days compared to controls. The treatment of G93A mice with 8 mg/kg/day celastrol dramatically increased survival from 125 days to 141 days. Administration of celastrol (2 mg/kg/day) to G93A mice dramatically reduced neuronal loss in the lumbar spinal cord. At 110 days, the number of surviving neurons in G93A mice treated with celastrol was considerably greater than in G93A mice fed a control diet.
3-NP and MPTP (3-Nitropropionic Acid and 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine)
In a study conducted by Cleren et al,Citation10 treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on Swiss Webster mice resulted in a 37% decrease in striatal dopamine (DA) concentration compared to dimethylsulfoxide/phosphate-buffered saline (DMSO/PBS)-treated animals. Additionally, treatment with celastrol (3 mg/kg) effectively prevented the depletion of striatal DA produced by MPTP. Significant decrease was also observed in striatal 3,4-dihydroxyphenylacetic acid and homovanillic acid following treatment with MPTP. Celastrol reduced the MPTP-induced depletion of 3,4-dihydroxyphenylacetic acid.
In light of celastrol’s significant anti-inflammatory properties, its effects on the generation of tumour necrosis factor a (TNF-α) and nuclear factor κ B (NF-κB) were examined. In the substantia nigra, treatment with DMSO and MPTP enhanced immunostaining for the inflammatory cytokine TNF-α. Although the brightly labelled cells for TNF-α were counted stereologically in the substantia nigra, no intensely stained cells were detected in the control groups. The number of TNF-α immunoreactive cells was considerably lower in mice treated with celastrol/MPTP when compared to mice treated with DMSO/MPTP.
β-Amyloid
Paris et alCitation11 investigated the effect of celastrol on brain β-amyloid (Aβ) levels in 6-month-old Tg PS1/APPsw mice. Mice of the Tg PS1/APPsw strain were intraperitoneally injected with celastrol (1 mg/kg) of the vehicle for four days. After one hour, the mice were euthanized, and the brains were retrieved. The β-amyloid reducing abilities of celastrol were evaluated by quantifying the pools of soluble and insoluble Aβ species in mice administered with either celastrol or a placebo. After four days of treatment with celastrol (1 mg/kg/day), brain-soluble Aβ (1–40) and Aβ (1–42) decreased by approximately 40%, whereas insoluble Aβ decreased by nearly 50% when compared to placebo-treated rats.
Transient Global Cerebral Ischemia Reperfusion (tGCI/R)
Transient global cerebral ischemia reperfusion (tGCI/R) results in quantifiable alterations in neurodeficit scores. Four vessel occlusion (4-VO) generated an apparent neurological loss in vehicle-treated tGCI/R rats, with a 7-point drop in the neurological score as compared to sham-treated mice (12 points). However, neurological scores improved in tGCI/R rats treated with celastrol, with the greatest improvement shown in the animals from the 4 mg/kg group. Celastrol (1, 2 and 4 mg/kg) dose-dependently suppressed the accumulation of pro-inflammatory cytokines induced by tGCI/R including TNF-α, IL-1β and IL-6. Treatment with celastrol concurrently, restored the decrease in hippocampus IL-10 level which is an anti-inflammatory cytokine.Citation12
Cadmium (Cd)
In an in vitro study conducted by Chen et alCitation13 to determine the effect of celastrol on cadmium (Cd), PC12 cells were pre-incubated with celastrol (0–1 µM) for 1 h, followed by treatment with/without Cd (10 and 20 µM) for 24 h. Upon exposure to Cd alone, phase-contrast microscopy revealed that many PC12 cells had become rounded or were shrunken. Although celastrol did not appear to modify cell morphology on its own, it significantly inhibited Cd-induced morphological change in cells. Additionally, the Cd + celastrol group showed a considerably greater cell viability than the Cd alone group. Celastrol provided the greatest protection against Cd-induced reductions in cell viability and morphological changes at 1 µM implying that celastrol may inhibit neuronal cell death triggered by Cd.
Rotenone
The 24 h effect of rotenone (500 nM) on cell death was mitigated by pre-treatment with celastrol (200, 500 and 1000 Nm) for 1 hour. Compared to the control group, rotenone treatment decreased cell viability and increased cell apoptosis. Celastrol pre-treatment mitigated these alterations, increased cell viability and decreased cell apoptosis relative to the Rotenone-group thus suggesting that celastrol prevented rotenone-induced cell death in SH-SY5Y cells.Citation14
Lipopolysaccharides (LPS)
Zhao et alCitation15 evaluated celastrol’s ability to defend H4-APP cells from lipopolysaccharide (LPS)-induced oxidative stress and induction of apoptosis. Celastrol treatment (10 nM) greatly lowered the formation of ROS. Cell viability tests found that treatment of 100 g/mL of LPS alone resulted in a 40% reduction in cell survival, a considerable reduction as compared to that seen in control. Additionally, celastrol dramatically reduced this LPS-induced increase in cellular death, overall suggesting that it is cytoprotective against a cell death as induced by LPS. Moreover, immunofluorescence is used to evaluate the expression of cyclooxygenase-2 (COX-2), which is closely related with neuroinflammation and Aβ amyloidosis. H4-APP cells treated with LPS exhibited elevated levels of COX-2. Celastrol therapy inhibited COX-2 expression, hence preventing further inflammation and Aβ synthesis.
Overview of Mechanisms of Action of Celastrol Against Memory Impairment
Multiple in vivo and in vitro investigations have shown that MI can be produced by a variety of variables including specific diseases (eg, ALS and tGCI/R),Citation9,Citation12 neurotoxicants (eg, 3-NP, MPTP, Cd and rotenone)Citation10,Citation13,Citation14 and neurodegenerative disorders (eg, AD caused by LPS and β-amyloid).Citation11,Citation15 In these investigations, the utility of celastrol in enhancing cognitive performance, particularly in memory domains such as spatial, recognition, retention and reference memories, have been successfully demonstrated both in vivo and in vitro ( and ). Overall, celastrol protects against MI via reducing oxidative stress,Citation12,Citation14 preventing neuroinflammation,Citation12 inducing autophagy,Citation14 inhibiting apoptosis,Citation9,Citation12–15 enhancing HSP expressionCitation10,Citation15 and suppressing Aβ production.Citation11,Citation15
Table 1 A Summary of in-vivo Studies Investigated the Efficacy of Celastrol on Memory Impairment
Table 2 A Summary of in-vitro Studies Investigated the Efficacy of Celastrol on Memory Impairment
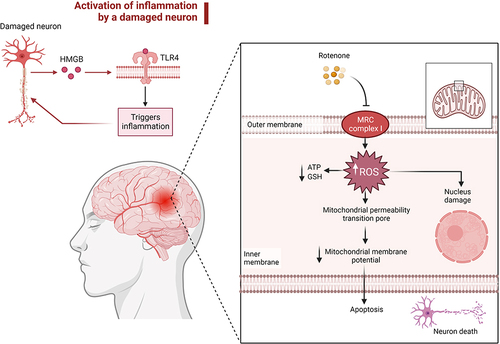
Oxidative stress has a crucial role in the aetiology of neurodegenerative diseases. The overproduction of ROS leads to oxidative stress, which in turn leads to mitochondrial malfunction by oxidising proteins in the mitochondria, resulting in the production of further ROS. Mitochondria are not only ROS producers, but also ROS targets. Certain neurotoxins such as rotenone and diseases can harm the mitochondria (). Damaged mitochondria release ROS and other apoptosis inducers, thus promoting neuronal damage.Citation14 Boosting the antioxidant defence system, which includes SOD and CAT, may facilitate neural recovery from oxidative stress.
Figure 2 The neurotoxic rotenone induces mitochondrial dysfunction, which leads to neurodegenerative diseases. Reactive oxygen species (ROS) are generated as a result of rotenone’s inhibition of the MRC complex I. Overproduction of ROS reduces the levels of ATP and GSH, which sets off a series of events that lessen its permeability and ultimately lead to the death of brain cells. Created with BioRender.com.
GSH is a key antioxidant in cells. Celastrol has been shown to produce antioxidant benefits by decreasing ROS production and enhancing the GSH redox cycle.Citation12 Zhang et alCitation12 investigated the effect of celastrol on transient global cerebral ischemia reperfusion (tGCI/R) rat model. The transcription factor NF-κB is related with inflammation, oxidative damage and apoptosis. Antioxidants prevented the phosphorylation and reduction of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα) in the cytoplasm and decreased NF-κB activity in the tGCI/R rat model. Celastrol restored tGCI/R-induced phosphorylation, degradation of IκBα and recovered nuclear translocation of NF-κB subunit p65, according to the findings. Celastrol can prevent the activation of the NF-κB pathway and reduce the release of inflammatory cytokines. Celastrol appeared to reduce neuroinflammation and oxidative stress, as evidenced by the upregulation of anti-inflammatory cytokine IL-10 and anti-oxidative substances GSH, SOD and CAT and the downregulation of pro-inflammatory cytokines TNF-α, IL-1β and IL-6.
In an in vitro study by Deng et al,Citation14 the presence of celastrol induced autophagy reaction in rotenone-treated SH-SY5Y cells. Autophagy is viewed as a survival mechanism of cells that is triggered under stress conditions to increase cell survival. Numerous studies indicate that autophagy has a crucial neuroprotective role in neurodegenerative disorders. Rotenone, an inhibitor of mitochondrial complex I, has numerous deleterious effects, including ATP depletion and oxidative stress. Therefore, the rotenone-induced cellular model in this study can recapitulate the primary pathophysiology of neurodegenerative disorders, confirming celastrol’s potential as a neuroprotective drug through the positive results (celastrol-induced autophagy reaction as well as protection of SH-SY5Y cells from rotenone-induced apoptosis). Celastrol can also protect against apoptosis induced by other neurotoxicant such as cadmium (Cd).Citation13
Zhao et alCitation15 concluded that celastrol protects cells from apoptosis through two mechanisms: upregulation of heat shock protein 70 (HSP 70) and reduction in Aβ production. HSP 70, the principal inducible heat shock protein, is induced by oxidative stress and neuronal damage. HSP 70 protects against protein misfolding and aggregation, as well as oxidative damage and apoptosis.Citation10 Celastrol is believed to produce the said effect by activating the heat shock transcription factor 1 (Hsf 1) and HSP 70. In addition to limiting protein aggregation, HSP-70 prevents apoptosis by inhibiting multiple stages along the apoptotic cascade. Alternatively, amelioration of apoptosis by celastrol may be attributable to the reduction in Aβ production. Aβ is directly harmful to cultured neurons and can induce apoptosis.Citation15 Furthermore, Paris et alCitation11 also investigated the effect of celastrol on its Aβ reduction properties with similar positive findings, thus confirming that celastrol can inhibit the production and aggregation of both soluble and insoluble Aβ species.
Bioavailability of Celastrol
Zhang et alCitation23 investigated the oral bioavailability of celastrol by comparing the equivalent area under the curve (AUC) of oral and intravenous celastrol administrations. The researchers indicated that oral administration of pure celastrol results in only 17.06% a bioavailability. It is plausible that celastrol has a low absolute bioavailability since it is poorly soluble in water and has a high rate of in vivo metabolism or tissue distribution. Additionally, significant gender disparity where females tend to absorb more celastrol. Therefore, therapeutic regimens should take gender differences in pharmacokinetic profiles into account since it impacts celastrol toxicity and therapy.
Challenges and Opportunities to Improve the Drug Delivery of Celastrol for Memory Impairment
Celastrol can interact with a wide range of cellular targets and can treat a variety of memory impairment models, but it also has several drawbacks, including poor water stability, low bioavailability, a narrow therapeutic window and undesirable side effects that hampers its clinical applicability. Similar with many phytochemical compounds, celastrol has a low water solubility (13.25 ± 0.83 mg/mL at 37°C). Additionally, several investigations have shown that oral administration of celastrol to rats indicated low absolute bioavailability (17.06%) with poor absorption into the systemic circulation.Citation24 In vivo metabolism and/or tissue distribution may also contribute to the said low bioavailability in addition to low aqueous solubility. Celastrol’s narrow therapeutic window of dose and occurrence of side effects are two major concerns related to its clinical application. Celastrol can ameliorate a range of memory impairment models when administered between 3 and 5 mg/kg. Lower doses, however, quickly lose their effectiveness, while greater amounts exhibit hazardous toxic effects thus suggesting its narrow therapeutic window. Celastrol also has various adverse effects, including as nephrotoxicity, cardiotoxicity, hepatotoxicity and toxicity to the haematological system. At therapeutic dosages, the potential for celastrol-drug interactions should raise serious concerns.Citation25 Therefore, its application and related preparations need to be further investigated.
To overcome the physical, chemical and pharmacokinetic limitations of celastrol and to lower the effective dose, researchers have tried out several useful attempts which include exosomes, lipid nanospheres, nanoencapsulation, polyamidoamine dendrimer nanocarriers, liposomes, polymeric micelles, cell-penetrating peptides-coated nanostructure. For example, celastrol-loaded lipid nanospheres, liposomal celastrol and solid self-microemulsifying dispersible tablets of celastrol greatly increase its oral bioavailability, boost effectiveness and decrease the incidence of side effects in animals ().Citation6
Figure 3 Future perspectives on strategies for the delivery of celastrol using nanocarriers. Celastrol can be loaded onto or conjugated to nanocarriers, boosting its ability to pass across blood-brain barriers and increasing its solubility and bioavailability through the digestive tract. Created with BioRender.com.
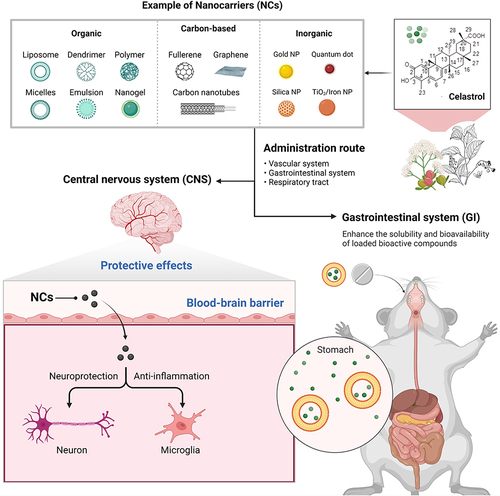
There has been a continuous rise in the research and publication of animal models using a variety of novel natural product-based nanotechnology therapy treatments that can attenuate MI.Citation26–35 This review will lead to the cutting-edge of novel celastrol-based nanotechnology treatments for MI in animal models and the future work for successful treatment of MI in humans.
Structurally Relevant Molecules of Celastrol
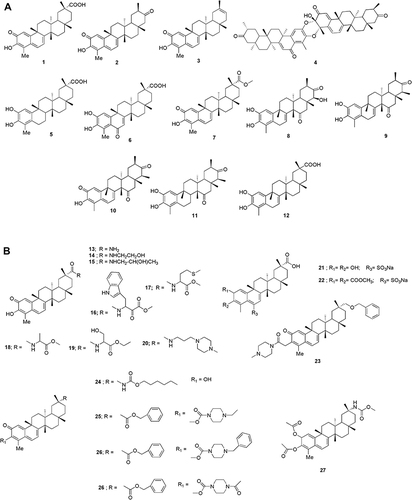
Celastrol (1) was initially isolated from Tripterygium wilfordii Hook. f., (thunder of god vine) and later identified in a variety of plant species in the Celastraceae family.Citation36 The related celastroid natural product, tingenone (2), has been investigated in clinical trials for lymphoepithelioma, skin, stomach and uterine cancers and has been shown to pose moderate activity with minimal side effects.Citation36,Citation37 Iguesterin (3) and a dimer of tingenone known as xuxuarine (4) were also reported to be present from Tripterygium wilfordii Hook. f. When a similar compound (wilforic acid) (5) was subjected to double oxidation followed by tautomerization, it failed to yield celastrol in a single operation. However, on sequential oxidations, wilferol is formed initially (6). On further oxidation, it yielded celastrol, which is further esterified into pristimerin (7).Citation38 Norfriedelane-type triterpenes, salaquinone A (8), salaquinone B (9), tingenine B (10) and regeol A (11) have similar structures to celastrol. They were reported to originate from the S. chinensis collected in Thailand and are important due to their antioxidant activities.Citation25,Citation39 Another similar compound Triptohypol C (12) was also reported as a precursor to celastrol during its biosynthesis ().Citation40
Figure 4 (A-D) Structurally relevant molecules of celastrol. The main body text contains information on the numbered molecules.
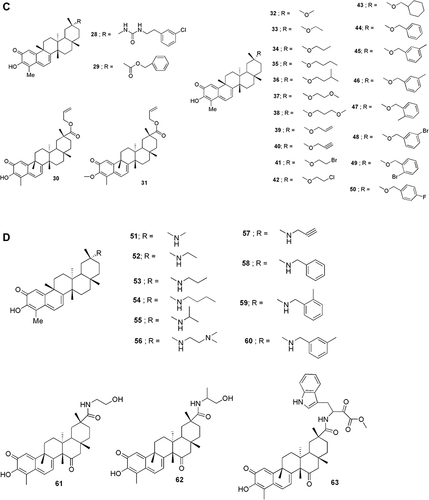
Celastrol (1) has poor water-solubility, short half-life, narrow therapeutic window and low bioavailability, which creates a serious setback on its clinical application. With the development of synthetic technology, the structure of celastrol was chemically modified and several derivatives having biological activities were synthesized. For example, the A/B ring is the group related to the activity, while C-2 and C-3 are modified to improve the activity. C-6 mainly introduces sulfonic acid and indole through nucleophilic reaction. C-20 carboxyl group is the main modifying group where its physicochemical properties can be improved mainly through esterification reaction and amide reactions.Citation41 Generally, the solubility of a compound is directly related to the number of polar groups. Therefore, enhancing the solubility of celastrol becomes a strategy to improve bioactivity. Hence, hydrophilic groups such as amino, hydroxyl, amide and sulfonic acid are introduced to improve the solubility of many compounds 13–23. Celastrol easily polymerizes and is unstable in acidic or alkaline environments, in which ring A is prone to ring opening and rearrangement. The structure of celastrol has been modified in compounds to improve its stability 24–27 ().
Celastrol has the duality of “toxin-effect” where its active part is also the toxic part. By enhancing the selectivity of celastrol, the toxicity can be decreased, and the property of drug can be increased. To achieve this, the C-20 position was modified in compounds 28 and 29. The C-20 ester derivatives 30 and 31 were evaluated with several human cancer cell lines.Citation42 A series of celastrol derivatives (32–50) were designed and synthesized for their immunosuppressive properties ().Citation43
The amide derivatives 51–60 were synthesized and tested as immunosuppressive agents. Compound 51, with a selectivity index value that was 31 times higher than that of celastrol, was selected as a lead compound. Three hypoxia-inducible factor-1 alpha (HIF-1a) functioning derivatives 61–63 stemming from modifications to the C-29 carboxyl group of celastrol were also reported. Subsequently, a series of C-29 derivatives were synthesized. Then, their anticancer activities were evaluated ().Citation44
All the above molecules (2–63) () have been structurally relevant to celastrol. Since then, investigations against MI have only been done using celastrol. Through this review, we propose that Celastrol’s structurally relevant molecules, which have been associated with various biological activities, be used in molecular docking and quantitative structure–activity relationship (QSAR) investigations, and then with the chosen molecules to be investigated against MI in the near future.
Conclusions
In general, celastrol is a promising natural lead for enhancing the cognitive performance of three animal models of MI (pharmacological, toxicological and genetic models). Celastrol attenuates neuronal damage via many mechanisms and has shown promise as a novel therapeutic drug for the treatment of MI. Our review indicates that celastrol acts primarily through the antioxidant defence mechanism, protecting against inflammation, inducing autophagy, preventing apoptosis, increasing the expression of neuroprotective genes, and suppressing amyloid beta production in in vivo and in vitro MI models. An in-silico strategy for selecting more effective compounds produced from celastrol may pave the way for the creation and discovery of novel drugs. To confirm that all semisynthetic celastrol derivatives are safe and effective, additional in vivo and in vitro studies are required. In the future, research into the QSAR of celastrol is necessary to develop a variety of other distinct compounds. Overall, celastrol is a viable natural lead for the design and development of a novel neuroprotective drug, especially to treat MI.
Disclosure
The authors declare that they have no conflict of interest for this work.
Acknowledgments
All the authors of this manuscript are thankful to their respective Departments/Universities for the successful completion of the study. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for financial support through Research Group (Large) (Project number RGP.2/54/44). Graphical abstract is created with BioRender.com.
References
- Brown P. Chapter 43 - transmissible spongiform encephalopathy. In: Textbook of Clinical Neurology. Vol. 355. Elsevier Health Sciences; 2007:953–965.
- Caselli RJ, Boeve BF. Chapter 33 - the degenerative dementias. In: Textbook of Clinical Neurology. Vol. 355. Elsevier Health Sciences; 2007:687–717.
- Jankovic J. Chapter 34–movement disorders. In: Goetz CG, editor. Textbook of Clinical Neurology. Amsterdam, The Netherlands: Elsevier; 2007:722–750.
- Kinsella LJ, Riley DE. Nutritional deficiencies and syndromes associated with alcoholism. In: Textbook of Clinical Neurology. Elsevier; 2007:897–917.
- Brewer JB, Gabrieli JDE, Preston AR, Vaidya CJ, Rosen AC. Chapter 5 - memory. In: Textbook of Clinical Neurology. Elsevier; 2007:71–86.
- Cascao R, Fonseca JE, Moita LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med. 2017;1–18. doi:10.3389/fmed.2017.00001
- Venkatesha SH, Moudgil KD. Chapter 12 celastrol and its role in controlling chronic diseases. In: Anti-Inflammatory Nutraceuticals and Chronic Diseases. Switzerland: Springer; 2016:267–289.
- Schiavone S, Morgese MG, Tucci P, Trabace L. The therapeutic potential of celastrol in central nervous system disorders: highlights from in vitro and in vivo approaches. molecules. 2021;26:1–13. doi:10.3390/molecules26154700
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegen Dis. 2005;2(5):246–254. doi:10.1159/000090364
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi:10.1111/j.1471-4159.2005.03253.x
- Paris D, Ganey NJ, Laporte V, et al. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2010;7:1–15. doi:10.1186/1742-2094-7-1
- Zhang B, Zhong Q, Chen X, et al. Neuroprotective effects of celastrol on transient global cerebral ischemia rats via regulating HMGB1/NF-kB signaling pathway. Front Neurosci. 2020;14:1–17. doi:10.3389/fnins.2020.00001
- Chen S, Gu C, Xu C, et al. Celastrol prevents cadmium-induced neuronal cell death via targeting JNK and PTEN-Akt/mTOR network. J Neurochem. 2013;128:256–266. doi:10.1111/jnc.12474
- Deng Y-N, Shi J, Liu J, Qu Q-M. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem Int. 2013;63(1):1–9. doi:10.1016/j.neuint.2013.04.005
- Zhao Y, Zhao H, Lobo N, Guo X, Gentleman SM, Ma D. Celastrol enhances cell viability and inhibits amyloid-beta production induced by lipopolysaccharides in vitro. J Alzheimers Dis. 2014;41:835–844. doi:10.3233/JAD-131799
- Sousa ÂRC. Efficacy and toxicity evaluation of celastrol in adjuvant-induced arthritis rat model. 2016.
- Levin ED, Buccafusco JJ. Introduction. In: Animal Models of Cognitive Impairment. CRC Press; 2006:1–2.
- Fahn S. Hypokinesia and hyperkinesia. In: Textbook of Clinical Neurology. Elsevier; 2007:292–309.
- Alvin V, Jerry J. Muscarinic receptor antagonist in rats. Anim Models Cognitive Impair. 2006;3:5–20.
- Roegge CS, Levin ED. Nicotinic receptor antagonists in rats. In: Animal Models of Cognitive Impairment. CRC Press; 2006:21–35.
- Rezvani AH. Involvement of the NMDA system in learning and memoy. In: Animal Models of Cognitive Impairment. CRC Press; 2006:56–67.
- Su P, Zhang J, Wang S, et al. Genistein alleviates lead-induced neurotoxicity in vitro and in vivo: involvement of multiple signaling pathways. NeuroToxicology. 2016;53:153–164. doi:10.1016/j.neuro.2015.12.019
- Zhang J, Li C-Y, Xu M-J, et al. Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J Ethnopharmacol. 2012;144(1):195–200. doi:10.1016/j.jep.2012.09.005
- Zhang J, Li CY, Xu MJ, et al. Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J Ethnopharmacol. 2012;144:195–200.
- Shi J, Li J, Xu Z, et al. Celastrol: a review of useful strategies overcoming its limitation in anticancer application. Front Pharmacol. 2020;11:1–25. doi:10.3389/fphar.2020.558741
- Giacomeli R, Izoton JC, Dos Santos RB, Boeira SP, Jesse CR, Haas SE. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by beta-amyloid 1-42 peptide in aged female mice. Brain Res. 2019;1721:146325. doi:10.1016/j.brainres.2019.146325
- Carradori D, Balducci C, Re F, et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomedicine. 2018;14:609–618. doi:10.1016/j.nano.2017.12.006
- Parikh A, Kathawala K, Li J, et al. Curcumin-loaded self-nanomicellizing solid dispersion system: part ii: in vivo safety and efficacy assessment against behavior deficit in Alzheimer disease. Drug Deliv. 2018;8:1406–1420. doi:10.1007/s13346-018-0570-0
- Hou K, Zhao J, Wang H, et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat Commun. 2020;11:4790. doi:10.1038/s41467-020-18525-2
- Cano A, Ettcheto M, Chang JH, et al. Dual-drug loaded nanoparticles of epigallocatechin-3-gallate (egcg)/ascorbic acid enhance therapeutic efficacy of egcg in a appswe/ps1de9 Alzheimer’s disease mice model. J Control Release. 2019;301:62–75. doi:10.1016/j.jconrel.2019.03.010
- Kim D, Kwon HJ, Hyeon T. Magnetite/ceria nanoparticle assemblies for extracorporeal cleansing of amyloid-beta in Alzheimer’s disease. Adv Mater. 2019;31:e1807965. doi:10.1002/adma.201807965
- Aso E, Martinsson I, Appelhans D, et al. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomedicine. 2019;17:198–209. doi:10.1016/j.nano.2019.01.010
- Cheng CH, Lin KJ, Hong CT, et al. Plasmon-activated water reduces amyloid burden and improves memory in animals with Alzheimer’s disease. Sci Rep. 2019;9:13252. doi:10.1038/s41598-019-49731-8
- Heydari S, Hedayati CM, Saadat F, et al. Diphtheria toxoid nanoparticles improve learning and memory impairment in animal model of Alzheimer’s disease. Pharmacol Rep. 2020;72:814–826. doi:10.1007/s43440-019-00017-w
- Palle S, Neerati P. Quercetin nanoparticles attenuates scopolamine induced spatial memory deficits and pathological damages in rats. Bull Fac Pharm Cairo Univ. 2017;55:101–106. doi:10.1016/j.bfopcu.2016.10.004
- Camelio AM, Johnson TC, Siegel D. Total synthesis of celastrol, development of a platform to access celastroid natural products. J Am Chem Soc. 2015;137(37):11864–11867. doi:10.1021/jacs.5b06261
- de Santana C, Asfora JJ, Cotias CT. Primeiras observações sobre o emprego da maitenina em pacientes cancerosos. Rev Inst Antibiot. 1971;11:37–49.
- Melo A, Jardim M, De Santana C, Lacet Y, Lobo Filho J. First observations on the topical use of Primin, Plumbagin and Maytenin in patients with skin cancer. Revista Do Instituto de Antibioticos. 1974;14(1–2):9–16.
- He Q-W, Feng J-H, Hu X-L, et al. Synthesis and biological evaluation of celastrol derivatives as potential immunosuppressive agents. J Nat Prod. 2020;83(9):2578–2586. doi:10.1021/acs.jnatprod.0c00067
- Zhou J, Hu T, Gao L, et al. Friedelane‐type triterpene cyclase in celastrol biosynthesis from Tripterygium wilfordii and its application for triterpenes biosynthesis in yeast. N Phytologist. 2019;223(2):722–735. doi:10.1111/nph.15809
- Kishi A, Morikawa T, Matsuda H, Yoshikawa M. Structures of new friedelane-and norfriedelane-type triterpenes and polyacylated eudesmane-type sesquiterpene from Salacia chinensis L INN. (S. prinoides DC., Hippocrateaceae) and radical scavenging activities of principal constituents. Chem Pharm Bull (Tokyo). 2003;51(9):1051–1055. doi:10.1248/cpb.51.1051
- Xu S, Fan R, Wang L, et al. Synthesis and biological evaluation of celastrol derivatives as potent antitumor agents with STAT3 inhibition. J Enzyme Inhib Med Chem. 2022;37(1):236–251. doi:10.1080/14756366.2021.2001805
- Morikawa T, Kishi A, Pongpiriyadacha Y, Matsuda H, Yoshikawa M. Structures of new friedelane-type triterpenes and eudesmane-type sesquiterpene and aldose reductase inhibitors from Salacia c hinensis. J Nat Prod. 2003;66(9):1191–1196. doi:10.1021/np0301543
- Shang -F-F, Wang JY, Xu Q, et al. Design, synthesis of novel celastrol derivatives and study on their antitumor growth through HIF-1α pathway. Eur J Med Chem. 2021;220:113474. doi:10.1016/j.ejmech.2021.113474