Abstract
Purpose
To investigate roxadustat’s preventive effects on hypoxia damage in the quick ascent to high altitude.
Methods
The roxadustat (7.8 mg/kg, 15.6 mg/kg, and 31.2 mg/kg) and control groups of BALB/C mice were distributed at random. To evaluate roxadustat’s anti-hypoxic effectiveness at the recommended dose, an atmospheric pressure closed hypoxic experiment was used. Wistar rats were randomly assigned to groups that received normal oxygen, hypoxic, acetazolamide, or roxadustat in order to evaluate the protective effects against hypoxic damage. Animal blood was obtained for arterial blood-gas analysis, inflammatory factors, and the identification of oxidative stress indicators. Animal tissues were removed for pathological investigation.
Results
In each group, the mice’s survival time was noticeably extended compared to the normal oxygen group. The medium dose had the best time extension rate at 19.05%. Blood SatO2 and PaO2 were significantly higher in the roxadustat group compared to the hypoxic group. Erythrocyte content, hemoglobin content, and hematocrit were also significantly higher. Plasma levels of IL-6, TNF-α, and IFN-γ were also significantly lower in the roxadustat group. Roxadustat can also improve the level of oxidative stress in the tissues of hypoxic rats. According to the results of HE staining, roxadustat could greatly lessen the harm done to rat heart, brain, lung, liver, and kidney tissue as a result of hypoxia.
Conclusion
Roxadustat can greatly reduce inflammation, oxidative stress, and tissue damage brought on by hypoxia, showing that it can significantly enhance the body’s ability to adapt to high altitude exposure.
Introduction
Hypoxia damages erythrocytes, vascular endothelial cells, and other tissues, such as the lung, brain, and myocardial. High-altitude cerebral edema (HACE) or high-altitude pulmonary edema (HAPE), which can be fatal, may occur from this. These conditions are brought on by an accumulation of reactive oxygen species (ROS), the production of inflammatory mediators, and damage to erythrocytes.Citation1
The Phase III clinical trial for roxadustat, an inhibitor of the hypoxia-inducible factor-proline hydroxylase (HIF-PHI) used to treat renal anemia, was completed in China on December 18, 2018. Roxadustat was authorized.Citation2 Proline hydroxylase (PHD) is the rate-limiting enzyme for the decomposition of hypoxia-inducible factor (HIF), a regulator of erythropoietin (EPO). EPO promotes the erythropoiesis process.Citation3 Roxadustat stabilizes HIF protein expression to encourage the expression of EPO by reducing the rate at which PHD degrades HIF.Citation4,Citation5 Hemoglobin, which is abundant in erythrocytes and is essential for oxygen combination, consumption, transit, and release, is present in high concentrations.Citation6 By encouraging EPO production and raising erythrocyte and hemoglobin content, roxadustat can ameliorate hypoxemia and alleviate hypoxia in hypoxic circumstances.
The pharmacodynamics of roxadustat were initially investigated through atmospheric pressure closed hypoxic and acute altitude field hypoxia experiments. In the current study, we conducted a randomized controlled experimental study based on the experimental animals to evaluate, the anti-hypoxia effect of roxadustat.
Research Design and Methods
Ethical Statement
This study was approved by the Scientific Research Management Ethics Committee of the 940th Hospital of the Joint Logistic Support Force of the People’s Liberation Army (Approval No. 2022KYLL155). All animal work was approved by the Animal Care and Treatment Committee of the 940th Hospital of the Joint Logistic Support Force of the People’s Liberation Army (Lanzhou, China). In the use of laboratory animals and research adhere to the 3R principle (Replacement, Reduction, and Refinement), optimize the design of good experiments, standardize the feeding environment and way to ensure the reliability, accuracy and scientific nature of experimental data.
Chemical and Reagents
Roxadustat Capsules (Specification: 20 mg × 3 tablets, AstraZeneca Pharmaceutical Co., Ltd.); Normal Saline (500 mL: 4.5 g, Shijiazhuang Siyao Pharmaceutical Co., Ltd. Batch No. 2007122002); Heparin (Shanghai First Biochemical Pharmaceutical Co., Ltd.); Acetazolamide (Purity >98.0%, Shanghai Yuanye Biotechnology Co., Ltd. CAS#59-66-5); Rat TNF-α Elisa kit, Rat IL-6 Elisa kit, MDA kit (Nanjing Jincheng Institute of Bioengineering); SOD kit (Nanjing Jiancheng Institute of Biological Engineering); GSH Kit (Nanjing Jiancheng Institute of Biological Engineering); 4% Paraformaldehyde (500 mL, Wuhan Xaver Biotechnology Co., Ltd.).
Animals and Experiment Design
Thirty-two male BALB/c mice, weighing 18–22 g each, were supplied for the study by the 940th hospital (SYXK (Military) 2020–0032). Thirty-two mice were subsequently split into 4 groups (n=8) at random: control group, low-dose group with roxadustat (7.8 mg/kg), medium-dose group (15.6 mg/kg), and high-dose group (31.2 mg/kg).
We acquired 24 healthy Wistar male rats, weighing 18–22 g each, from Liaoning Changsheng Biotechnology Co., Ltd. (SCXK (Liaoning) 2020–0001). The rats were randomly assigned to one of the four groups: the normal oxygen group, the hypoxic group, the acetazolamide group (22.5 mg/kg), and the roxadustat group (10.8 mg/kg) (n=6).
Atmospheric Pressure Closed Hypoxic Experiment
Thirty-two BALB/c mice were administered intragastrically for one week, and the equivalent dose of roxadustat was 15.6 mg/kg by the body surface area conversion method (conversion coefficient of mice = (body size coefficient mouse ×W2/3 mouse)/(body size coefficient man ×W2/3 human)), according to the clinical dose of humans. Roxadustat low, medium, and high dose groups were given 0.5 times, 1 time, and 2 times the equivalent dose, namely 7.8 mg/kg, 15.6 mg/kg, and 31.2 mg/kg, dissolved in normal saline and assisted with 0.5% carboxymethylcellulose sodium (CMC-Na) and 0.1% tween-80. The control group was given the same amount of solvent. The experiment was started 1 hour after the fourth administration. Forty mice were put into 250 mL jar (put filter paper and sodium lime into the bottle in advance, smear vaseline evenly on the bottle mouth and stopper). Per mouse into the bottle and screw down the lid as began to hypoxic time, stop breathing in mice, legs no longer tic as a time of death, statistics the survival time of mice and prolong the rate.
Experiment on Field Anoxia at a High Altitude
Rats from Lanzhou to gavage in advance to medicine 3 times, the fourth day morning by constant temperature after dosing van rats to Xining airport, and then accelerated to Yushu Tibetan autonomous prefecture in Qinghai province high altitude field laboratory (33.1 °N, 96.7 °E, at an altitude of 4010 m. The temperature was 10°C, the humidity was 80%), and the water was freely supplemented with jelly on the way. After arriving in Yushu, the rats underwent acute hypoxia for 3 days. On the fourth day, blood was collected from the ocular venous plexus, anaesthesia with chloral hydrate, and blood was collected from the abdominal aorta. The myocardial, liver, brain, lung, and renal tissues were collected for index determination.
Determination of Blood Routine Index
One hour after administration, 0.5 mL of orbital venous plexus blood was collected from the centrifuge tube rinsed with heparin, and immediately put into an automatic hematology analyzer to measure relevant indexes of blood routine.
Measure Inflammatory Factor Index and Erythropoietin
One hour after administration, 0.5 mL of orbital venous blood was collected in a heparin-rinsed centrifuge tube, centrifuged at room temperature at 3500 r/min for 10 min. The supernatant was taken and stored in a liquid nitrogen tank, which was transported to Lanzhou in strict accordance with IL-6, IFN-γ, TNF-α and EPO ELISA kit for operation.
Measure Blood Gas Index
After anesthesia, the abdominal cavity of rats was opened and 0.5 mL of abdominal aortic blood was collected, which was immediately determined by a blood gas analyzer.
Changes in Oxidative Stress Indicators
After collecting the abdominal aortic blood from rats, the myocardial, liver, brain, lung, and renal tissues were extracted completely on ice. The blood was washed in pre-cooled normal saline, and the water was drained by filter paper before being stored in liquid nitrogen tanks. During the determination, the tissues were weighed and added with pre-cooled normal saline (M:V=1:9) in proportion, fully ground in a homogenizer, centrifuged at 4°C at a speed of 4000 r/min for 10 min. The supernatant was taken to obtain 10% tissue homogenate. The homogenate was determined in strict accordance with the instructions of the SOD, MDA and GSH kit.
HE Dyed
After collecting the abdominal aortic blood from rats, the myocardial, liver, brain, lung and renal tissues of 2 rats in each experimental group were extracted completely on ice. The blood stains were washed in pre-cooled normal saline and immediately fixed in 4% paraformaldehyde. The tissues were dehydrated, embedded, sliced, and stained with HE.
Statistical Analysis
SPSS 21.0 software was used for statistical analysis, one-way analysis of variance, and an independent sample T-test were used for analysis. The statistical results were expressed by mean ± standard deviation, P<0.05 is a statistical difference.
Results
Effect of Roxadustat on Survival Time of Mice under Atmospheric Pressure
As shown in , the survival time of mice treated with roxadustat at atmospheric pressure was significantly increased. The survival time of low, medium, and high dose roxadustat groups were significantly prolonged than that of the control group, with an increased rate of 13.73% (32.72±5.24 vs 28.77±1.91, P<0.05), 19.05% (34.25±3.64 vs 28.77±1.91, P<0.01) and 14.15% (32.84±3.40 vs 28.77±1.91, P<0.05). The results indicate that roxadustat can increase the survival time of mice in atmospheric hypoxia, and the effect of medium-dose is the best ().
Table 1 Effects of Different Doses of Roxadustat on the Survival Time of Mice. Error Bars Indicate SD (n=8/per Group )
Effects of Roxadustat on Blood Gas in Field Hypoxia Rats at High Altitude
After entering the high altitude area for a period of time, the body of normal rats will return to the steady state through a series of compensatory adjustment due to the stimulation of hypoxia, such as the appropriate increase of hemoglobin, the decrease of blood oxygen saturation and oxygen partial pressure, etc. As shown in , compared with normal oxygen group, blood SaO2, PaO2 in the hypoxic group were significantly decreased (P<0.01), and Hct, pH and HGB contents were significantly increased (P<0.01). The SaO2 and PaO2 in the roxadustat group were significantly increased than that of the hypoxic group by 3.21% (94.00±0.72 vs 91.08±0.46, P<0.01) and 11.56% (65.93±2.84 vs 59.10±1.64, P<0.01). The Hct, pH and HGB contents were significantly decreased than that of the hypoxic group by 12.46% (36.33±0.52 vs 41.50±0.55, P<0.01), 0.27% (7.42±0.03 vs 7.44±0.01, P<0.01) and 11.85% (121.50±0.84 vs 137.83±2.14, P<0.01) (). The results showed that roxadustat could improve blood gas index to prevent overcompensation and adapt the body to hypoxic environment.
Table 2 Comparison of Blood Gas Indexes in Each Group. Error Bars Indicate SD (n=6/per Group )
Effects of Roxadustat on Blood Routine in Field Hypoxia Rats at High Altitude
Hypoxia at high altitude will affect the physiological indexes of rats, and blood routine is an important mean to analyze whether the physiological indexes are abnormal. As shown in , compared with normal oxygen group, RBC and HGB in the hypoxic group were significantly decreased (P<0.01), and MCV was significantly increased (P<0.01). The RBC, HGB, and HCT in the roxadustat group were significantly increased than that of the hypoxic group by 7.99% (8.65±0.19 vs 8.01±0.14, P<0.01), 7.62% (10.87±0.15 vs 10.10±0.24, P<0.01), and 5.09% (50.13±0.78 vs 47.70±0.81, P<0.01) (). Chronic hypoxia can lead to chronic mountain sickness. The abnormalities of RBC, HGB and HCT in blood routine are important indicators to determine whether chronic mountain sickness will develop. The results indicate that roxadustat can significantly improve the blood routine indexes of acute altitude hypoxic rats. Although both blood gas and routine blood tests tested for HGB, blood gas analysis for arterial blood and routine blood analysis for venous blood. While the HGB data are different, the overall trend is consistent.
Table 3 Comparison of Blood Routine Indexes in Each Group. Error Bars Indicate SD (n=6/per Group )
Effects of Roxadustat on Plasma Inflammatory Factors and EPO in Field Hypoxia Rats at High Altitude
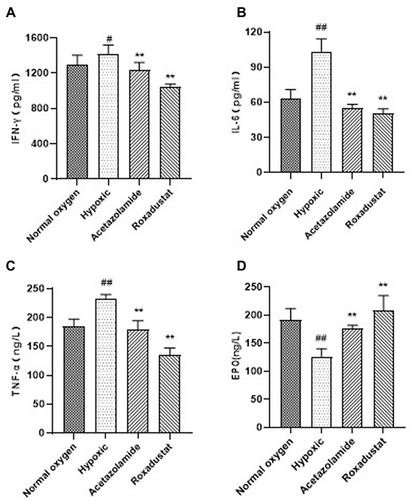
Inflammation is regulated by many factors in the body, one of which is hypoxia. Hypoxia can cause inflammation of tissue cells, excessive inflammation will also cause cell death, thus damaging the body tissue. After 3 days of hypoxia, the results of plasma inflammatory factors showed that the levels of IFN-γ, IL-6 and TNF-α in the hypoxia model group were significantly higher than those in the normal oxygen group. The content of IFN-γ, IL-6 and TNF-α in the roxadustat group significantly decreased than that of the hypoxic group by 26.66% (1038.97±38.44 vs 1416.62±101.79, P<0.01), 51.44% (50.28±4.13 vs 103.54±10.63, P<0.01), 42.06% (135.18±11.81 vs 233.30±6.67, P<0.01) (). EPO is produced by renal tubule interstitial cells to increase the number of erythrocytes during hypoxia. Compared with normal oxygen group, the content of EPO was significantly decreased (P<0.01). The content of EPO was significantly increased than that of the hypoxic group by 64.40% (207.54±27.09 vs 126.24±13.79, P<0.01) (). The results showed that roxadustat reduced hypoxic-induced inflammation and increased EPO levels to promote erythrocyte production.
Figure 1 Effects of roxadustat on inflammatory factor and EPO in rats plasma. The content of IFN-γ (A), IL-6 (B), TNF-α (C) and EPO (D). Error bars indicate SD (n=6/per group).
Effects of Roxadustat on Oxidative Stress in Field Hypoxia Rats at High Altitude
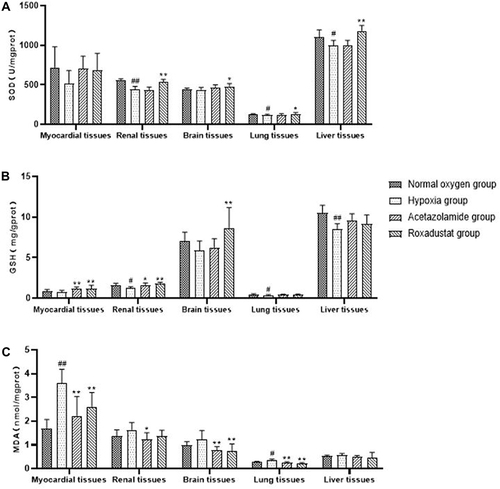
In order to explore whether roxadustat has a protective effect on hypoxic rat tissues, we measured the changes of SOD activity, GSH and MDA content in each tissue of rats. After 3 days of hypoxia, oxidative stress results showed that SOD activity in myocardial, renal, lung and liver tissues of rats in hypoxic group was significantly decreased compared with that in normal oxygen group (). MDA contents in myocardial, renal, brain and lung were significantly increased in hypoxic group (). The contents of GSH in myocardial, renal, brain, lung and liver tissues was significantly decreased in hypoxic group (). Compared with the hypoxic group, the SOD activity of brain, lung, liver, and renal tissue in the roxadustat group was significantly increased by 9.64% (480.16±37.19 vs 437.96±29.84, P<0.05), 21.61% (134.15±17.06 vs 110.31±9.12, P<0.05), 18.68% (1182.57±69.81 vs 996.40±66.30, P<0.01) and 34.98% (535.28±34.27 vs 396.55±60.13, P<0.01) (). The contents of GSH in myocardial, brain, and renal tissue were significantly increased by 53.85% (1.20±0.41 vs 0.78±0.20, P<0.01), 47.03% (8.66±2.25 vs 5.89±1.05, P<0.01) and 48.80% (1.86±0.13 vs 1.25±0.16, P<0.01) (). The MDA contents in the myocardial, brain, and lung tissue was significantly decreased by 27.70% (2.61±0.60 vs 3.61±0.51, P<0.01), 40.32% (0.74±0.30 vs 1.24±0.37, P<0.01), and 36.11% (0.23±0.03 vs 0.36±0.05, P<0.01) (). The results suggest that roxadustat can ameliorate oxidative stress injury induced by acute altitude hypoxia in rats.
Figure 2 Effects of roxadustat on SOD activity (A), GSH (B) and MDA (C) content in myocardial, renal, brain, lung and liver of hypoxic rats. Error bars indicate SD (n=6/per group).
Effect of Roxadustat on the Pathology of Field Hypoxia Rats at High Altitude
In the normal oxygen group, the cardiac fibers were properly organized and the nucleus remained unharmed (). The hypoxic group’s myocardial fibers were disorganized, with wavy fiber breaks at the arrow and inflammatory cell infiltration. Roxadustat group myocardial fibers were cleanly organized, inflammation was diminished, and cytoplasmic staining was consistent.
Figure 3 Pathological results of myocardial (A), brain (B), lung (C), liver (D), and renal (E) tissues in rats (HE, 400.0×).
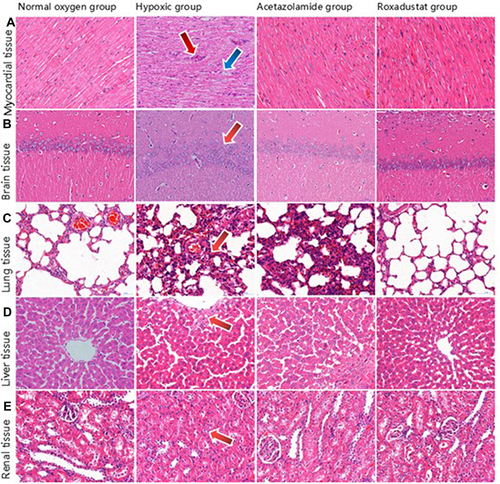
In the rats in the normal oxygen group, the pyramidal cells in the hippocampus of the cerebral tissue were neatly and densely packed, with normal cell structure (). In the hypoxic group, pyramidal cells in hippocampal region were disordered and loose at the arrow. There was no visible damage, and the cells in the hippocampus of the roxadustat group were packed neatly and thickly.
Rats in the normal oxygen group had distinct nuclei and thin-walled vacuolar alveoli in their lung tissue (). In the hypoxic group of arrowhead rats, the alveolar walls had thicker, incompletely developed structures, inflammatory cell infiltration, and fibrous hyperplasia at the arrow. The roxadustat group’s alveolar wall was thinner, the structure was normal, and the number of inflammatory cells was decreased.
The normal oxygen group’s liver tissue cells were organized in a cord-like pattern around the central venous canal (). The liver tissue’s nucleus was coyoted, vacuolated at the arrow, and pierced necrosis was seen in the hypoxic group. The liver tissue cells in the roxadustat group did not exhibit vacuolation or spot-like necrosis.
The normal oxygen group’s renal tissue’s cell structure was normal (). Renal tubule epithelial cells exhibited edema at the arrow, a narrow and convoluted lumen, and interstitial capillary dilatation in the hypoxic group. The glomeruli exhibit erythrocyte exudation. In the roxadustat group, there was reduced exudation of glomerular red blood cells and edema of renal tubule epithelial cells.
Discussion
The amount of oxygen that the alveoli could take up was fixed when the control and administered mice were in the same volume of the confined bottle for the atmospheric pressure confinement hypoxia experiment. The results showed that the high, medium, and low doses of roxadustat significantly prolonged the survival time of hypoxia-sensitive mice, with the medium dose having the highest survival rate, which was consistent with the human equivalent dose. Blood gas analysis was performed to detect arterial blood and routine blood analysis to detect venous blood. Blood gas analysis is commonly used in medicine to determine whether the body has an imbalance in acid–base balance or the degree of hypoxia. Abnormalities of RBC, HGB, and HCT in routine blood are important indicators to determine the occurrence of chronic plateau disease. The results showed that roxadustat could improve the degree of hypoxia in the body and thus prevent the appearance of chronic plateau disease. High-altitude hypoxia can induce the release of inflammatory factors, and conversely, inflammatory lesions can be hypoxic. Inflammatory factors can be used to indicate inflammatory damage in the mouse organism. MDA reflects the extent of lipid peroxidation triggered by oxygen radicals and thus reflects cellular damage, and SOD and GSH can improve cellular oxidative stress-induced cell death by scavenging toxic substances such as ROS and reactive nitrogen species produced in excess and stabilizing intracellular DNA and protein content. The results showed that roxadustat could reduce the release of tissue inflammatory factors and the production of oxidative stress damage by hypoxia, as also seen on histopathological sections. In conclusion, roxadustat alleviates hypoxia-induced damage in rat organisms and has an anti-hypoxic protective effect.
Roxadustat inhibits PH activity by mimicking the substrate of prolyl hydroxylase (PH) to maintain the balance between HIF production and degradation and is commonly used to treat anemic patients with chronic kidney disease (CKD) who are on dialysis therapy. In diabetic nephropathy, where inflammatory cytokines and reactive oxygen species (ROS) are increased,Citation7 and if proteinuria is increased in obese and insulin-resistant states,Citation8 it remains to be further investigated whether roxadustat can be used as an effective anti-inflammatory and antioxidant agent in the treatment of diabetic nephropathy.
Roxadustat could also potentially be used therapeutically to prevent retinopathy of prematurity, promote bone and tendon regeneration,Citation9 and treat bronchial dysplasia in premature infants.Citation10 By exposing rats to 7% oxygen, Yukiko Yasuoka et al found that roxadustat produced EPO in some tissues stimulated by hypoxia, specifically regulating EPO production in the kidney.Citation11 Hypoxia stimulates various adaptive responses, many of which are mediated through transcriptional complexes of the HIF family, directly upregulating the transcription of genes involved in erythropoiesis, angiogenesis, vasomotor tone, metabolic pathways, and processes related to cell reproduction and survival. What is the mechanism by which roxadustat counteracts high-altitude hypoxia? A review of the literature yielded the following findings.
Hypoxia-inducible factor 1 (HIF-1) consists of two subunits, HIF-1α and HIF-1β. Hif-1α is a major regulator of oxygen homeostasis, and its oxygen-regulated alpha chain binds to the constitutive aryl hydrocarbon receptor nuclear transporter. PHD is central to the oxygen-sensing pathway that leads to HIF-1α activation. The hydroxylation of iron and 2-oxoglutarate proline occurs in the presence of sufficient amounts of oxygen. The resulting hydroxyproline residues allow binding to the von Hippel-Lindau E3 ubiquitin ligase complex, leading to polyubiquitination of the HIF-1α chain on some lysine residues, which in turn leads to its complete disappearance.Citation12 Hypoxia inhibits the stability of PHD and HIF-1α. Roxadustat, as HIF-PHI, stabilizes HIF and inhibits its degradation, reversing the hypoxia-induced reduction in transcription of related genes, restoring the corresponding physiological response under normoxia, and reducing hypoxia-induced damage. Roxadustat can also increase cellular IKKβ activity and NF-κB activity by inhibiting PHD activity. NF-κβ directly regulates HIF-1α, an event that amplifies the ability of cells to respond to cytokines.Citation13
During hypoxia or inflammation, HIFs drive an increase in extracellular adenosine levels, and extracellular adenosine signaling plays an important role in reducing hypoxia-induced tissue damage and inflammation.Citation14 Inflammatory and hypoxic conditions increase the release of extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP), and HIF-1α drives the adenosine signaling molecules ATP and AMP. Extracellular adenosine triphosphate bis-phosphatase (CD39, which metabolizes ATP to AMP) and 5’-extracellular nucleotidase (CD73, which metabolizes AMP to adenosine) metabolizeCitation15 to produce adenosine that activates endothelial adenosine receptors.Citation16 Extracellular adenosine receptor (ARs)-mediated effects are protective of the heart and play a protective role in myocardial reperfusion injury in various species.Citation17–20 Myocardial ischemia is associated with oxygen supply deficit, and induction of CD39 by specific protein-1 (SP-1) and induction of CD73 and adenosine receptors by HIF-1α can adapt cardiac tissue to ischemia and treat myocardial ischemia and reperfusion injury.Citation21 Intestinal epithelial cells also have adenosine receptors, and increasing HIF-1α by PHD inhibitors or adenosine signaling by adenosine receptor agonists can contribute to inflammatory bowel disease, intestinal ischemia/reperfusion injury and colon cancer patients.Citation22 Adenosine signaling activates adenosine receptors in the alveolar epithelium and protects lung tissue during acute lung injury.Citation23 Administration of adenosine or adenosine receptor antagonists that inhibit oxygen sensor compounds, such as PHDs, thereby preventing HIF-1α hydroxylation and leading to HIF stabilization, is an attractive therapeutic approach. HIF regulates extracellular adenosine Whether this mechanism of HIF regulation of extracellular adenosine levels is related to the anti-hypoxic effect exerted by rosadustat needs further investigation.
Conceptually, there are two pathways that can target the stabilization of HIFs. Firstly, drugs that promote the stabilization of HIFs, mainly by inhibiting prolyl hydroxylase, such as HIF-PHI drugs like Roxadustat. Secondly, direct activation of HIF target genes, such as adenosine receptors. Our results show that Roxadustat increases blood RBC and Hb levels, reduces hypoxemia and inflammatory factor expression, decreases oxidative stress damage, and has pharmacological effects on hypoxic injury under high altitude conditions. HIF-PHIs can increase total iron binding and reduce inflammatory oxidative stress to treat patients with renal anemia with relatively few side effects.Citation24 So it is proven from both theoretical and experimental studies that roxadustat exerts anti-hypoxic effects. This paper provides evidence for further clinical applications.
Contacts to Participate
No human subjects were involved in this study.
Contacts to Publish
The work described has not been published before. This publication has been approved by all co-authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose in this study.
About Data Availability Statements
All data generated or analysed during this study are included in this published article.
Additional information
Funding
References
- Leaf DE, Goldfarb DS. Mechanisms of action of Acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102(4):1313–1322. doi:10.1152/japplphysiol.01572.2005
- Dhillon S. Roxadustat: first Global Approval. Drugs. 2019;79(5):563–572. doi:10.1007/s40265-019-01077-1
- Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int. 2017;21(Suppl1):S110–s124. doi:10.1111/hdi.12567
- Long G, Chen H, Wu M, et al. Antianemia Drug Roxadustat (FG-4592) Protects Against Doxorubicin-Induced Cardiotoxicity by Targeting Antiapoptotic and Antioxidative Pathways. Front Pharmacol. 2020;11:1191. doi:10.3389/fphar.2020.01191
- Deguchi H, Ikeda M, Ide T, et al. Roxadustat markedly reduces myocardial ischemia reperfusion injury in mice. Circ J. 2020;84(6):1028–1033. doi:10.1253/circj.CJ-19-1039
- Wang B, Zhang YB, Zhang F, et al. On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One. 2011;6(2):e17002. doi:10.1371/journal.pone.0017002
- Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res. 2013;2013:248563. doi:10.1155/2013/248563
- Mima A, Yasuzawa T, King GL, Ueshima S. Obesity-associated glomerular inflammation increases albuminuria without renal histological changes. FEBS Open Bio. 2018;8:664–670. doi:10.1002/2211-5463.12400
- Miao M, Wu M, Li Y, et al. Clinical potential of hypoxia inducible factors prolyl hydroxylase inhibitors in treating nonanemic diseases. Front Pharmacol. 2022;13:837249. doi:10.3389/fphar.2022.837249
- Kirschner KM, Kelterborn S, Stehr H, et al. Adaptation of the oxygen sensing system during lung development. Oxid Med Cell Longev. 2022;2022:9714669. doi:10.1155/2022/9714669
- Yasuoka Y, Izumi Y, Fukuyama T, et al. Effects of roxadustat on erythropoietin production in the rat body. Molecules. 2022;27(3):1119. doi:10.3390/molecules27031119
- Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi:10.1016/j.molcel.2008.04.009
- Cummins EP, Berra E, Comerford KM, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154–18159. doi:10.1073/pnas.0602235103
- Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol. 2017;123(5):1303–1320. doi:10.1152/japplphysiol.00101.2017
- Eltzschig HK, Weissmüller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi:10.1385/1-59745-113-4:73
- Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med. 2013;91(2):141–146. doi:10.1007/s00109-013-0999-z
- McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant. J Cardiovasc Pharmacol Ther. 2012;17(1):21–33. doi:10.1177/1074248410396877
- Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4(8):e6784. doi:10.1371/journal.pone.0006784
- Yang Z, Day YJ, Toufektsian MC, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111(17):2190–2197. doi:10.1161/01.Cir.0000163586.62253.A5
- Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J Mol Cell Cardiol. 2010;49(2):280–286. doi:10.1016/j.yjmcc.2010.01.018
- Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med. 2013;19(6):345–354. doi:10.1016/j.molmed.2013.02.005
- Bowser JL, Phan LH, Eltzschig HK. The Hypoxia-Adenosine Link during Intestinal Inflammation. J Immunol. 2018;200(3):897–907. doi:10.4049/jimmunol.1701414
- Eckle T, Hughes K, Ehrentraut H, et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 2013;27(8):3078–3089. doi:10.1096/fj.13-228551
- Mima A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: advantages and disadvantages. Eur J Pharmacol. 2021;912:174583. doi:10.1016/j.ejphar.2021.174583
