?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Evaluation of the potential antimutagenic activities of new compounds by Ames assay has been of great interest for the development of novel therapeutics for many diseases including cancer. Ten novel bichalcophenes with in vitro and in vivo broad spectrum activities against various microbial strains were investigated throughout the present study for their cytotoxic, antioxidant, and antimutagenic potential in a Salmonella reverse mutation assay system against sodium azide (NaN3) and benzo[a]pyrene (B[a]P). At nontoxic concentrations, all bichalcophenes alone or in combination with NaN3 (1 μg/plate) or B[a]P (20 μM) with S9 mix were not mutagenic. The bichalcophenes significantly reduced NaN3- and B[a]P-induced mutagenicity under pre-exposure and co-exposure conditions in a concentration-independent manner. However, the antimutagenic activity of bichalcophenes against B[a]P varied depending on the exposure regimen, being more effective under pre-exposure conditions. The antimutagenic activity was correlated with a high antioxidant activity that could promote the DNA repair system. Bichalcophenes are least likely to interfere with the microsomal bioactivation of B[a]P. Monocationic bichalcophenes were superior to the corresponding mononitriles as antimutagenic agents against both mutagens investigated, possibly due to the higher nucleophilic centers they have which could bind and protect the bacterial DNA. Three monocationic compounds were shown to have a strong anticancer activity against the 58 cell line. Based on the results of the present investigation, monocationic compounds (1, 4, and 5B) will be selected for further time consuming and costly chemoprevention studies in animal models.
Introduction
Human exposure to genotoxic substances present in food and the environment is inevitable. Therefore, humans are provided with many defenses. The failure of these defenses could lead to DNA damage and failure of repair systems, leading to cancer. Novel potential drugs are usually screened for their possible mutagenic/antimutagenic activities in many systems including the Salmonella typhimurium microsome assay. This fruitful assay is very efficient in screening for anticancer activities of novel compounds and proved a strong relation between the mutagenicity in Salmonella and the carcinogenicity in animal models.Citation1
Several pentamidine derivatives have antiproliferative activity against several human cancer cell lines.Citation2,Citation3 However, these exhibit a number of serious side effects, toxicity problems, and poor availability.Citation4 Recently, a series of pentamidine-related 4-substituted phenyl bichalcophenes and aza-analogs () were synthesized,Citation5 and shown to exhibit structurally related broad-spectrum antimicrobial activities against Gram positive and Gram negative bacteria.Citation6 In addition, these novel bichalcophenes were not toxic to the mouse hepa1c1c7 cells up to 200 μM and the IC50 values were >450 μM suggesting their possible clinical use. Two of these bichalcophenes (designated as 1A and 1B in the present study) were found to elevate the enzymatic and non-enzymatic antioxidants in mice and caused a conversion of methicillin-resistant bacteria into methicillin-susceptible bacteria.Citation7
We pursued an investigation of antimutagenic activities of novel bichalcophenes and aza-analogs against two mutagens that act in different ways: (1) sodium azide (NaN3) which is a powerful direct mutagen that does not require a metabolic activation; and (2) benzo[a]pyrene (B[a]P), a known ubiquitous environmental carcinogen, which is one of the polycyclic aromatic hydrocarbons that needs metabolic activation mainly by cytochrome P4501A1.Citation8 Benzo[a]pyrenediolepoxide and bibenzo[a,l]pyrene are the major metabolites of B[a]P responsible for cancer.Citation9,Citation10 The mutagenic activity of NaN3 and B[a]P were inhibited by the addition of several antioxidants.Citation10–Citation13 Therefore, we conducted this study to evaluate the antimutagenic and antioxidant activities of novel bichalcophenes using the Salmonella reverse mutation assay system against NaN3 and B[a]P as a prelude to the time consuming and costly in vivo evaluation of anticancer properties of these compounds.
Materials and methods
The S. typhimurium TA1535, a histidine-mutant bacterial strain was obtained from the American Type Culture Collection (Manassas, VA, USA). NaN3, B[a]P, and nicotinamide adenine dinucleotide phosphate reduced (NADPH) were obtained from Sigma-Aldrich (St Louis, MO, USA). Ten bichalcophene derivatives () were kindly provided by Professor MA Ismail to be used throughout the present study. The S9 mix consisted of filter-sterilized NADPH (1.25 mM) and hepatic S9 fraction (4 mg protein/mL) prepared from male Sprague Dawley rats treated with a single dose (25 mg/kg, intraperitoneal in corn oil) of Aroclor 1254 for three days.
Cytotoxicity assays
The cytotoxicity assays were performed using two exposure conditions: bacterial growth in broth and bacterial colony growth on nutrient agar plates. In the former, an overnight growing S. typhimurium TA1535 was grown in Oxoid Nutrient Broth No 2 in 96-well plates in the presence and absence of bichalcophenes (12.5–100 μM) for 15 hours at 37°C. The final optical density of each well was recorded at 570 nm. In the latter, the experiment was designed with conditions that mimic those of the revertant mutagenesis/antimutagenesis assay. A preliminary experiment was performed to identify the optimal bacterial dilution using three dilutions (10−6, 10−7, and 10−8). A 10−7 dilution was the appropriate dilution; therefore, it was selected to proceed with. Briefly, 100 μL of 10−7 dilution of the overnight growing bacterial culture in Luria Broth medium was incubated with each of the bichalcophenes (0, 5, 10, and 20 μM) and mixed with 2.5 mL of warm 0.6% top agar (NaCl/agar). The mix was then added to nutrient agar plates and the plates were incubated at 37°C for 24 hours. After the incubation period, colonies on triplicate plates were counted and compared to control plates containing no bichalcophenes. Concentrations investigated hereafter for all bichalcophenes were 10 and 20 μM except for compounds 1B and 5B, in which the concentrations used were 5 and 10 μM.
Bichalcophenes cytotoxicity in combination with selected mutagens
To rule out the possible toxic effect exerted by a combination of the mutagens used and bichalcophene derivatives in the mutagenicity/antimutagenicity evaluation assays, an evaluation of the number of colonies/plate was performed. A 100 μL sample of 10−7 dilution of overnight growing S. typhimurium TA1535 was incubated with each bichalcophene at 37°C for 30 minutes on a shaking incubator in the presence of 1 μg/plate of NaN3 or 20 μM of B[a]P and S9 mix in 400 μL of phosphate buffer. Control experiments were carried out simultaneously – the S9 mix alone had no effect on the bacterial viability. Colonies on triplicate plates were counted after 24 hours.
Mutagenicity assays
The Ames Salmonella/microsome mutagenicity assay was performed for screening the mutagenic potential of the bichalcophenes as described elsewhere.Citation1 Briefly, 100 μL of 15-hour overnight growing S. typhimurium TA1535 (1 × 109 colony forming units/mL) in Luria Broth medium was pre-incubated with each bichalcophene within the non-toxic range of concentrations at 37°C for 30 minutes in phosphate buffer (pH 7.4) in the presence of S9 mix. The incubate (0.6 mL) was then added to top agar containing a growth-initiating concentration (50 μM) of histidine/biotin, and poured on to minimal glucose agar plates. Revertant colonies were counted after incubation at 37°C for 48 hours. Spontaneous revertant colonies arising on plates containing neither mutagens nor bichalcophenes were also counted. Revertant colonies seen with 20 μM B[a]P with S9 mix were used as a positive control. All assays were performed in triplicates.
Antimutagenicity assays
For assessing the antimutagenic properties of bichalcophenes against NaN3 or B[a]P, pre-exposure and co-exposure assays were carried out simultaneously by a modified method of Maron and Ames.Citation1 Under pre-exposure conditions, each bichalcophene was incubated with S. typhimurium TA1535 at 37°C for 30 minutes before the addition of B[a]P (20 μM) or NaN3 (1 μg/plate). Co-exposure assays were performed by incubating the bacteria, the mutagen, and each bichalcophene together at 37°C for 30 minutes prior to plating on minimal glucose agar plates. In all assays, positive and negative controls were used. All antimutagenesis determinations were performed in triplicate. Revertant colonies were counted after 48 hours of incubation and the antimutagenic potential of the tested compounds was expressed as a percentage of reduction in mutagenicity,Citation14 and calculated according to the following equation:
A 20% or less reduction means no antimutagenic activity, 20%–40% reduction means a moderate activity, and 40% or more reduction means a strong antimutagenic activity.
The mutant frequency or mutation rate was then calculated from the mutant colonies/viable colonies for both exposure conditions for the mutagens investigated. All procedures were approved by the University of King Faisal Committee of Scientific Research Ethics.
Determination of total antioxidant activity
The antioxidant capacity of bichalcophenes was measured spectrophotometrically using a phosphomolybdenum method,Citation15 based on the reduction of Mo(VI) to Mo(V) and the subsequent formation of specific green phosphate/Mo(V) compounds. A 0.3 mL aliquot of sample solution (20 μM final concentration) was combined with 2.7 mL of the reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The sample was capped and incubated in a boiling water bath at 95°C for 90 minutes. After cooling to room temperature, the absorbance was measured at 695 nm. Stock solution of ascorbate was freshly prepared and used as a standard antioxidant.
Assay of reducing power
The reducing power of bichalcophenes was determined according to the method described by Oyaizu.Citation16 The samples (20 μM final concentration) were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (2.5 mL, 10 g/L). The mixtures were incubated at 50°C for 20 minutes. Trichloroacetic acid (2.5 mL, 10%) was added to each mixture and centrifuged at 3000 g for 3 minutes. Finally, the supernatants (2.5 mL) were mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). The absorbance of the solution was measured at 700 nm. Ascorbate solution was freshly prepared and used as a positive control.
Statistical analysis of data
Statistical analyses were performed using ANOVA, followed by Fisher’s protected least significant difference multiple range test. Differences were considered significant at P values of <0.05.
Results and discussion
In the current study, the mutagenic and antimutagenic properties of novel bichalcophenes against NaN3 and B[a]P were investigated using the Salmonella reverse bacterial assay. Since mutation is an early key step in cancer development, the Salmonella reverse assay is of great importance in the detection of mutagenic/carcinogenic and antimutagenic/anticarcinogenic agents in the pipeline of drug discovery and development.
Effect of bichalcophenes on the viability of S. typhimurium
It was crucial to first examine the effect of bichalcophenes on the viability of bacteria to validate the antimutagenic data and be assured that the reduction in the number of mutant colonies is not due to the inhibition of the growth of bacteria due to overt toxicity. The non-toxic/non-inhibitory concentrations of the tested bichalcophene derivatives against S. typhimurium TA1535 were determined using two independent exposure conditions. In the liquid broth method, with the exception of compounds 1B and 5B, all compounds up to a 100 μM had no significant toxic effects on the viability of bacteria (). Compounds 1B and 5B showed a concentration-dependent reduction in the bacterial growth. Compound 1B significantly reduced the viability at 25, 50, and 100 μM and compound 5B had a significant cytotoxic effect at 50 and 100 μM. In a previous study,Citation6 the minimum inhibitory concentration (MIC) values of all bichalcophenes were at 32–64 μM against Escherichia coli, another Gram negative bacteria. Compounds 1B and 5B discerned themselves and the MIC values for these compounds were 16 and 8 μM, respectively. It was shown that the monoamidines (B compounds) are more active as antibacterial agents than the corresponding mononitriles (A compounds) and the presence of thiophene rings as in compound 5B adds more activity, while the presence of pyridyl rings (as in compounds 2B and 4B) negatively affects activity.Citation6 Compound 2A at 12.5 μM was unique in causing a significant elevation (∼40%) in the viability of bacteria; whether it acts as a nutrient to the bacteria, a source of nitrogen, or promotes the maximal growth through a different mechanism is unknown. From the previous data and to avoid any cytotoxicity concerns, we decided to reduce the concentrations to 10 and 20 μM for all bichalcophenes and 5 and 10 μM for compounds 1B and 5B for the following experiments. In the colony/plate assay, none of the bichalcophene derivatives exhibited any cytotoxicity (diminution in the number of colonies) at 10 and 20 μM for all compounds and 5 and 10 μM for compounds 1B and 5B (). On the other hand, a significant increase in the number of colonies was recorded with compound 2A (193%) at 10 μM and with compound 4A (139%) at 20 μM. Compounds 2A and 4A have similar chemical structures – both are mononitrile aza derivatives of bichalcophenes.
Table 1 Cytotoxicity of bichalcophenes to Salmonella typhimurium TA1535 in liquid medium
Table 2 Effect of bichalcophenes on Salmonella typhimurium TA1535 viability as assessed by colony formation on plate
Effect of combination of bichalcophenes and mutagens on the viability of S. typhimurium
Two mutagens were selected for antimutagenic studies: NaN3 and B[a]P. Both act through different pathways. NaN3 is a direct mutagen that does not need metabolic activation and B[a]P is a known polycyclic aromatic hydrocarbon that needs metabolic activation by P4501A1.Citation8 The S. typhimurium TA1535 strain contains the base-pair substitution mutation hisG46,Citation17 which is known to be more responsive to sodium azide than other direct mutagens.Citation1
To rule out any possible toxic effects developed from the concurrent exposure of bacteria to bichalcophenes and any of the tested mutagens, S. typhimurium TA1535 cells were grown in the presence of either NaN3 (1 μg/plate) or B[a]P (20 μM) with S9 mix alone or in combination with non-toxic concentrations of the bichalcophenes. None of the bichalcophenes were toxic when used in combination with NaN3 (). A statistically significant increase in the number of colonies was observed by a combination of compound 1B with NaN3 at 5 μM. When bacteria were exposed to B[a]P, S9 mix and bichalcophenes and evaluated for colony formation the results were similar to those seen with bichalcophenes alone and the number of colonies were not affected reflecting the nontoxic effects of the combination () and validating the results of the latter experiments.
Table 3 Effect of bichalcophenes and sodium azide (NaN3) on Salmonella typhimurium TA1535 viability as assessed by colony formation on plate
Table 4 Effect of bichalcophenes, benzo[a]pyrene (B[a]P), and S9 mix on Salmonella typhimurium TA1535 viability as assessed by colony formation on plate
Mutagenicity of bichalcophenes
On examining the mutagenic activity at or well below the non-toxic concentrations, none of the bichalcophenes caused a doubling in the number of colonies over the spontaneous number in the presence of S9 mix (); ie, all of the bichalcophenes were not mutagenic. The experiment was repeated in the absence of S9 mix and very similar results were obtained (data not shown). Similar compounds such as pentamidine derivatives, in spite of their binding to DNA minor grooves, have been shown to cause no mutagenic activity in S. typhimurium strains TA98 and TA100 with and without metabolic activation.Citation18,Citation19
Table 5 Determination of mutagenic activity of bichalcophenes in Salmonella typhimurium TA1535 in presence of S9 mix
Antimutagenic activity of bichalcophenes against NaN3 and B[a]P
The potential antimutagenic activity of bichalcophenes was evaluated using a modified Ames assay in pre-exposure and co-exposure treatments. All bichalcophenes showed strong (>40%) antimutagenic activity against the NaN3-induced mutagenicity (). In a few instances, the reduction in mutagenicity was concentration-dependent. The absence of concentration dependency could reflect a saturation effect. The monocationic bichalcophenes were more effective in reducing the azide-induced mutagenicity than the corresponding mononitriles (3B > 3A and 5B > 5A). Compound 4B was superior to 4A only at the pre-exposure regimen while 4A was better than 4B in reducing the azide-induced mutagenicity at the co-exposure treatment ().
Table 6 Determination of anti-mutagenic activity of bichalcophenes in Salmonella typhimurium TA1535 against sodium azide (NaN3; 1 μg/plate)
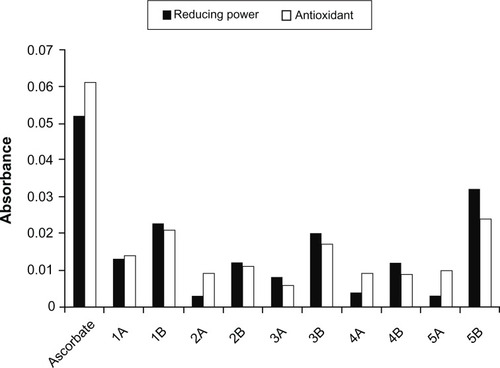
Upon evaluation of the antimutagenic potential of bichalcophenes against B[a]P-induced mutagenicity, compounds 1B and 5B resulted in significant reductions in the mutagenicity induced by B[a]P in pre-exposure and co-exposure treatments at 5 and 10 μM (). However, Compounds 3A and 5A failed to reduce the induced mutagenicity and caused no significant reduction in the number of revertant colonies. Pre-exposure was much more effective than the co-exposure protocol. Some of the bichalcophenes were effective only under pre-exposure conditions (2A, 4A, 2B, and 4B) at 10 and 20 μM. The reductions in mutagenicity were concentration-independent. Compound 1A significantly reduced the mutagenicity in pretreatment experiments only at 20 μM. Compound 3B significantly reduced the mutagenicity in pre-exposure at 10 and 20 μM and in co-exposure only at 20 μM. Again, monocationic bichalcophenes (1B, 3B, and 5B) were more effective than the corresponding mononitriles (1A, 3A, and 5A) in reducing B[a]P-induced mutagenicity (). Bichalcophenes could exert their antimutagenic activities through various mechanisms. One such mechanism is the direct binding and protection of DNA from the electrophilic mutagens or metabolites,Citation20 given that bichalcophenes are nucleophilic. The monocationic bichalcophenes provide more nucleophilic centers than mononitriles which could explain their superior antimutagenic activity. Another mechanism could be the elevation in the antioxidant milieu of the cells thus, promoting the DNA repair systems.Citation21 The total antioxidant activity and reducing power of bichalcophenes showed that compounds 1B, 3B, and 5B are in the lead with antioxidant activity of ∼30%–50% () of that of ascorbic acid at the same concentration. In our previous study, compound 1B showed a noticeable antioxidant activity in vivo and elevated the hepatic activities of glutathione reductase and glutathione peroxidase and reduced the elevated hepatic malondialdehyde and serum interleukin-6 in mice infected with methicillin-resistant Staphylococcus aureus.Citation7 A third mechanism could be the direct interaction with the mutagens/metabolites and preventing their damaging effects.Citation22 Since B[a]P is activated by CYP4501A1, one of the possible mechanisms would be the inhibition of this bioactivation. The antimutagenic activity of some heterocyclic aromatic amines was attributed to this mechanism.Citation23 However, if this is the scenario, we would expect to see no difference between the two exposure regimens (pre-exposure and co-exposure) but most bichalcophenes exerted their antimutagenic activity against B[a]P under pre-exposure conditions. In the pre-exposure protocol, bichalcophenes were incubated with the bacteria 30 minutes before the addition of B[a]P. Therefore, it is possible that the antimutagenic efficiency of bichalcophenes against B[a]P is due to the effects on the bacteria, not on the mutagen or mutagen metabolism.
Table 7 Determination of anti-mutagenic activity of bichalcophenes in Salmonella typhimurium TA1535 against benzo[a]pyrene (B[a]P; 20 μM) in presence of S9 mix
Figure 2 The reducing power and total antioxidant activity of bichalcophenes at 25 μM compared to ascorbate at the same concentration.
Mutation frequency is highly correlated to mutation rate. All bichalcophenes reduced the mutant frequency caused by NaN3 by 40%–95%. Monocationic bichalcophenes were superior to the corresponding mononitriles resulting in a 95% reduction in NaN3-mutant frequency at the higher concentration investigated under the co-exposure protocol (). However, the antimutagenic activity of bichalcophenes against B[a]P mutagenicity was less evident. Most of bichalcophenes were more effective only in the pre-exposure treatment and compounds 2A, 2B, and 4B were only effective under this treatment. Under these conditions, compounds 1B, 2B, 4B, and 5B, in addition to 2A and 4A, reduced the mutant frequency by 28%–63%; while in the co-exposure protocol, compounds 1B, 3B, 4B, and 5B reduced the frequency by 15%–53% (). Again monocationic (B compounds) derivatives were more effective than the analogous mononitriles ().
Table 8 Effects of bichalcophenes on sodium azide (NaN3) mutant frequency
Table 9 Effects of bichalcophenes on benzo[a]pyrene (B[a]P) mutant frequency
Conclusion
At non-toxic concentrations, bichalcophenes were not mutagenic to S. typhimurium. All bichalcophenes exerted strong antimutagenic activity against NaN3 and many showed antimutagenic activity against B[a]P, especially in the pre-exposure conditions. This activity is most likely due to the direct effect of bichalcophenes on the bacterial DNA or the antioxidant activity of bichalcophenes rather than the interference in the bioactivation and metabolism of the mutagens. Although there are some differences in specificity between the human and bacterial systems in the response to B[a]P mutagenic effects and in the processing of DNA adducts formed, this survey in the Salmonella system is of essential help as a platform to select compounds for further investigations. Monocationic B compounds were superior to the equivalent mononitrile A compounds. Out of the ten bichalcophenes, it is important to select the prime compounds for the time consuming and costly chemoprevention studies in animal models. The National Cancer Institute (Developmental Therapeutics Program) has recently examined these bichalcophenes for anticancer activity in the 58 cell line in the concentration range defined in the present study and found that the most active compounds we identify in the present study (1, 4, and 5B) were the most active compounds in the NCI study (unpublished data).
Based on the results of the present investigation, monocationic compounds (1, 4, and 5B) will be selected for further in vivo anticancer studies.
Acknowledgemnts
The authors acknowledge Dr Mohamed Ismail of King Faisal University for providing the bichalcophene compounds. This project was supported by King Faisal University, Deanship of Scientific Research, Grant 130156.
Disclosure
The authors report no conflicts of interest in this work.
References
- Maron DM Ames BN Revised methods for the Salmonella mutagenicity test Mutat Res 1983 113 3–4 173 215 6341825
- Jarak I Marjanović M Piantanida I Kralj M Karminski-Zamola G Novel pentamidine derivatives: synthesis, anti-tumor properties and polynucleotide-binding activities Eur J Med Chem 2011 46 7 2807 2815 21546133
- Kobayashi J Kato A Ota Y Ohba R Komatsu K Bisbenzamidine derivative, pentamidine represses DNA damage response through inhibition of histone H2A acetylation Mol Cancer 2010 9 34 44 20144237
- Paliwal SK Verma AN Paliwal S Neglected disease – african sleeping sickness: recent synthetic and modeling advances Sci Pharm 2011 79 3 389 428 21886894
- Youssef MM Al-Omair MA Ismail MA Synthesis, DNA affinity, and antimicrobial activity of 4-substituted phenyl-2,20-bichalcophenes and aza-analogues Med Chem Res 2012 21 12 4074 4082
- Hussin WA Ismail MA El-Sayed WM Novel 4-Substituted Phenyl-2,2′-Bichalcophenes and Aza-Analogues as Antibacterial Agents: Structural Activity Relationship Drug Des Devel Ther In press.
- El-Sayed WM Hussin WA Ismail MA Efficacy of two novel 2,2′-bifurans to inhibit methicillin-resistant Staphylococcus aureus infection in male mice in comparison to vancomycin Drug Des Devel Ther 2012 6 279 287
- Luch A Baird WM Metabolic activation and detoxification of polycyclic aromatic hydrocarbons Luch AA The Carcinogenic Effects of Polycyclic Aromatic Hydrocarbons London Imperial College Press 2005 19 96
- Yoon JH Besaratinia A Feng Z DNA damage, repair, and mutation induction by (+)-Syn and (−)-anti-dibenzo[a,l]pyrene-11,12-diol-13,14-epoxides in mouse cells Cancer Res 2004 64 20 7321 7328 15492252
- Tesfai Y Davis D Reinhold D Chromium can reduce the mutagenic effects of benzo[a]pyrenediolepoxide in normal human fibroblasts via an oxidative stress mechanism Mutat Res 1998 416 3 159 168 9729358
- Katoh Y Nemoto N Tanaka M Takayama S Inhibition of benzo[a] pyrene-induced mutagenesis in Chinese hamster V79 cells by hemin and related compounds Mutat Res 1983 121 2 153 157 6308440
- Neffati A Limem I Kilani S A comparative evaluation of mutagenic, antimutagenic, radical scavenging and antibacterial activities of essential oils of Pituranthos chloranthus (Coss et Dur) Drug Chem Toxicol 2009 32 4 372 380 19793030
- Ajith T Subin JP Jacob J Sanjay PS Babitha NV Antimutagenic and anti-oxidant activities of the non-steroidal anti-inflammatory drug celecoxib Clin Exp Pharmacol Physiol 2005 32 10 888 893 16173952
- Hong CE Cho MC Jang HA Lyu SY Mutagenicity and anti-mutagenicity of Acanthopanax divaricatus var albeofructus J Toxicol Sci 2011 36 5 661 668 22008541
- Prieto P Pineda M Aguilar M Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E Anal Biochem 1999 269 2 337 341 10222007
- Oyaizu M Studies on products of browning reaction: Antioxidative activity of product of browning reaction prepared from glucosamine Jpn J Nutr 1986 44 307 315
- Zeiger E Identification of rodent carcinogens and noncarcinogens using genetic toxicity tests: Premises, promises, and performance Regul Toxicol Pharmacol 1998 28 85 95 9927558
- Stauffert I Paulini H Steinmann U Sippel H Estler CJ Investigations on mutagenicity and genotoxicity of pentamidine and some related trypanocidal diamidines Mutat Res 1990 245 2 93 98 2215556
- Connor TH Trizna Z Pentamidine isethionate is negative in tests for microbial mutagenicity and chromosomal breakage in vitro Toxicol Lett 1992 63 1 69 74 1412524
- Marnewick JL Gelderblom WC Joubert E An investigation on the antimutagenic properties of South African herbal teas Mutat Res 2000 471 1–2 157 166 11080671
- Collins AR Azqueta A Langie SA Effects of micronutrients on DNA repair Eur J Nutr 2012 51 3 261 279 22362552
- Watanabe M Kobayashi H Ohta T Rapid inactivation of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX), a potent mutagen in chlorinated drinking water, by sulfhydryl compounds Mutat Res 1994 312 2 131 138 7510825
- Schwab CE Huber WW Parzefall W Search for compounds that inhibit the genotoxic and carcinogenic effects of heterocyclic aromatic amines Crit Rev Toxicol 2000 30 1 1 69 10680768