Abstract
Purpose
Remimazolam has demonstrated the potential as a valuable medication for procedural sedation. However, there were some shortcomings for higher doses of remimazolam during hysteroscopy in spite of less frequent adverse events. The aim of this study was to find the 50% and 95% effective dose (ED50 and ED95) of remimazolam when combined with propofol for intravenous sedation during day-surgery hysteroscopy.
Patients and Methods
Patients were randomly assigned evenly (20 per group) to one of five different dosage of remimazolam: group A (0.05mg/kg), group B (0.075mg/kg), group C (0.1mg/kg), group D (0.125mg/kg) or group E (0.15mg/kg). Intravenous injection of sufentanil 0.1µg/kg was administered before sedative medication. Intravenous anesthesia was commenced with remimazolam. Subsequently, propofol was administered at 1mg/kg and maintained at 6mg/kg/h. Success was defined when the patient did not move during cervical dilation, had sufficient sedation as judged by SE <60 and no requirement for rescue doses. The success rate, induce and average dosage of propofol, the induction time, total surgery time, recovery time, and adverse events were recorded. Estimate of ED50 and ED95 with 95% confidence interval (CI) was performed by probit regression.
Results
The mean (95% CI) values for ED50 and ED95 of remimazolam in patients were 0.09 (0.08−0.11) mg/kg and 0.21 (0.16–0.35) mg/kg, respectively. There was no difference in the induction time, total surgery time, and recovery time among groups. No serious adverse events occurred in all patients.
Conclusion
The dose–response effects of remimazolam were evaluated for intravenous sedation during hysteroscopy. A combination of remimazolam and propofol was recommended to produce stabler sedation, reduce the total dosage and have less effect on cardiovascular and respiratory depression.
Introduction
Propofol combined with opioids remains the most widely intravenous anesthesia for day-surgery hysteroscopy.Citation1 However, propofol may induce significant hemodynamic and respiratory depression at effect site concentration close to those needed for procedural sedation because of narrow therapeutic index.Citation2
Remimazolam is an ultra-short-acting benzodiazepine with characteristics of rapid onset and offset, short duration, and predictable recovery times.Citation3 The efficacy and safety profile of remimazolam has been widely validated in both healthy and high-risk patients with minimal respiratory depression and less pronounced hemodynamic changes.Citation4,Citation5 In most Phase II and III trials, remimazolam was administered from 2.5 to 8mg for initial dose and followed by 1.25–3mg for top-up doses. The doses were weight adjusted in some studies and not weight adjusted in other studies.Citation6–9 The regimen of remimazolam for procedural sedation had not been a consistent opinion. There were some shortcomings for higher doses of remimazolam during hysteroscopy, such as more body movement and longer recovery time. The body movement increased the difficulty in operation with high risk of uterine perforation and longer recovery time reduced the efficiency of operating room. Considering the synergistic effect, remimazolam combined with propofol would produce safer and stabler sedation with a slight effect on cardiorespiratory depression.
There is a growing need for intravenous anesthesia during day-surgery hysteroscopy. It is necessary to find a more ideal sedation method to improve the patients’ comfort and safety. Previous studies on the dose of remimazolam cannot satisfy demand for the hysteroscopy, and the dose finding study is required. The purpose of this study was to find the ED50 and ED95 of remimazolam combined with propofol for intravenous sedation during day-surgery hysteroscopy.
Materials and Methods
Design and Study Subjects
The study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (No. IRB-20220059-R) and was registered at the Chinese Clinical Trials.gov (No. ChiCTR2200058865).
Written informed consent was obtained from all patients before enrollment in this study from May 9 to July 7, 2022. Patients were eligible if they met the inclusion criteria: (1) scheduled to hysteroscopy requiring intravenous anesthesia; (2) American Society of Anesthesiologists (ASA) physical status I-II; (3) aged 20–45 years; and (4) body mass index (BMI) 18–30kg/m2. Patients were excluded if they had any of following conditions: (1) cardiopulmonary disease; (2) alcohol abuse; (3) liver and kidney dysfunction; (4) obstructive sleep apnea–hypopnea syndrome (OSAHS); (5) chronic use of tranquillizers/opioids/antidepressants; (6) anesthetic drug allergy; and (7) difficulty in cervical dilatation which was defined as the duration of cervical dilatation >5 minutes.
Randomization was based on a computer-generated random numbers table. Randomized results were sealed in sequentially numbered envelopes until the end of the study. The drugs used in this study were prepared by a nurse who was not involved in the anesthesia process. Attending anesthesiologists, surgeons, resuscitation room nurses, and patients were all blinded to the grouping assignments.
Study Protocol
The intramuscular injection of phloroglucinol (80mg) was used for cervical ripening each patient 2 hours before operation. All the patients had routine noninvasive monitoring of blood pressure (1 min cycle), electrocardiogram (ECG), and pulse oximetry (SpO2) in the operating room. In addition, end-tidal carbon dioxide monitoring measured respiratory rate (RR). Spectral entropy (SE) measured the depth of anesthesia by monitor (CARESCAPE Monitor B650, GE Healthcare, Helsinki, Finland). All patients inhaled oxygen (5L/min) through a Venturi oxygen mask.
Patients were randomly assigned evenly (20 per group) to one of five different dosage of remimazolam (Yichang Humanwell Pharmaceutical Co. LTD., China): group A (0.05mg/kg), group B (0.075mg/kg), group C (0.1mg/kg), group D (0.125mg/kg) or group E (0.15mg/kg). Intravenous injection of sufentanil (Yichang Humanwell Pharmaceutical Co. LTD., China) 0.1µg/kg was administered before sedative medication. Intravenous anesthesia was commenced with remimazolam. Subsequently, propofol (Aspen Pharma Co. LTD., Ireland) was administered at 1mg/kg and maintained at 6mg/kg/h. The surgeon was allowed to start cervical dilation when adequate sedation (SE<60) was achieved. The cervix was dilated to 1cm in diameter and hysteroscope with 27-Fr (9mm) outer sheath was inserted. SE was maintained at 40–60 during the operation. If patients did not achieve adequate sedation after the initial dose of remimazolam and propofol, they were given top-up doses of propofol (0.5mg/kg per time) and the top-up interval was more than 1min. Visual analog scale (VAS) was used to assess pain after surgery. If VAS >4, ketorolac 30mg was administered intravenously.
Outcomes and Definitions
The primary outcome was the procedure success rate. Success was defined when patient did not move during cervical dilation, had sufficient sedation as judged by SE <60 and no requirement for rescue doses.
The secondary outcomes included various anesthesia induction-, maintenance-, recovery-related parameters and some adverse events. The induce dosage of propofol was defined as the dosage of propofol for cervical dilation. The average dosage of propofol was defined as the dosage of propofol for operation. The induction time was defined as the time from injection of sedation drugs to reach SE <60. The total surgery time was defined as the time from the insertion of the speculum to the time of the hysteroscope removal. Recovery time was defined as the time from the stop of propofol injection to the time that the patient was awake and opened eyes. Respiratory depression was defined as RR <8 breaths per minute and/or SpO2<95%. Hypotension was defined as a decrease in systolic pressure to <90mmHg for 2 consecutive readings. Bradycardia was defined as heart rate (HR) <50 beats per minute. These events were treated by intravenous injection of atropine or norepinephrine, or mask ventilating.
Statistical Analysis
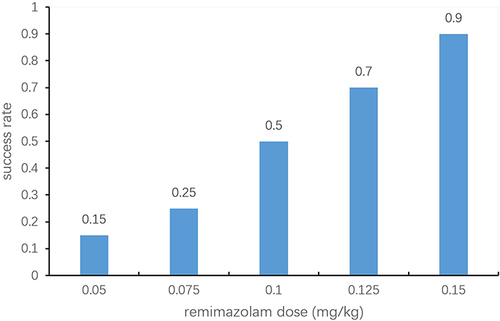
A pilot study: fifty patients were divided randomly into five groups, 10 in each group. The proportions of patients with successful anesthesia for cervical dilation were 0.2, 0.4, 0.6, 0.8, 0.9 in patients who received remimazolam at doses of 0.05, 0.075, 0.1, 0.125 or 0.15mg/kg, respectively. Sample size was calculated using the Cochran–Armitage test for trend in proportions using PASS 11 (NSCC, LCC, Kaysville, UT). Number of groups was 5. Group sample size pattern was equal. Proportions were 0.2, 0.4, 0.6, 0.8, 1.0. We calculated that 11 patients per dosage group were required at a significance level of 0.05 and power of 0.80 using the Z-test with continuity correction. We arbitrarily increased the sample size to 20 per dosage group in order to achieve narrower confidence intervals for the derived estimates.
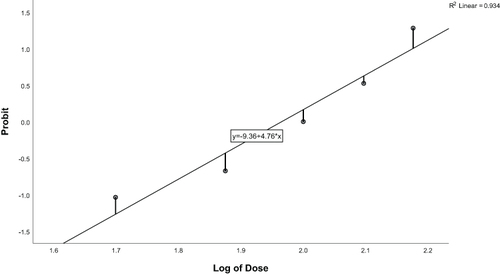
Demographic data of patients were expressed as mean±standard deviation (SD) or number of patients N (%). Different groups were compared by one-way ANOVA. The dose–response relationship was performed using probit regression. Following logarithmic transformation of dose values, the proportions of successes at each dose level were converted to probit and regression analysis was performed. The Pearson goodness-of-fit chi-square statistic was used to test the null hypothesis that the regression model adequately fitted the data. Values for ED50 and ED95 with 95% CIs were derived by interpolation. Analyses were performed using IBM SPSS Statistics for Windows version 26. Values of P<0.05 were considered statistically significant.
Results
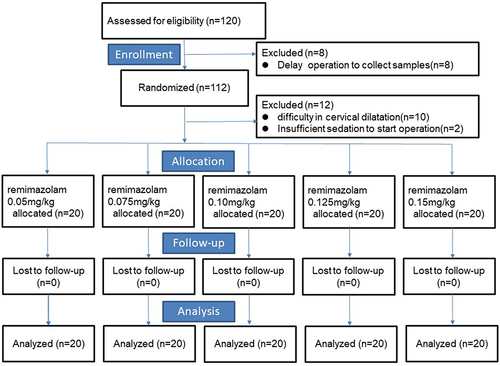
A total of 120 patients were included in the study, and 20 patients were excluded (). Eight patients were removed prior to randomization for delay operation to collect samples. Twelve patients were randomized but excluded for difficulty in cervical dilation (n=10) and insufficient sedation to start operation (n=2). The demographic characteristics of patients are presented in . There was no difference in age, weight, and BMI among the groups (P>0.05).
Table 1 Demographic Data
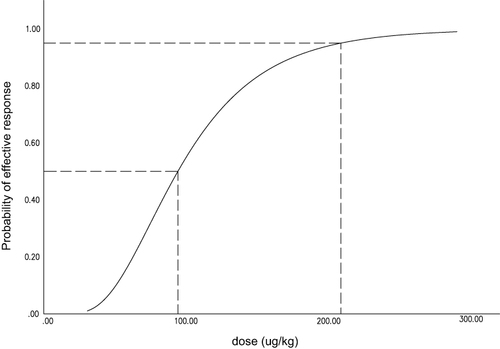
The proportions of successes for different doses of remimazolam are shown in . Dose values were logarithmically transformed and success rates were converted to probits for analysis (). The corresponding probit regression curve was shown in . The Pearson goodness-of-fit chi-square statistic confirmed that the model adequately fitted the data (P=0.566). The derived dose–response curve is presented in . The mean (95% CI) values for ED50 and ED95 of remimazolam in patients were 0.09 (0.08−0.11) mg/kg and 0.21 (0.16–0.35) mg/kg, respectively.
Table 2 Success Rate for Different Doses of Remimazolam
Figure 4 Dose–response curves for remimazolam derived from probit analysis. Dashed line indicates the position of the estimate of ED50 and ED95.
The secondary outcomes are shown in . There was a trend toward increasing loading dose of remimazolam with the lower demand of propofol (F=14.399 and 7.719 for induce and average dosage of propofol, respectively, P=0.000). There was no difference in the induction time (P=0.251), total surgery time (P=0.682), and recovery time (P=0.972) among groups. No serious adverse events occurred in all patients. The incidence of respiratory depression had no difference among groups (P=0.926). The incidence of hypotension was 13% in total. The number of hypotension was 4 and 5 in group A and E respectively versus 1 or 2 in other groups (P=0.217). All the values returned to normal within 3min. None of patient appeared bradycardia and nobody received any medication for hypotension.
Table 3 Comparison of Secondary Outcomes Among Groups
Discussion
In this study, we found that the ED50 and ED95 values of remimazolam for intravenous sedation during hysteroscopy when combined with propofol (1mg/kg) and sufentanil (0.1µg/kg) was 0.09 (0.08–0.11) mg/kg and 0.21 (0.16–0.35) mg/kg, respectively.
Remimazolam is an ester-based benzodiazepine which is rapidly hydrolyzed into an inactive metabolite by carboxyesterase.Citation3 As carboxylesterases are ubiquitous and unlikely to be saturated, remimazolam showed high clearance even with hepatic and renal dysfunction, a small steady-state volume of distribution (Vdss) and a short terminal phase half-life.Citation10,Citation11 Remimazolam is better than midazolam for procedural sedation from existing clinical trials.Citation12 Wiltshire et al suggested that dosing of remimazolam by body weight maybe not better than fixed doses for no clinically relevant covariate effects observed.Citation13 It was proved to be safe and effective for moderate sedation with no-weight adjusted doses in gastrointestinal endoscopy.Citation9,Citation14 Another study reported by Antonik et al observed that the depth and duration of sedation for remimazolam were dose dependent.Citation10 Remimazolam appeared to produce deeper sedation at the higher doses of 0.10–0.20 mg/kg, as evidenced by the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scores of <2 and mean bispectral index (BIS) scores of 60–70 soon after dosing.
There have been imperfections for higher doses of remimazolam in hysteroscopy. As a result of short duration of action, additional doses or continuous intravenous infusion are required for remimazolam in prolonged sedation. Zhang et al observed remimazolam had non-inferiority in sedative efficacy and less adverse events than propofol in hysteroscopy.Citation15 The regimen was based on the research by Doi et al as a general anesthesia.Citation16 However, there was still 36.6% body movement during the short procedures with higher (0.4mg/kg) mean doses of remimazolam. This would be due partly to insensitive to the effects of remimazolam whose minimum MOAA/S score was higher than 2 and in part for the lesser effects with persistence of the evoked response during apparently satisfactory anesthesia.Citation17 Recently, Park et al reported a combination of remimazolam and remifentanil was administered to insert supraglottic airway without a neuromuscular blocking agent in hysteroscopy.Citation18 In spite of the maximum dose (0.4mg/kg) of remimazolam, 21.6% patients required supplementary midazolam during hysteroscopy to keep BIS <60. Additionally, 3 (8.1%) patients were administered flumazenil because recovery was delayed 15 minutes or MOAA/S was <2 in PACU. Recovery would take up to 50 minutes for 0.4mg/kg remimazolam.Citation19 Yamamoto et al reported a case in which one patient fell asleep again after remimazolam was reversed with flumazenil.Citation20
To achieve the objectives of a stable intraoperative course and rapid recovery to consciousness with adequate spontaneous ventilation, hypnotic drugs should ideally be administered at concentrations not significantly higher than required to achieve loss of consciousness. Remimazolam combined with propofol maybe a good choice to improve sedation and safety. According to the maximally synergistic doses and duration effect of midazolam–propofol combination, it is an appropriate choice for propofol 1mg/kg bolus and continuous infusion 6mg/kg/h.Citation21 The values for ED50 and ED95 of remimazolam in our study were similar to the previous research for loss of consciousness (0.11 and 0.14mg/kg).Citation22 Because the ED50 and ED95 of bolus remimazolam for respiratory depression were 0.14 and 0.27mg/kg, respectively, no more than 0.2mg/kg remimazolam was recommended in procedural sedation for less respiratory depression.Citation22
Adverse events were mild and required no reversal agents. The incidence of respiratory depression was lower and the degree was minor. The one in 0.15mg/kg remimazolam group, RR was lower than 8 breaths per minute for 3min and SpO2 decreased to 94%. The other one in 0.125mg/kg remimazolam group, RR increased to 20 from 12 breaths per minute and minimum SpO2 was 91%. The numbers were too small to draw precise conclusions and respiratory parameters were recovered soon with no supported ventilation. In our present study, the incidences of hypotension were 20–25% in 0.15mg/kg and 0.05mg/kg remimazolam group. In 0.05mg/kg remimazolam group, patients required more propofol doses and hypotension maybe due to the effect of propofol. However, patients had no additional rescue dose in 0.15mg/kg remimazolam group and hypotension may be due to the effect of remimazolam. Although remimazolam is less hypotension than propofol, it should be noted that the incidence of hypotension is high in vulnerable patients.Citation23 The number of hypotension was only 1 in 0.125mg/kg remimazolam group compared with 5 in 0.15mg/kg remimazolam group. Although there was no statistical difference among groups, further large-sample clinical studies are needed to confirm it.
We chose the entropy to estimate the depth of sedation. Electroencephalogram (EEG) changes during remimazolam-induced sedation were reported to have higher signal-to-noise ratios in the alpha band than the beta band and predominantly a monotonic beta activation especially in frontal areas.Citation24 The average power in the alpha band was least affected by noise, had stable baseline readings and showed a graded response consistent with the observed sedative effects. However, the BIS is designed for propofol to focus on the beta band and does not correlate well with the depth of sedation after benzodiazepine administration.Citation25 When adult patients were dosed with midazolam 0.2 or 0.3mg/kg, BIS remained around 60 and there was no correlation between BIS and predicted effect-site midazolam concentration.Citation26 The entropy can reflect the irregularity, complexity, or unpredictability characteristics of signals from any particular frequency range.Citation27 It has been confirmed to be a valid indicator of the hypnotic effect of propofol, thiopental, sevoflurane and desflurane and has a good correlation with the MOAA/S score, especially SE.Citation28,Citation29 To compared the sedative effects of propofol and benzodiazepines, the entropy was more accurate in clinical.Citation30
Our study had several limitations. First, our study was conducted at a single tertiary university hospital and all patients were women who underwent hysteroscopy at the day surgery center. The surgeons have rich clinical experience and are familiar with the operation. The results of this study might be different in lower-level hospitals due to technical differences. Second, specious situation were excluded, such as ASA III-IV, obesity, old, postmenopausal. The efficacy and safety of remimazolam were shown in many clinical trials. However, the dose–response relationships remain unclear in these special populations. Further study is needed to compare and clarify. Finally, we chose the fixed ratios of propofol and sufentanil by the commendation. Various combinations were not discussed. The application of response-surface methodology to the study of drug interactions has the potential to overcome the limitations.
Conclusion
In summary, the current study evaluated the dose–response relationships between remimazolam and propofol. A combination of remimazolam and propofol was recommended to produce stabler sedation, reduce the total dosage and have less effect on cardiovascular and respiratory depression.
Data Sharing Statement
The data supporting the study findings are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (No. IRB-20220059-R) and was registered at the Chinese Clinical Trials.gov (No. ChiCTR2200058865). All patients provided written informed consent. We confirm our study complies with the Declaration of Helsinki.
Disclosure
The authors declared no funding sources or conflicts of interest to support this article.
Acknowledgments
The authors thank the colleagues in the Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
References
- Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low‐dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. 2019;125:460–465. doi:10.1111/bcpt.13280
- Eleveld DJ, Colin P, Absalom AR, Struys MM. Pharmacokinetic–pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018;120:942–959. doi:10.1016/j.bja.2018.01.018
- Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. doi:10.1097/01.anes.0000267503.85085.c0
- Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers. Anesthesiology. 2020;132:636–651. doi:10.1097/ALN.0000000000003103
- Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. 2021;53:94–101. doi:10.1016/j.dld.2020.10.039
- Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093–1100. doi:10.1213/ANE.0b013e3182a705ae
- Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771–780. doi:10.1213/ANE.0000000000000548
- Rex DK, Bhandari R, Desta T, et al. A Phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427–437.e6. doi:10.1016/j.gie.2018.04.2351
- Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36:474–481. doi:10.1111/jgh.15188
- Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled Phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–283. doi:10.1213/ANE.0b013e31823f0c28
- Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127:415–423. doi:10.1016/j.bja.2021.05.027
- Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127:41–55. doi:10.1016/j.bja.2021.03.028
- Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115:284–296. doi:10.1213/ANE.0b013e318241f68a
- Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12:4594–4603.
- Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021;21:156. doi:10.1186/s12871-021-01373-y
- Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–553. doi:10.1007/s00540-020-02788-6
- Schwender D, Klasing S, Madler C, Pöppel E, Peter K. Effects of benzodiazepines on mid-latency auditory evoked potentials. Can J Anaesth. 1993;40:1148–1154. doi:10.1007/BF03009604
- Park I, Cho M, Nam SW, Hwang JW, Do SH, Na HS. Total intravenous anesthesia induced and maintained by a combination of remimazolam and remifentanil without a neuromuscular blocking agent: a prospective, observational pilot study. BMC Anesthesiol. 2022;22:237. doi:10.1186/s12871-022-01779-2
- Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76:383–391. doi:10.1007/s00228-019-02800-3
- Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021;35:322. doi:10.1007/s00540-021-02915-x
- Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL. Response surface model for anesthetic drug interactions. Anesthesiology. 2000;92:1603–1616. doi:10.1097/00000542-200006000-00017
- Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129:49–57. doi:10.1016/j.bja.2022.02.040
- Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501. doi:10.1007/s00540-020-02776-w
- Upton R, Martinez A, Grant C. A dose escalation study in sheep of the effects of the benzodiazepine CNS 7056 on sedation, the EEG and the respiratory and cardiovascular systems. Br J Pharmacol. 2008;155:52–61. doi:10.1038/bjp.2008.228
- Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001;95:1151–1159. doi:10.1097/00000542-200111000-00019
- Miyake W, Oda Y, Ikeda Y, Hagihira S, Iwaki H, Asada A. Electroencephalographic response following midazolam-induced general anesthesia: relationship to plasma and effect-site midazolam concentrations. J Anesth. 2010;24:386–393. doi:10.1007/s00540-010-0907-4
- Viertiö-Oja H, Maja V, Särkelä M, et al. Description of the entropy algorithm as applied in the Datex-Ohmeda S/5 entropy module. Acta Anaesthesiol Scand. 2004;48:154–161. doi:10.1111/j.0001-5172.2004.00322.x
- Vakkuri A, Yli-Hankala A, Talja P, et al. Time-frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anesthesia. Acta Anaesthesiol Scand. 2004;48:145–153. doi:10.1111/j.0001-5172.2004.00323.x
- Schmidt GN, Bischoff P, Standl T, Hellstern A, Teuber O, Schulte Esch J. Comparative evaluation of the Datex-Ohmeda S/5 entropy module and the bispectral index monitor during propofol-remifentanil anesthesia. Anesthesiology. 2004;101:1283–1290. doi:10.1097/00000542-200412000-00007
- Dinu AR, Rogobete AF, Popovici SE, et al. Impact of general anesthesia guided by state entropy (SE) and response entropy (RE) on perioperative stability in elective laparoscopic cholecystectomy patients-a prospective observational randomized monocentric study. Entropy Basel Switz. 2020;22:E356. doi:10.3390/e22030356