Abstract
SGLT1 and SGLT2 are the two main members of the sodium-glucose cotransporters (SGLTs), which are mainly responsible for glucose reabsorption in the body. In recent years, many large clinical trials have shown that SGLT2 inhibitors have cardiovascular protection for diabetic and non-diabetic patients independent of lowering blood glucose. However, SGLT2 was barely detected in the hearts of humans and animals, while SGLT1 was highly expressed in myocardium. As SGLT2 inhibitors also have a moderate inhibitory effect on SGLT1, the cardiovascular protection of SGLT2 inhibitors may be due to SGLT1 inhibition. SGLT1 expression is associated with pathological processes such as cardiac oxidative stress, inflammation, fibrosis, and cell apoptosis, as well as mitochondrial dysfunction. The purpose of this review is to summarize the protective effects of SGLT1 inhibition on hearts in various cell types, including cardiomyocytes, endothelial cells, and fibroblasts in preclinical studies, and to highlight the underlying molecular mechanisms of protection against cardiovascular diseases. Selective SGLT1 inhibitors could be considered a class of drugs for cardiac-specific therapy in the future.
Introduction
The global incidence of cardiovascular diseases (CVD) has nearly doubled in the past three decades, and the prevalence of CVD is still increasing in the young population aged 18–50 years old, which is the leading cause of death and disability for people worldwide.Citation1,Citation2 Diabetes mellitus (DM) is an important risk factor for CVD, and the risk of CVD in patients with type 2 diabetes mellitus (T2DM) is more than twice as high as that in those without DM.Citation3,Citation4 In recent years, with advances in prevention and treatment for DM, the trend towards CVD in people with T2DM has been decreasing. However, it remains the primary reason for death in patients with DM. The management and treatment for CVD is still a difficult and long-term process. Therefore, the search for novel targets of treatment for CVD will be more valuable in the future.
Sodium-glucose cotransporters (SGLTs) transport glucose into cells via a sodium concentration gradient established by Na+/K+-ATPase pump. Facilitative glucose transporters (GLUTs) utilize the diffusion gradient of glucose across plasma membranes to participate in glucose reabsorption.Citation5,Citation6 Two important transport proteins involved in glucose reabsorption, SGLT1 and SGLT2, are found to belong to the SGLT family (SLC5A), and both are expressed in kidney.Citation7 It was found that inhibition of renal glucose reabsorption allowed glucose to be excreted in the urine, lowering blood glucose, such as SGLT2 inhibitors on the market. Recent findings have highlighted that SGLT1 can also act as a potential pharmacological target in DM. SGLT1 inhibition improves glucose homeostasis by reducing glucose absorption in the intestine and increasing the release of glucagon-like peptide-1 (GLP-1), which contributes to glucose-dependent insulin release from the pancreas.Citation8,Citation9 Therefore, dual SGLT1/SGLT2 inhibitors or a combination of SGLT1 and SGLT2 inhibitors are also good options for the treatment of DM, and the combination of SGLT1 inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors might also be an effective way in increasing GLP-1 activity.Citation10 Furthermore, numerous large clinical trials have shown that SGLT inhibitors may have cardiovascular benefits independent of glycemic control. The SCORED and SOLOIST-WHF trials showed that sotagliflozin, a dual SGLT1/SGLT2 inhibitor, was associated with a lower risk of the composite of cardiovascular deaths and hospitalizations for heart failure (HF) in patients with T2DM combined with chronic kidney disease (CKD) or recent worsening HF.Citation11–13 In addition, a growing number of clinical trials such as EMPA-REG OUTCOME, CANVAS Program, CREDENCE, and DECLARE-TIMI have shown that SGLT2 inhibitors not only provide protective effects on kidneys but also have cardiovascular benefits.Citation14–17 Moreover, DAPA-HF, EMPEROR-Reduced, and EMPEROR-Preserved all suggest whether the ejection fraction is reduced and SGLT2 inhibitors exert cardioprotective effects on HF patients with or without T2DM.Citation18–20 According to VERTIS CV clinical trial, ertugliflozin was non-inferior to placebo in terms of major cardiovascular adverse events in patients with T2DM and atherosclerotic cardiovascular disease (ASCVD).Citation21 A recent meta-analysis included 13 randomized clinical trials showed that SGLT2 inhibitors significantly reduced the risk of hospitalization for HF in patients with and without DM.Citation22 Although SGLT2 was not detected in cardiomyocytes at either gene or protein level, the commonly used SGLT2 inhibitors such as empagliflozin, dapagliflozin, and canagliflozin show different selectivity of SGLT2 over SGLT1.Citation23–25 Kondo et alCitation26 paid attention to canagliflozin and found that it has anti-inflammatory and anti-apoptotic effects on the human myocardium, these effects are related to significant affinity of canagliflozin with SGLT1, which enhance the bioavailability of tetrahydrobiopterin (BH4) through SGLT1/AMPK/Rac1-dependent mechanism and improve the coupling of nitric oxide synthase (NOS) in primary human cardiomyocytes and myocardial tissues. These all suggest that the cardiac benefits of SGLT2 inhibitors may not be achieved through inhibition of SGLT2, but most likely through inhibition of SGLT1.Citation23,Citation27
In this review we summarize the effects of SGLT1 inhibition on myocardium and potential mechanisms in preclinical studies to provide a target for treatment of CVD ().
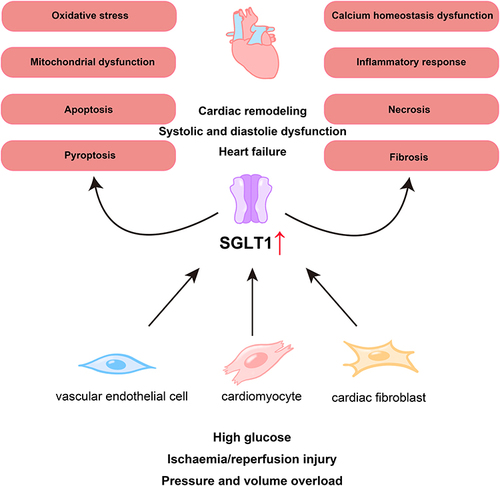
Figure 1 SGLT1 is involved in the pathological process of myocardial injury. Under some conditions, such as high glucose, ischemia/reperfusion injury, and pressure and volume overload, the expression of SGLT1 are increased in vascular endothelial cells, cardiomyocytes, and cardiac fibroblasts, which participate in the pathological processes of myocardial damage such as mitochondrial dysfunction, oxidative stress, and fibrosis, ultimately leading to cardiac remodeling, systolic and diastolic dysfunction, and the occurrence of heart failure.
Function of SGLTs and Drugs for SGLT Inhibition
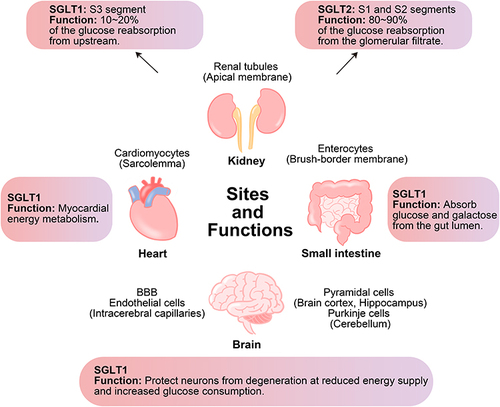
Under physiological conditions, almost all of the glucose filtered by the glomerulus (~180 g/day) is reabsorbed in the proximal tubule.Citation28,Citation29 In the kidney, low affinity/high capacity SGLT2 is located mainly in the apical membrane of the renal proximal tubules (S1 and S2 segments) and is responsible for most of the glucose reabsorption (80%–90%) in the kidney, while high affinity/low capacity SGLT1 is located in the straight renal proximal tubules (S3 segment) where it reabsorbs the remaining glucose (10%–20%) from upstream.Citation8,Citation30–32 SGLT inhibition can lower blood sugar in a non-insulin-dependent manner. However, SGLT1 is also expressed in the small intestine, heart, and brain.Citation33 SGLT1 located in the brush-border membrane of the small intestine has a higher expression compared to the kidney,Citation34 which is essential for the rapid absorption of glucose and galactose in the intestine.Citation35 In the brain, information on the expression and function of SGLT2 is still uncertain, but SGLT1 is mainly expressed in pyramidal cells of brain cortex and hippocampus, Purkinje cells of cerebellum, endothelial cells of intracerebral capillaries, and the blood–brain barrier (BBB), protecting neurons from degeneration in the presence of reduced energy supply or increased glucose consumption.Citation36–38 The expression of SGLT1, which is primarily localized in the sarcolemma, is approximately 10-fold higher in the human hearts than that in the kidneys.Citation34 The physiological function of SGLT1 in the heart remains unclear.Citation8 In cases of myocardial ischemia or hypoglycemia, SGLT1 may increase the concentration of ATP in myocardium by enhancing the availability of glucose. However, increased glucose transport may also increase the toxic effects of hyperglycemia. Thus, SGLT1 may play an important role in myocardial energy metabolism.Citation39,Citation40 In addition, SGLT1 expression was increased in cardiomyocytes in patients with T2DM, but there was no difference in SGLT1 expression between healthy and ischemic/hypertrophic hearts.Citation23,Citation41 The expression of SGLT1 in cardiomyocytes from mice appears to increase with age.Citation41 Moreover, functional changes in SGLT1 may be associated with altered cardiac pathology, particularly in diseases characterized by increased glucose consumption.Citation41 The sites and functions of SGLT1 and SGLT2 are shown in .
Figure 2 Sites and functions of SGLT1 and SGLT2 in kidney and of SGLT1 in small intestine, heart and brain. SGLT1 is mainly expressed in the small intestine and also expressed in the kidney, heart, and brain, while SGLT2 is mainly expressed in the kidney. In the kidney, both SGLT1 and SGLT2 are responsible for glucose reabsorption. In the small intestine, SGLT1 is responsible for the absorption of glucose and galactose from the gut lumen. In the heart and brain, SGLT1 plays an important role in energy metabolism.
Phlorizin, a non-selective inhibitor of SGLT, was first isolated from the root bark of the apple tree in 1835.Citation42 Phlorizin with Ki values are 300 and 39 nM in human SGLT1 and SGLT2, respectively,Citation43 and the order of inhibiting effects on SGLT is SGLT2 > SGLT1 > SGLT4 > SGLT3 ≫ νSGLT.Citation44 In addition to its anti-inflammatory, antioxidant, and anti-cancer effects, phlorizin has been reported to correct hyperglycemia and improve insulin sensitivity without altering insulin levels.Citation42,Citation45 However, phlorizin is hydrolyzed by intestinal glucosidase and has poor oral bioavailability, so novel phlorizin-based analogs derived from O glucoside and from C glucoside have emerged, both of which exhibit good pharmacokinetic profiles and are resistant to hydrolysis by intestinal glucosidase.Citation28,Citation46 Currently, compounds derived from C glycoside, such as empagliflozin, canagliflozin, and dapagliflozin, are all approved by European Medicines Agency (EMA), Food and Drug Administration (FDA), Pharmaceuticals and Medical Devices Agency, Japan (PMDA), and National Medical Products Administration, China (NMPA) for marketing for the treatment of T2DM.Citation28 Sotagliflozin is currently approved in Europe as an adjunct to optimize insulin therapy in adults with type 1 diabetes mellitus (T1DM) and a body mass index (BMI) ≥27 kg/m2, helping to achieve glycemic control and weight loss without increasing the risk of hypoglycemia.Citation47 Although the relevant clinical data are currently limited to short-term studies, sotagliflozin remains a promising new drug for treatment of T2DM and T1DMCitation13 ().
Table 1 Current/Under Development SGLT2, Dual SGLT1/SGLT2, and SGLT1 Inhibitors
SGLT2 inhibitors were originally designed for glycemic control in patients with DM, and SGLT2 inhibitors have also been shown to have cardioprotective effects. When it comes to glucose-lowering drugs, it is important to consider whether there is a risk of hypoglycemia, which can activate sympathetic nerves and thus damage the heart.Citation48 One of the advantages of SGLT2 inhibitors is the lower risk of hypoglycemia during their treatment. No increased risk of hypoglycemia has been observed in patients with CKD or HF without DM, while these drugs do not increase the risk of acute kidney injury (AKI).Citation49 This may be related to the enhanced glucose transport capacity of SGLT1 in the S3 segment and increased plasma glucagon concentration, gluconeogenesis, and lipolysis after SGLT2 inhibition.Citation50,Citation51 SGLT2 inhibitors have recently been recommended as basic therapy for HF with reduced ejection fraction (HFrEF), and Tomasoni et alCitation52 also recommend early initiation of SGLT2 inhibitors to rapidly improve clinical outcomes and quality of life in patients with HFrEF. However, some side effects of SGLT2 inhibitors still deserve attention, such as genital and urinary tract infections, AKI, diabetic ketoacidosis, bone fracture, and lower limb amputation.Citation53–56
In the small intestine, the uptake of dietary glucose is mainly mediated by SGLT1. SGLT1 inhibition enhanced GLP-1 and peptide YY (PYY) release, reducing the risk of worsening DM and its complications.Citation57 However, mutations or defects in the SGLT1 gene can cause the retention of unabsorbed galactose and glucose in the intestine, leading to dehydration and hyperosmolar diarrhoea.Citation58–60 In a clinical trial on the treatment of functional constipation with mizagliflozin, a selective SGLT1 inhibitor, it was found that of all adverse effects, only diarrhea and abdominal distention were deemed to be related to mizagliflozin treatment. The selective SGLT1 inhibitor KGA-2727 is still in preclinical studies. However, it is worth noting that sotagliflozin, a SGLT1/SGLT2 dual inhibitor, is well tolerated, and no evidence of increased gastrointestinal side effects was found.Citation61 The pathophysiology of the diabetic heart shows that cardiac inhibition of SGLT1 can also have beneficial effects on cardiac reactive oxygen species (ROS) formation and glycogen accumulation.Citation8 More data on their safety and efficacy are needed when evaluating their role in cardiovascular-related diseases.
The Effects of SGLT1 Inhibition on Diabetes-Related Heart Injury
Patients with DM have more than twice the risk of HF and a worse prognosis than that in those without DM.Citation62 Many commonly used glucose-lowering drugs have been successful in glycemic control in patients with T2DM, but not all of them provide cardiovascular benefits to patients. Sulphonylureas have a higher incidence of cardiovascular adverse events compared to other hypoglycemic agents, and insulin sensitizers also significantly increase the risk of HF in T2DM patients.Citation63,Citation64 Recently, SGLT2 inhibitors and GLP-1 agonists have been reported to have a significant reduction in cardiovascular mortality.Citation65–67 A growing number of studies have also shown that SGLT1 inhibition does have cardiovascular benefits.
The Effects of SGLT1 Inhibition on Cardiomyocytes
Diabetic cardiomyopathy (DCM) is characterized by cardiomyocyte apoptosis, hypertrophy, and myocardial fibrosis, leading to firstly diastolic dysfunction, later systolic dysfunction, and eventually clinical HF, independent of coronary artery disease, hypertension, or valvular heart diseases.Citation68,Citation69 Increasing evidence demonstrates that oxidative stress, mitochondrial dysfunction, and impaired calcium homeostasis are related to the development of DCM and are the primary mechanisms of myocardial injury.Citation70,Citation71 SGLT1 is also involved in the occurrence of DCM to some extent. Lin et al demonstrated that SGLT1 levels were significantly elevated in high glucose (HG)-induced H9C2 cells and in patients with DCM. Mizagliflozin, a selective SGLT1 inhibitor, attenuated myocardial fibrosis and apoptosis by suppressing JNK and p38 MAPK pathways.Citation72 Mitochondrial dysfunction and free fatty acids induced by HG generate large amounts of ROS.Citation73 Increased oxidative stress exacerbates the inflammatory response, causing multiple abnormal changes in the structure and function of cardiomyocytes, including cell apoptosis, necrosis, loss of intercellular contacts, and malformation of contractile structures.Citation74 A significant increase in SGLT1 expression was found in H9C2 exposed to HG and palmitic acid. However, SGLT2 expression was not detected in H9C2. The inhibition of SGLT1 by canagliflozin and dapagliflozin reduced the generation of ROS and alleviated H9C2 apoptosis. Dasari et alCitation75 consider that glucolipotoxicity mediates cardiomyocyte injury through upregulation of SGLT1 to augment oxidative stress, cell apoptosis, and necrosis. Therefore, inhibition of SGLT1 may be a strategy to inhibit cardiomyocyte injury. Moreover, Balteau et alCitation40 first described that HG led to NADPH oxidase activation and ROS production by interacting with SGLT1 rather than GLUTs. Overexpression of GLUT1 in myocardium did not affect the morphology and function of the hearts.Citation76 NADPH oxidase is involved in hyperglycemia-induced ROS production. NOX2 is an important component of NADPH oxidase, and activation of NOX2 is associated with p47phox, p67phox, p40phox, and Rac1. HG activates NOX2 by Rac1 activation and p47phox translocation and then increases the production of ROS, which induces cell death.Citation77 Phlorizin not only counteracted HG-induced ROS production but also blocked p47phox translocation and Rac1 activation.Citation40 These suggest that HG promotes oxidative stress in cardiomyocytes through SGLT1-mediated signaling pathways. Inhibition of SGLT1 can alleviate the degree of oxidative stress to some extent and play a role in protecting cardiomyocytes from injury. Ng et alCitation78 found that HG upregulated the expression of SGLT1 and SGLT2 in human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes, which increased Na+ into the cardiomyocytes. Through the reverse mode of sodium-calcium exchanger (NCX), the increased sodium entry into cardiomyocytes leads to an increase in cytosolic calcium, which affects the excitation-contraction coupling of cardiomyocytes. In addition, HG-induced intracellular calcium overload could promote oxidative stress.Citation79 Ng et alCitation78 also found that HG significantly induced hypertrophic changes and impaired excitation-contraction coupling in both hiPSC-derived cardiomyocytes and IMR90 cell line. The contractility of hiPSC-derived cardiomyocytes was significantly diminished. Empagliflozin effectively reduced HG-induced cardiac abnormalities and did not alter hiPSC-derived cardiomyocytes viability or glucose metabolism.
Intermittent hyperglycemia is more harmful than persistent hyperglycemia and is closely associated with cardiovascular complications of DM.Citation80 Glucose fluctuation exacerbates mitochondrial damage, oxidative stress, fibrosis, and inflammation in cardiomyocytes, which ultimately result in cardiac diastolic dysfunction.Citation81–84 Mitochondrial fusion and fission maintain the stability of mitochondrial dynamics.Citation85 Wu et alCitation86 demonstrated that glucose fluctuation induced SGLT1 upregulation and mitochondrial dysfunction in myocardium from streptozotocin-induced diabetic mice. SGLT1 knockdown promoted mitochondrial fusion and inhibited mitochondrial fission in the hearts of diabetic mice, which ultimately improved cardiac mitochondrial dysfunction and attenuated cardiomyocyte apoptosis. Moreover, the knockdown of SGLT1 blocked the leakage of cytochrome c from mitochondria to cytoplasm and increased the ATP content and complex I–IV activity in diabetic hearts. Sun et alCitation87 found that glucose fluctuation increased the expression of IL-1β and IL-18 and activated the inflammatory response in left ventricular cardiomyocytes of diabetic mice. It is well known that NF-κB induces inflammatory response and pyroptosis. Pyroptosis is a form of programmed cell death associated with inflammatory response.Citation88 Pyroptosis requires activation of the nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which in turn activates caspase-1 to cleave gasdermin D (GSDMD) and convert the precursor cytokines pro-IL-1β and pro-IL-18 to mature IL-1β and IL-18. Inhibition of SGLT1 partially reversed the inflammatory response and pyroptosis of cardiomyocytes and alleviated glucose fluctuation-induced heart injury.Citation87 Chai et alCitation89,Citation90 established a model of glucose fluctuation by H9C2 and confirmed that intermittent HG could elevate SGLT1 expression. Knockdown of SGLT1 attenuated mitochondrial dysfunction and counteracted oxidative stress and inflammation-induced cell death.
In summary, the effects of SGLT1 inhibition on HG-related cardiomyocytes are shown in .
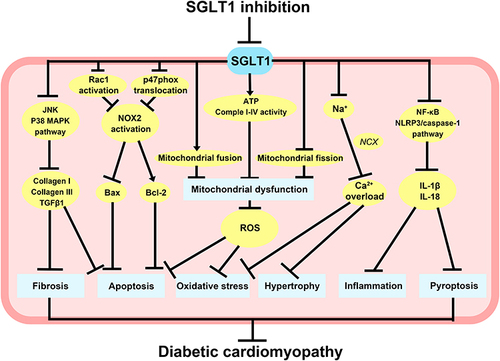
Figure 3 Potential molecular mechanisms of SGLT1 inhibition in diabetes-related heart injury. SGLT1 inhibition can reduce cardiomyocyte fibrosis and apoptosis through JNK and p38 MAPK pathways, as well as block Rac1 activation and p47phox translocation to reduce NOX2 activation and ROX production, thus reducing cardiomyocyte apoptosis. The knockdown of SGLT1 also promoted mitochondrial fusion, inhibited mitochondrial fission, and increased ATP content and complex I–IV activity, therefore improving mitochondrial dysfunction and attenuating cardiomyocyte oxidative stress and apoptosis. Inhibition of SGLT1 can reduce cardiomyocyte hypertrophy and oxidative stress caused by intracellular Ca2+ overload, and partially decrease the inflammatory response and pyroptosis in cardiomyocytes by suppressing NF-κB and NLRP3/caspase-1 pathway.
The Effects of SGLT1 Inhibition on Vascular Endothelial Cells
Endothelial dysfunction is a key factor leading to cardiovascular complications in DM.Citation91 Vascular endothelial cells (ECs) are sensitive to glucotoxicity and are important targets of HG injury.Citation92 Exposure of ECs to HG reduced NO production and diminished NO-mediated vasodilation, causing various pathological changes in blood vessels.Citation93 It was found that HG significantly upregulated the expression of SGLT1 and SGLT2 in coronary ECs and promoted SGLT1 and SGLT2-mediated glucose uptake. Empagliflozin and LX-4211, a dual SGLT1/SGLT2 inhibitor, were both strongly effective in blocking the stimulatory effects of HG on ECs, cell senescence, and cell glucotoxicity.Citation93
The Effects of SGLT1 Inhibition on Cardiac Fibroblasts
Cardiac fibroblasts (CFs), one of the main cells in hearts, play a crucial role in cardiac fibrosis and provide a pathological basis for cardiac remodeling. Changes in CFs from a resting type to an activated type increase their proliferation and migration capacity and secrete large amounts of extracellular matrix (ECM), contributing to cardiac fibrosis.Citation94 Under normal conditions, a dynamic balance between ECM catabolism and synthesis is maintained by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).Citation95 MMPs are responsible for the cleavage of ECM. During cardiac remodeling, ECM degradation and synthesis are simultaneously activated. Dysregulation of MMP-2 expression and activity alter the balance between ECM synthesis and degradation, resulting in excessive collagen deposition and destructive myocardial structural integrity. Increased ECM degradation provides space for the proliferation and migration of human CFs and other macrophages, further aggravating the cardiac remodeling process.Citation6,Citation95 Meng et alCitation6 found that HG may induce an increase in MMP-2 expression in human CFs by upregulating SGLT1. Extracellular signal-regulated kinase (ERK) 1/2 and p38 MAPK signaling pathways play an important role in myocardial fibrosis.Citation96,Citation97 Meng et alCitation6 also found that p38 MAPK and ERK1/2 phosphorylation were inhibited by SGLT1 knockdown, which decreased transforming growth factor-β1 (TGF-β1), collagen I, and collagen III. Knockdown of SGLT1 inhibited HG-induced activation of CFs and ameliorated cardiac fibrosis.Citation98 Similarly, Wu et alCitation99 also confirmed that inhibition of SGLT1 could attenuate the proliferation and activation of CFs and M1 polarization induced by glucose fluctuation. M1 macrophages are proinflammatory macrophages. M2 macrophages are anti-inflammatory macrophages.Citation100–102 SGLT1 inhibition significantly promotes the shift of macrophages to M2, and suppresses myocardial fibrosis.
The Effects of SGLT1 Inhibition on Non-Diabetes-Related Heart Injury
The Effects of SGLT1 Inhibition on Cardiomyocytes
Myocardial infarction (MI) is often secondary to coronary atherosclerosis, and when coronary blood flow is suddenly reduced, myocardial ischemia and hypoxia aggravate myocardial remodeling, which eventually leads to HF.Citation103 Myocardial remodeling not only leads to myocardial hypertrophy and fibrosis but also creates favorable conditions for the formation of reentry, which affect normal cardiac electrical conduction and induce arrhythmias.Citation104 Current interventional and pharmacological therapy is insufficient to reduce subsequent cardiac remodeling in HF.Citation105 Therefore, the identification of new targets to improve MI-induced HF is of significant importance. It is shown that SGLT1 protein expression was observably increased in myocardium from MI mice that undergo left anterior descending coronary artery ligation operation, leading to an increase in cardiomyocyte diameter and fibrosis in the left ventricle. The selective SGLT1 inhibitor, KGA-2727, inhibited cardiomyocyte hypertrophy gene expression, attenuated myocardial fibrosis and left ventricular shortening fraction. Moreover, there were no differences in blood glucose levels in mice, indicating that the cardioprotection of KGA-2727 is independent of inhibition of SGLT1 in the small intestine and kidney.Citation105 Sotagliflozin attenuated cardiac remodeling due to excessive activation of the TLR4/CaMKII pathway and significantly reduced the incidence of malignant ventricular arrhythmias.Citation106 5’-AMP-activated protein kinase (AMPK) upregulates SGLT1 expression through the ERK pathway in the process of ischemia, and SGLT1 interacts with epidermal growth factor receptor (EGFR), which in turn increases protein kinase C (PKC) and NADPH oxidase 2 (Nox2) activity as well as oxidative stress. However, in transgenic mice with cardiomyocyte-specific RNA interference knockdown of SGLT1, Li et al found a reduction in infarct size, necrosis, and oxidative stress.Citation107 SGLT1 may therefore be a novel target of therapeutic for ischemia/reperfusion (I/R) injury.
In the murine model of PRKAG2 cardiomyopathy, cardiac SGLT1 knockdown not only did not affect cardiac function but also attenuated glycogen storage, myocardial hypertrophy, and left ventricular dysfunction.Citation108 Myocardial hypertrophy is the most common adaptive response of the heart under pressure and volume overload. Sayour et alCitation109 induced chronic progressive pressure overload and volume overload in Wistar rats using transverse aortic constriction (TAC), abdominal aortic, and inferior vena cava shunts, respectively. They verified that SGLT1 protein expression was significantly upregulated in the left ventricle from HF rats by pressure overload or volume overload. Their results were consistent with the study of Matsushita et alCitation110, chronic stress overload led to a reduction in left ventricular fractional shortening and left ventricular dilatation in TAC-operated WT but not in TAC-operated SGLT1−/− mice. Increased gene expression of SGLT1, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), IL-18, connective tissue growth factor (CTGF), and collagen I was shown in TAC-operated WT mice. However, an increase in these factors was not observed in TAC-operated SGLT1−/− mice. These results suggest that increased SGLT1 expression is associated with chronic pressure overload-induced hypertrophic cardiomyopathy. Moreover, treatment of rats with sotagliflozin attenuated myocardial hypertrophy and fibrosis induced by pressure overload after TAC. This is the first study to demonstrate the cardioprotective effect of dual SGLT1/2 inhibition at therapeutic doses.Citation111
HF begins with myocardial injury. Initially, the body is able to maintain normal cardiac output through compensatory mechanisms, but these neurohumoral mechanisms eventually lead to direct cytotoxicity, causing arrhythmias and pump failure. HF with preserved ejection fraction (HFpEF) is usually associated with precursors of left atrial enlargement and atrial fibrillation.Citation112,Citation113 The study of Bode et alCitation114 has shown that sotagliflozin improves left atrial remodeling in metabolic syndrome-related HFpEF, and also improves spontaneous Ca2+ release events, mitochondrial Ca2+ buffer capacity, diastolic Ca2+ accumulation, and NCX activity, and exerts antiarrhythmic effects on left atrial cardiomyocytes. In patients with end-stage HF who were undergoing heart transplantation, Sayour et alCitation115 found that SGLT1 expression positively correlated with left ventricular end-diastolic diameter (LVEDD) and negatively correlated with left ventricular systolic function. However, SGLT2 expression was not detected in myocardium. These results suggest a potential of SGLT1 in the development of HF and possible cardioprotective effects of SGLT1 inhibitors ().
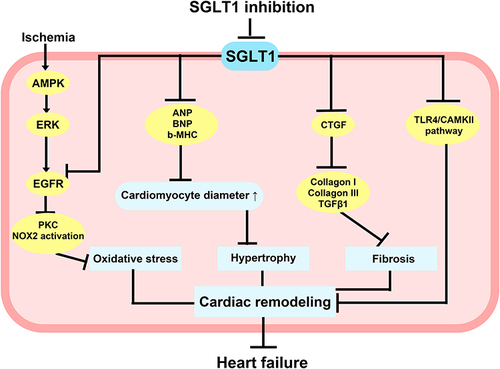
Figure 4 Potential molecular mechanisms of SGLT1 inhibition in non-diabetes-related heart injury. AMPK upregulated SGLT1 expression via the ERK pathway under ischemic conditions, and inhibition of SGLT1 attenuated the interaction of SGLT1 with EGFR, which in turn reduced PKC and NOX2 activity and alleviated oxidative stress in cardiomyocytes. Inhibition of SGLT1 reduced ANP, BNP, b-MHC, and CTGF, which contribute to preventing myocardial hypertrophy and fibrosis. Inhibition of SGLT1 also improved abnormal Ca2+ metabolism in cardiomyocytes, attenuated cardiac remodeling due to excessive activation of TLR4/CaMKII pathway, and eventually significantly reduced the incidence of heart failure.
The Effects of SGLT1 Inhibition on Vascular Endothelial Cells
Angiotensin II (Ang II) and blood-derived microparticles are major mediators of CVD.Citation116,Citation117 Ang II and microparticles from patients with coronary artery disease were also found to be potent inducers of SGLT1 and SGLT2 by Park et alCitation117, and SGLT1 and SGLT2 work in a feed-forward manner to maintain the stimulatory effect of the Ang II/AT1R/NADPH oxidase/ROS pathway on SGLT1 and SGLT2 expression in ECs. Sotagliflozin and empagliflozin abrogated Ang II–induced upregulation of SGLT1 and SGLT2 and alleviated oxidative stress, senescence, and dysfunction of ECs. Despite the presence of persistent hypertension, empagliflozin also effectively prevented Ang II–induced cardiac remodeling, macrophage infiltration, and activation of ECs. It was further noted that cardiovascular benefits were achieved by preventing the activation of the deleterious Ang II/NADPH oxidase/SGLT1 and SGLT2 pro-oxidant pathways in ECs and maintaining eNOS/ROS homeostasis, thereby endothelial SGLT1 and SGLT2 play a key role in the control of vascular and cardiac homeostasis.Citation118
Summary
Large clinical trials have shown that SGLT2 inhibitors not only lower blood glucose and body weight but also have cardioprotective effects. SGLT2 inhibitors have been widely used for diabetic patients in clinics, and are particularly recommended for patients with DM and ASCVD, HF or CKD. SGLT1 inhibition also shows great potential in the treatment of CVD regardless of the presence of DM. Evidence from studies targeting cardiomyocytes, ECs, and CFs suggests that inhibition of SGLT1 can be effective in cardiovascular protection by alleviating oxidative stress, inflammation, cell pyroptosis, myocardial fibrosis, and apoptosis. SGLT1 knockdown helps to prevent cardiovascular complications aggravated by glucose fluctuations in DM and to provide new insights into the role of SGLT1 in cardiomyocyte injury caused by glucose fluctuations, which also suggests the therapeutic potential of SGLT1 for DCM. Meanwhile, SGLT1 inhibition attenuates I/R injury, and would be a possible target of the treatment for arrhythmias and HF, but further evaluation in clinical trials is still needed. These experimental results also help to explain the underlying mechanisms of cardiovascular benefits of SGLT2 inhibitors observed in large clinical trials, which may be partially attributed to inhibition of SGLT1. In conclusion, SGLT1 may be implicated in the mechanisms of the development of CVD by acting on different cell types in myocardium. However, there is a need to further investigate the molecular mechanisms of SGLT1 inhibitors in CVD. We are looking forward to the development of more SGLT1 or dual SGLT1/SGLT2 inhibitors and to seeking their cardiovascular benefits in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
- Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4):230–240. doi:10.1038/nrcardio.2017.154
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
- Raghavan S, Vassy JL, Ho YL, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8(4):e011295. doi:10.1161/JAHA.118.011295
- Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838. doi:10.1016/j.metabol.2021.154838
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89(1):3–9. doi:10.1079/BJN2002763
- Meng L, Uzui H, Guo H, Tada H. Role of SGLT1 in high glucose level-induced MMP-2 expression in human cardiac fibroblasts. Mol Med Rep. 2018;17(5):6887–6892. doi:10.3892/mmr.2018.8688
- Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28(5):364–371. doi:10.1177/0148607104028005364
- Song P, Onishi A, Koepsell H, Vallon V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets. 2016;20(9):1109–1125. doi:10.1517/14728222.2016.1168808
- Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Arch. 2020;472(9):1177–1206. doi:10.1007/s00424-020-02433-x
- Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig. 2020;11(4):770–782. doi:10.1111/jdi.13255
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021;384(2):129–139. doi:10.1056/NEJMoa2030186
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384(2):117–128. doi:10.1056/NEJMoa2030183
- Cariou B, Charbonnel B. Sotagliflozin as a potential treatment for type 2 diabetes mellitus. Expert Opin Investig Drugs. 2015;24(12):1647–1656. doi:10.1517/13543784.2015.1100361
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi:10.1056/NEJMoa1611925
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381(21):1995–2008. doi:10.1056/NEJMoa1911303
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413–1424. doi:10.1056/NEJMoa2022190
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi:10.1056/NEJMoa2107038
- Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi:10.1056/NEJMoa2004967
- Hasan MT, Awad AK, Shih M, et al. Meta-analysis on the safety and efficacy of sodium glucose cotransporters 2 inhibitors in patients with heart failure with and without diabetes. Am J Cardiol. 2023;187:93–99. doi:10.1016/j.amjcard.2022.10.027
- Di Franco A, Cantini G, Tani A, et al. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol. 2017;243:86–90. doi:10.1016/j.ijcard.2017.05.032
- Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62(10):3324–3328. doi:10.2337/db13-0604
- Anker SD, Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail. 2018;5(4):549–551. doi:10.1002/ehf2.12345
- Kondo H, Akoumianakis I, Badi I, et al. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42(48):4947–4960. doi:10.1093/eurheartj/ehab420
- Bertrand L, Auquier J, Renguet E, et al. Glucose transporters in cardiovascular system in health and disease. Pflugers Arch. 2020;472(9):1385–1399. doi:10.1007/s00424-020-02444-8
- Kalra J, Mangali SB, Dasari D, et al. SGLT1 inhibition boon or bane for diabetes-associated cardiomyopathy. Fundam Clin Pharmacol. 2020;34(2):173–188. doi:10.1111/fcp.12516
- Li N, Zhou H. SGLT2 inhibitors: a novel player in the treatment and prevention of diabetic cardiomyopathy. Drug Des Devel Ther. 2020;14:4775–4788. doi:10.2147/DDDT.S269514
- Ferrannini E. Sodium-Glucose Co-transporters and Their Inhibition: clinical Physiology. Cell Metab. 2017;26(1):27–38. doi:10.1016/j.cmet.2017.04.011
- Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61(10):2087–2097. doi:10.1007/s00125-018-4656-5
- Hummel CS, Lu C, Loo DDF, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300(1):C14–21. doi:10.1152/ajpcell.00388.2010
- Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467(9):1881–1898. doi:10.1007/s00424-014-1619-7
- Zhou L, Cryan EV, D’Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003;90(2):339–346. doi:10.1002/jcb.10631
- Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013;34(2–3):183–196. doi:10.1016/j.mam.2012.11.002
- Poppe R, Karbach U, Gambaryan S, et al. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem. 1997;69(1):84–94. doi:10.1046/j.1471-4159.1997.69010084.x
- Szablewski L. Brain Glucose Transporters: role in Pathogenesis and Potential Targets for the Treatment of Alzheimer’s Disease. Int J Mol Sci. 2021;22(15). doi:10.3390/ijms22158142
- Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther. 2009;328(2):487–495. doi:10.1124/jpet.108.146589
- Kashiwagi Y, Nagoshi T, Yoshino T, et al. Expression of SGLT1 in Human Hearts and Impairment of Cardiac Glucose Uptake by Phlorizin during Ischemia-Reperfusion Injury in Mice. PLoS One. 2015;10(6):e0130605. doi:10.1371/journal.pone.0130605
- Balteau M, Tajeddine N, de Meester C, et al. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res. 2011;92(2):237–246. doi:10.1093/cvr/cvr230
- Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84(1):111–118. doi:10.1093/cvr/cvp190
- Habtemariam S. The molecular pharmacology of phloretin: anti-inflammatory mechanisms of action. Biomedicines. 2023;11(1). doi:10.3390/biomedicines11010143
- Pajor AM, Randolph KM, Kerner SA, Smith CD. Inhibitor binding in the human renal low- and high-affinity Na+/glucose cotransporters. J Pharmacol Exp Ther. 2008;324(3):985–991. doi:10.1124/jpet.107.129825
- Raja M, Kinne RKH. Identification of phlorizin binding domains in sodium-glucose cotransporter family: SGLT1 as a unique model system. Biochimie. 2015;115:187–193. doi:10.1016/j.biochi.2015.06.003
- Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510–1515. doi:10.1172/JCI112981
- Hardman TC, Dubrey SW. Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther. 2011;2(3):133–145. doi:10.1007/s13300-011-0004-1
- Danne T, Edelman S, Frias JP, et al. Efficacy and safety of adding sotagliflozin, a dual sodium-glucose co-transporter (SGLT)1 and SGLT2 inhibitor, to optimized insulin therapy in adults with type 1 diabetes and baseline body mass index ≥ 27 kg/m(2). Diabetes Obes Metab. 2021;23(3):854–860. doi:10.1111/dom.14271
- Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38(2):316–322. doi:10.2337/dc14-0920
- van der Aart-van der Beek AB, de Boer RA, Heerspink HJL. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022;18(5):294–306. doi:10.1038/s41581-022-00535-6
- Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–225. doi:10.1007/s00125-016-4157-3
- Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014;306(2):F188–193. doi:10.1152/ajprenal.00518.2013
- Tomasoni D, Fonarow GC, Adamo M, et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2022;24(3):431–441. doi:10.1002/ejhf.2397
- Garofalo C, Borrelli S, Liberti ME, et al. SGLT2 Inhibitors: nephroprotective Efficacy and Side Effects. Medicina. 2019;55(6). doi:10.3390/medicina55060268
- Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019;21(2):434–438. doi:10.1111/dom.13531
- Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(3):348–355. doi:10.1111/dom.12825
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. doi:10.2337/dc15-0843
- Dobbins RL, Greenway FL, Chen L, et al. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol. 2015;308(11):G946–954. doi:10.1152/ajpgi.00286.2014
- Alruwaili NW, Alshdayed F. Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: a Literature Review. Diagnostics. 2023;13(2). doi:10.3390/diagnostics13020294
- Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350(6316):354–356. doi:10.1038/350354a0
- Martín MG, Turk E, Lostao MP, Kerner C, Wright EM. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat Genet. 1996;12(2):216–220. doi:10.1038/ng0296-216
- Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012;92(2):158–169. doi:10.1038/clpt.2012.58
- Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124(1):121–141. doi:10.1161/CIRCRESAHA.118.311371
- Bain S, Druyts E, Balijepalli C, et al. Cardiovascular events and all-cause mortality associated with sulphonylureas compared with other antihyperglycaemic drugs: a Bayesian meta-analysis of survival data. Diabetes Obes Metab. 2017;19(3):329–335. doi:10.1111/dom.12821
- Asleh R, Sheikh-Ahmad M, Briasoulis A, Kushwaha SS. The influence of anti-hyperglycemic drug therapy on cardiovascular and heart failure outcomes in patients with type 2 diabetes mellitus. Heart Fail Rev. 2018;23(3):445–459. doi:10.1007/s10741-017-9666-8
- Nyström T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Novel oral glucose-lowering drugs are associated with lower risk of all-cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):831–841. doi:10.1111/dom.12889
- Schnell O, Rydén L, Standl E, Ceriello A. Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol. 2017;16(1):128. doi:10.1186/s12933-017-0610-y
- Thompson PL, Davis TME. Cardiovascular Effects of Glucose-lowering Therapies for Type 2 Diabetes: new Drugs in Perspective. Clin Ther. 2017;39(5):1012–1025. doi:10.1016/j.clinthera.2016.10.008
- Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–28. doi:10.1007/s00125-017-4390-4
- Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–638. doi:10.1161/CIRCRESAHA.117.311586
- Murtaza G, Virk HUH, Khalid M, et al. Diabetic cardiomyopathy - A comprehensive updated review. Prog Cardiovasc Dis. 2019;62(4):315–326. doi:10.1016/j.pcad.2019.03.003
- Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–153. doi:10.1038/nrendo.2015.216
- Lin N, Lin H, Yang Q, et al. SGLT1 Inhibition Attenuates Apoptosis in Diabetic Cardiomyopathy via the JNK and p38 Pathway. Front Pharmacol. 2020;11:598353. doi:10.3389/fphar.2020.598353
- Fiordaliso F, Bianchi R, Staszewsky L, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37(5):959–968. doi:10.1016/j.yjmcc.2004.07.008
- Dyntar D, Sergeev P, Klisic J, Ambühl P, Schaub MC, Donath MY. High glucose alters cardiomyocyte contacts and inhibits myofibrillar formation. J Clin Endocrinol Metab. 2006;91(5):1961–1967. doi:10.1210/jc.2005-1904
- Dasari D, Bhat A, Mangali S, et al. Canagliflozin and Dapagliflozin Attenuate Glucolipotoxicity-Induced Oxidative Stress and Apoptosis in Cardiomyocytes via Inhibition of Sodium-Glucose Cotransporter-1. ACS Pharmacol Transl Sci. 2022;5(4):216–225. doi:10.1021/acsptsci.1c00207
- Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116(8):901–909. doi:10.1161/CIRCULATIONAHA.107.691253
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi:10.1152/physrev.00044.2005
- Ng KM, Lau YM, Dhandhania V, et al. Empagliflozin Ameliorates High Glucose Induced-Cardiac Dysfunction in Human iPSC-Derived Cardiomyocytes. Sci Rep. 2018;8(1):14872. doi:10.1038/s41598-018-33293-2
- Kumar S, Kain V, Sitasawad SL. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochim Biophys Acta. 2012;1820(7):907–920. doi:10.1016/j.bbagen.2012.02.010
- Kuricová K, Pácal L, Šoupal J, Prázný M, Kaňková K. Effect of glucose variability on pathways associated with glucotoxicity in diabetes: evaluation of a novel in vitro experimental approach. Diabetes Res Clin Pract. 2016;114:1–8. doi:10.1016/j.diabres.2016.02.006
- Saito S, Teshima Y, Fukui A, et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res. 2014;104(1):5–14. doi:10.1093/cvr/cvu176
- Ying C, Liu T, Ling H, et al. Glucose variability aggravates cardiac fibrosis by altering AKT signalling path. Diab Vasc Dis Res. 2017;14(4):327–335. doi:10.1177/1479164117698917
- Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076–2082. doi:10.2337/dc12-0199
- Saito S, Thuc LC, Teshima Y, et al. Glucose Fluctuations Aggravate Cardiac Susceptibility to Ischemia/Reperfusion Injury by Modulating MicroRNAs Expression. Circ J. 2016;80(1):186–195. doi:10.1253/circj.CJ-14-1218
- Schrepfer E, Scorrano L. Mitofusins, from Mitochondria to Metabolism. Mol Cell. 2016;61(5):683–694. doi:10.1016/j.molcel.2016.02.022
- Wu W, Chai Q, Zhang Z. Glucose fluctuation accelerates cardiac injury of diabetic mice via sodium-dependent glucose cotransporter 1 (SGLT1). Arch Biochem Biophys. 2021;709:108968. doi:10.1016/j.abb.2021.108968
- Sun Z, Chai Q, Zhang Z, Lu D, Meng Z, Wu W. Inhibition of SGLT1 protects against glycemic variability-induced cardiac damage and pyroptosis of cardiomyocytes in diabetic mice. Life Sci. 2021;271:119116. doi:10.1016/j.lfs.2021.119116
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi:10.1111/imr.12534
- Chai Q, Meng Z, Lu D, Zhang Z, Liu M, Wu W. Intermittent high glucose induces pyroptosis of rat H9C2 cardiomyocytes via sodium-glucose cotransporter 1. Mol Cell Biochem. 2021;476(6):2479–2489. doi:10.1007/s11010-021-04104-6
- Chai Q, Miao J, Liu M, Zhang Z, Meng Z, Wu W. Knockdown of SGLT1 prevents the apoptosis of cardiomyocytes induced by glucose fluctuation via relieving oxidative stress and mitochondrial dysfunction. Biochem Cell Biol. 2021;99(3):356–363. doi:10.1139/bcb-2020-0491
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl 2):S314–321. doi:10.2337/dc09-S330
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi:10.1038/35008121
- Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24(3):2109–2122. doi:10.1111/jcmm.14233
- Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117(6):1450–1488. doi:10.1093/cvr/cvaa324
- Meng L, Liu L, Zhou C, et al. Polyphenols and Polypeptides in Chinese Rice Wine Inhibit Homocysteine-induced Proliferation and Migration of Vascular Smooth Muscle Cells. J Cardiovasc Pharmacol. 2016;67(6):482–490. doi:10.1097/FJC.0000000000000370
- Tao L, Bei Y, Chen P, et al. Crucial Role of miR-433 in Regulating Cardiac Fibrosis. Theranostics. 2016;6(12):2068–2083. doi:10.7150/thno.15007
- Xu Y, Xiao H, Luo H, et al. Inhibitory effects of oxymatrine on TGF‑β1‑induced proliferation and abnormal differentiation in rat cardiac fibroblasts via the p38MAPK and ERK1/2 signaling pathways. Mol Med Rep. 2017;16(4):5354–5362. doi:10.3892/mmr.2017.7277
- Lin H, Guan L, Meng L, Uzui H, Guo H. SGLT1 Knockdown Attenuates Cardiac Fibroblast Activation in Diabetic Cardiac Fibrosis. Front Pharmacol. 2021;12:700366. doi:10.3389/fphar.2021.700366
- Wu W, Chai Q, Zhang Z. Inhibition of SGLT1 Alleviates the Glycemic Variability-Induced Cardiac Fibrosis via Inhibition of Activation of Macrophage and Cardiac Fibroblasts. Mol Cell Biol. 2022;42(2):e0028221. doi:10.1128/MCB.00282-21
- Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci. 2018;19(6). doi:10.3390/ijms19061801
- Jing R, Long TY, Pan W, Li F, Xie QY. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur Rev Med Pharmacol Sci. 2019;23(14):6283–6291. doi:10.26355/eurrev_201907_18450
- Yang M, Zheng J, Miao Y, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1675–1686. doi:10.1161/ATVBAHA.112.248732
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
- Gibb AA, Hill BG. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ Res. 2018;123(1):107–128. doi:10.1161/CIRCRESAHA.118.312017
- Sawa Y, Saito M, Ishida N, et al. Pretreatment with KGA-2727, a selective SGLT1 inhibitor, is protective against myocardial infarction-induced ventricular remodeling and heart failure in mice. J Pharmacol Sci. 2020;142(1):16–25. doi:10.1016/j.jphs.2019.11.001
- Gong Y, Kong B, Shuai W, Chen T, Zhang J, Huang H. Effect of sotagliflozin on ventricular arrhythmias in mice with myocardial infraction. Eur J Pharmacol. 2022;936:175357. doi:10.1016/j.ejphar.2022.175357
- Li Z, Agrawal V, Ramratnam M, et al. Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc Res. 2019;115(11):1646–1658. doi:10.1093/cvr/cvz037
- Ramratnam M, Sharma RK, D’Auria S, et al. Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J Am Heart Assoc. 2014;3(4). doi:10.1161/JAHA.114.000899
- Sayour AA, Ruppert M, Oláh A, et al. Left Ventricular SGLT1 Protein Expression Correlates with the Extent of Myocardial Nitro-Oxidative Stress in Rats with Pressure and Volume Overload-Induced Heart Failure. Antioxidants. 2021;10(8). doi:10.3390/antiox10081190
- Matsushita N, Ishida N, Ibi M, et al. Chronic Pressure Overload Induces Cardiac Hypertrophy and Fibrosis via Increases in SGLT1 and IL-18 Gene Expression in Mice. Int Heart J. 2018;59(5):1123–1133. doi:10.1536/ihj.17-565
- Young SL, Ryan L, Mullins TP, et al. Sotagliflozin, a Dual SGLT1/2 Inhibitor, Improves Cardiac Outcomes in a Normoglycemic Mouse Model of Cardiac Pressure Overload. Front Physiol. 2021;12:738594. doi:10.3389/fphys.2021.738594
- Hohendanner F, Messroghli D, Bode D, et al. Atrial remodelling in heart failure: recent developments and relevance for heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5(2):211–221. doi:10.1002/ehf2.12260
- Skanes AC, Tang ASL. Atrial Fibrillation and Heart Failure: untangling a Modern Gordian Knot. Can J Cardiol. 2018;34(11):1437–1448. doi:10.1016/j.cjca.2018.07.483
- Bode D, Semmler L, Wakula P, et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc Diabetol. 2021;20(1):7. doi:10.1186/s12933-020-01208-z
- Sayour AA, Oláh A, Ruppert M, et al. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol. 2020;19(1):159. doi:10.1186/s12933-020-01141-1
- Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16(6):431. doi:10.1007/s11906-014-0431-2
- Park SH, Belcastro E, Hasan H, et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20(1):65. doi:10.1186/s12933-021-01252-3
- Bruckert C, Matsushita K, Mroueh A, et al. Empagliflozin prevents angiotensin II-induced hypertension related micro and macrovascular endothelial cell activation and diastolic dysfunction in rats despite persistent hypertension: role of endothelial SGLT1 and 2. Vascul Pharmacol. 2022;146:107095. doi:10.1016/j.vph.2022.107095
