Abstract
Small-cell lung cancer (SCLC) is the most aggressive form of lung cancer characterized by early metastasis and high mortality. In recent years, monotherapy and combination therapy of amrubicin with cisplatin or carboplatin has been actively studied and shown promise for the treatment of extensive disease SCLC (ED-SCLC). In this article, we summarize clinical trials of both monotherapy and combination therapy with amrubicin conducted in Japan, the USA, and the European Union. The results suggest that the clinical outcome of amrubicin therapy may be associated with genetic variations in patients. Further study of combination regimens in patients of different ethnicities is warranted.
Introduction
Lung cancer is the most common cancer and causes 1.38 million deaths annually worldwide.Citation1 The major types of lung cancer are small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC). SCLC comprises approximately 15% of all lung cancers and over 25,000 people are diagnosed with SCLC in the US each year.Citation2 Traditionally, SCLC is classified into “limited disease” (LD) or “extensive disease” (ED), depending on the degree of spread.Citation3 This type of lung cancer is highly aggressive and approximately two-thirds of SCLC patients have disease spread at diagnosis.
In the past few decades, SCLC has mainly been treated with combinations of a platinum agent (cisplatin or carboplatin) with etoposide or irinotecan.Citation4 The combination regimen has long been the standard chemotherapy; however, only little improvement in survival has been achieved over the last 30 years, reflecting a need to develop more effective therapies.Citation5,Citation6 In recent years, amrubicin alone and in combination with other chemotherapeutic agents, such as cisplatin and carboplatin, have been actively studied in Japan and have shown promise.Citation7,Citation8 Now more clinical studies have been conducted to evaluate the efficacy and safety of amrubicin in Western populations.Citation9 This review summarizes the clinical studies of amrubicin in combination with cisplatin or carboplatin for the treatment of ED-SCLC, aiming for further improvement of the combination regimens.
Chemistry and mechanisms of action
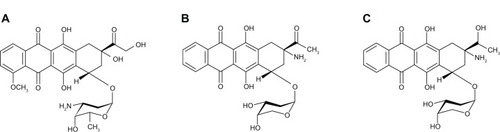
Amrubicin (brand name Calsed®, Sumitomo Pharmaceuticals, Japan) is a synthetic anthracycline used for treatment of lung cancer including SCLC and NSCLC. It was developed by Sumitomo Pharmaceuticals and first approved in Japan in 2002. Amrubicin has a structure similar to doxorubicin and works as a potent inhibitor of topoisomerase II.Citation10 Preclinical studies show that amrubicin has better antitumor activity than doxorubicin in several xenograft models of human cancers and causes almost no cardiotoxicity.Citation11,Citation12 The lower cardiotoxicity of amrubicin results from lower levels of accumulation and metabolic advantages over doxorubicin.Citation13,Citation14
It has been demonstrated that amrubicin itself has a weak antitumor effect and converts to the active form amrubicinol by reduction of the C-13 ketone group to a hydroxy group in vivo (). Amrubicinol has about 10- to 100-fold greater anticancer activity than the prodrug amrubicin.Citation15
The mechanism of action of anthracyclines has been extensively investigated. Several lines of evidence indicate that they interact with many macromolecules in the cell. Amrubicin is a potent inhibitor of topoisomerase II, which relaxes supercoils of deoxyribonucleic acid (DNA) for replication. Through facilitating the formation of a cleavable topoisomerase II-DNA complex, amrubicin prevents the double-helix of DNA from being resealed and thereby arrests DNA synthesis in rapidly-growing cancer cells.Citation10 In addition, as a member of the anthracyclines, the aromatic chromophore moiety of amrubicin intercalates between adjacent base-pairs of DNA, while the six-membered sugar interacts with flanking base-pairs near the intercalation site in the minor groove, resulting in the breakage of single-and double-strand DNA.Citation16–Citation18 Eventually, the anthracycline induces apoptosis in different cancer cells through several signaling pathways involved in nuclear factor kappa-B (NF-κB) activation, p53-dependence, and caspase-3 activation.Citation19
Pharmacokinetics and pharmacodynamics
Several pharmacokinetic studies of amrubicin and its active metabolite amrubicinol have been reported.Citation20–Citation22 When amrubicin was administered at a dose of 45 mg/m2/day in a bolus injection for 3 consecutive days, the area under the curve concentration-time (AUC) for amrubicin was 13.490 μg hour/mL and for amrubicinol was 2.585 μg hour/mL.Citation18 The apparent total clearance of amrubicin was 15.4 L/hour with large inter-individual variability (coefficient of variation = 49.8%). Another study showed that the plasma concentration peaked immediately after a bolus intravenous injection of amrubicin and declined rapidly in a biexponential manner thereafter, whereas the level of amrubicinol decreased more slowly. The apparent total clearance of amrubicin has a large inter-individual variability, despite adjustment of dosage for body surface area.Citation21 These studies suggest that monitoring plasma concentrations of amrubicin and amrubicinol may be necessary to obtain the optimal dosage for each patient.Citation20,Citation21
A relationship between the plasma concentration of the metabolite amrubicinol and hematological toxicity has been reported.Citation22 In 35 patients who received 30 or 40 mg/m2/day for 3 consecutive days, a significant relationship between amrubicinol concentration on day 4 and the level of toxicity (neutropenia, leukopenia, and anemia) was found.Citation22 Recently, a pharmacokinetic (PK) and pharmacodynamic (PD) study of amrubicin and amrubicinol in patients with lung cancer was conducted.Citation23 Amrubicin (35–40 mg/m2) was administered to 21 lung-cancer patients for 3 consecutive days every 3–4 weeks. The concentrations of amrubicin and amrubicinol in plasma were quantified with high-performance liquid chromatography (HPLC). The overall PK profile of amrubicin obeys a three-compartment model, while that of amrubicinol follows a one-compartment model with a first-order metabolic process. The PK-PD analysis indicated that the higher maximal plasma concentration (Cmax) and AUC of amrubicinol is directly correlated with major toxicity (neutropenia).Citation24 In the case of combination with cisplatin, an in vitro study showed that cisplatin does not influence the metabolism of amrubicin and amrubicinol in either human liver microsomes or the cytosol.Citation25 The metabolic pathway of amrubicin includes the reduction of the carbonyl group to a hydroxyl group at the C-13 site by carbonyl reductase, and inactivation by nicotinamide adenine dinucleotide phosphate (NADPH):quinone oxidoreductase (NQO) and NADPH-P450 reductase.Citation25 The quinones are reduced to hydroquinones in a single two-electron step. Recently, it was reported that C609T polymorphism of NADPH quinone oxidoreductase 1 (NQO1) in patients with lung cancer is correlated with amrubicinol levels in plasma and hematological toxicity of amrubicin.Citation26 A single nucleotide polymorphism in C609T of NQO1 enhanced the susceptibility of cancer cells to amrubicin, which may be used as a predictive biomarker for clinical response.Citation27
Clinical studies
Phase I trials
From 2005 to 2012, several Phase I trials have been reported for amrubicin alone and in combination with cisplatin or carboplatin for the treatment of lung cancer and SCLC ().Citation20,Citation28–Citation31 Phase I studies are designed to determine the maximum-tolerated doses (MTDs) and the recommended doses (RDs) for Phase II studies. Amrubicin was administered daily by intravenous injection (IV) for 3 consecutive days every 3–4 weeks. For monotherapy with amrubicin, the MTD was 40 mg/m2 and the RD was 35 mg/m2 while in the presence of cisplatin (60 mg/m2), the MTD increased to 45 mg/m2 and the RD to 40 mg/m2.20,29 Two studies of combination therapy with carboplatin have been conducted. The MTD and RD of amrubicin were the same as in monotherapy, while the dose of carboplatin in elderly patients was reduced from a fixed target AUC of five to four.Citation31,Citation32 The common toxicities, neutropenia, leukopenia, thrombocytopenia, febrile neutropenia, were observed in these trials. Recently, a Phase I trial of weekly administration of amrubicin was reported.Citation28 Sixteen patients (seven with SCLC and nine with NSCLC) participated and the MTD was 65 mg/m2 and the RD 60 mg/m2. The best response rate was 15.4%, and adverse events with this regimen were tolerable, so the adjusted regimen demands further study. In order to reduce the adverse effects of amrubicin, granulocyte colony-stimulating factor (G-CSF) has been used recently to support the therapy. The prophylactic use of G-CSF allowed the dose of amrubicin to be raised 40% in the original regimen.Citation32
Table 1 Phase I studies of monotherapy and combination therapy with amrubicin in NSCLC and SCLC
Phase II trials
Several Phase II studies have been conducted in Japan, the USA, and the European Union (EU) for the treatment of SCLC. The results are summarized in . For monotherapy with amrubicin, five studies have been reported (three in Japan and two in the USA and EU). In the three studies in Japan, patients with refractory or relapsed SCLC were enrolled.Citation33–Citation35 The first group contained patients who experienced progression of disease occurring within 3 months of completion of initial therapy, including people who never responded and people who responded but relapsed quickly (refractory group), and the second group contained patients who relapsed more than 3 months from completion of initial therapy (sensitive group).
Table 2 Phase II studies of amrubicin monotherapy or combination therapy with a platinum agent in SCLC patients
Amrubicin was administered at 35–40 mg/m2 IV for 3 consecutive days, every 3–4 weeks. The overall response rate (ORR) in the sensitive group was 52%–60% and in the refractory group 17%–50%. The median progression-free survivals (PFSs) in the refractory groups and the sensitive groups were 2.6–4.0 months and 3.9–4.5 months, respectively. The median survival times (MSTs) in the refractory groups were 5.3–11 months and in the sensitive groups were 9.2–12 months. The main toxicity was hematologic toxicities including neutropenia, thrombocytopenia, and anemia with febrile neutropenia, while nonhematologic toxicity was mild. These results indicated that amrubicin has significant efficacy against SCLC, with acceptable toxicity in Japanese patients.Citation33–Citation35 In the second trial, the effects of amrubicin and topotecan were compared.Citation34 Eligible patients with SCLC previously treated with platinum-based chemotherapy were randomly assigned to receive amrubicin (40 mg/m2, days 1 through 3) or topotecan (1.0 mg/m2, days 1 through 5). Of 60 patients enrolled, 59 (36 sensitive and 23 with refractory relapse) were assessable for efficacy and safety evaluation. Amrubicin treatment resulted in a significantly higher ORR than topotecan (38% versus 13%). The median PFS was 3.5 months for amrubicin and 2.2 months for topotecan treatment. One treatment-related death due to infection occurred in the amrubicin group. The results indicated that amrubicin is superior to topotecan for the treatment of previously-treated SCLC patients.
In order to investigate the efficacy of amrubicin in Western patients, recently two Phase II trials of amrubicin as second-line therapy in SCLC patients with refractory or sensitive relapse have been completed in the USA and EU.Citation33–Citation37 In the first trial, patients with refractory SCLC (either with progressive disease as the best response or progression within 90 days of first-line therapy) received amrubicin (40 mg/m2/day for 3 days every 21 days). Seventy-five eligible patients were enrolled and 69 patients received an average of four cycles of treatment. The ORR was 21.3% with one complete response (1.3%) and 15 partial responses (20%). The median PFS was 3.2 months and the overall survival (OS) 6.0 months. The ORR in 43 patients who did not respond to first-line therapy was 16.3%. Adverse events occurred as neutropenia (67%), thrombocytopenia (41%), and anemia (30%), with febrile neutropenia in 12%. No early cardiotoxicity was observed, even with cumulative amrubicin doses exceeding 750 mg/m2. These results demonstrated that monotherapy with amrubicin as second-line therapy shows promising activity and an acceptable safety in Western patients with refractory SCLC.Citation36 The second randomized Phase II trial to compare single-agent amrubicin with topotecan as second-line treatment in patients with SCLC sensitive to first-line platinum-based chemotherapy was conducted in the USA.Citation37 Seventy-six patients were enrolled in the study. Of them, 50 patients were randomly assigned to the amrubicin group and 26 to the topotecan group. Patients received amrubicin (40 mg/m2/day in a 5-minute IV infusion, days 1 through 3, every 21 days) or topotecan (1.5 mg/m2/day in a 30-minute IV infusion, days 1 through 5, every 21 days). Amrubicin resulted in a significantly higher ORR than topotecan (44% versus 15%). The median PFS was 4.5 months for amrubicin and 3.3 months for topotecan, while the median OS was 9.2 months for amrubicin, and 7.6 months for topotecan. Worse adverse effects (neutropenia and thrombocytopenia) occurred in the topotecan group.Citation37 These results demonstrated that amrubicin has better activity than topotecan as second-line treatment in Western patients with SCLC sensitive to first-line platinum-based chemotherapy.
Monotherapy with amrubicin has shown positive effects in patients with SCLC in previous studies. Since SCLC patients are traditionally sensitive to platinum agents, it would be interesting to evaluate the efficacy of combination therapy of amrubicin with cisplatin or carboplatin. Three Phase II trials of the combination therapy of amrubicin with platinum agents have been conducted in Japan. The first study in previously-untreated patients with ED-SCLC was reported in 2005.Citation29 Forty-one patients received amrubicin (40 mg/m2, days 1–3) and cisplatin (60 mg/m2, day 1), and 22 (54%) patients completed four cycles without dose modification. However, ten (31%) patients had a reduction in the amrubicin dose at the fourth cycle. The ORR was 87.8% and the MST was 13.6 months, while the 1-year survival rate was 56.1%. Adverse effects included grade 3/4 neutropenia and leukopenia occurred in 95.1% and 65.9% of patients, respectively. The combination therapy of amrubicin and cisplatin shows a significant efficacy in patients with previously-untreated ED-SCLC.
Recently, a European group reported a multicenter Phase II study of amrubicin as the single agent or in combination with cisplatin versus cisplatin etoposide as the first-line treatment in patients with ED-SCLC.Citation38 From June 2006 to July 2009, 99 patients with previously-untreated ED-SCLC were randomized: 33 in each arm. Eligible patients received 3 weekly cycles of either amrubicin alone (45 mg/m2 IV on days 1–3; A), cisplatin 60 mg/m2 IV on day 1 and amrubicin 40 mg/m2 IV on days 1–3 (PA), or cisplatin 75 mg/m2 IV on day 1 and etoposide 100 mg/m2 IV on day 1, oral administration 200 mg/m2 on days 2–3, or 100 mg/m2 IV on days 1–3 (PE). For the 88 eligible patients who started treatment, the ORR was 61% for the A arm, 77% for the PA arm, and 63% for the PE arm. The PFS was 5.2 months for A, 6.9 for PA , and 5.8 for PE, while OS (10–11 months) was similar in all arms. The major toxicity was grade three hematological in the A, PA, and PE arms, and included neutropenia (73%, 73%, 69%), thrombocytopenia (17%, 15%, 9.4%), anemia (10%, 15%, 3.1%) and febrile neutropenia (13%, 18%, 6%), respectively. One treatment-related death occurred in the A arm, three in the PA arm, and three in the PE arm. This study demonstrated that the combination therapy of amrubicin with cisplatin has the highest response rate in Western patients. Further evaluation of this therapy is warranted.
Since carboplatin has less nonhematological toxicity than cisplatin, a Phase II trial of amrubicin combined with carboplatin for chemotherapy-naïve elderly patients with SCLC was carried out.Citation31 The patients received amrubicin (35 mg/m2 for 3 days) and carboplatin (AUC 4.0, day 1) every 21 days. Thirty-six patients with an average age of 76 (range 70–83) were enrolled. The ORR was 89% with one complete response and 31 partial responses. The median PFS was 5.8 months and the MST was 18.6 months. Grade 3–4 neutropenia occurred in most patients (97%) and six (17%) had grade 3–4 febrile neutropenia, while other toxic effects were moderate. These results demonstrated that amrubicin combined with carboplatin is effective for SCLC with acceptable toxicity even in an elderly population. To evaluate the efficacy of the combination therapy in patients with previously-treated SCLC, another Phase II trial of combination therapy of amrubicin with carboplatin was recently completed.Citation39 Twenty-five eligible patients received amrubicin (30 mg/m2/day for 3 days) plus carboplatin (AUC 4 mg minute/mL using the Calvert formula on day 1) every 3 weeks. The ORR was 36.0% and the PFS was 3 months. The response rates differed significantly between patients with sensitive relapse (58.3%) and those with refractory relapse (15.4%). The median PFS time of patients with sensitive relapse (5 months) was significantly longer than that of patients with refractory relapse (2 months). The hematological toxicity was similar to previous reports, while nonhematologic toxicity was temporary and generally mild to moderately severe. To circumvent side-effects, it has been suggested that Pegfilgrastim, a long-lasting G-CSF, should be added to the regimen, and a trial with amrubicin and carboplatin with Pegfilgrastim in patients with ED-SCLC has been initiated in a population in the USA (NCT01076504).
Phase III trials
So far, only two Phase III trials of amrubicin as a single agent or in combination with cisplatin for the treatment of SCLC have been completed. The first Phase III trial of amrubicin in Western patients was reported at the American Society of Clinical Oncology annual meeting in 2011.Citation40 This multicenter trial was designed to compare the safety and efficacy of amrubicin versus topotecan for the second-line treatment of SCLC. A total of 637 patients were randomly assigned 2:1 to amrubicin treatment (40 mg/m2 IV on days 1–3, n = 424) or to topotecan treatment (1.5 mg/m2 IV on days 1–5, n = 213) with prophylactic white blood cell growth factors required in the last third of the trial. Amrubicin treatment resulted in a significantly higher ORR than topotecan (31% versus 17%). The median PFS was 4.1 months and the median OS was 7.5 months with amrubicin, and the corresponding values were 4.0 months and 7.8 months with topotecan. The 12-month, 18-month, and 24-month OS was 28%, 16%, 9% in the amrubicin group, and 25%, 9%, and 3% in the topotecan group, respectively. Adverse events (grade 3/4) in the amrubicin versus the topotecan groups were neutropenia (41% versus 53%), thrombocytopenia (21% versus 54%), anemia (16% versus 30%), infections (16% versus 10%), febrile neutropenia (10% versus 4%), and cardiac disorders (5% versus 5%).Citation40 The results showed that amrubicin is more effective in the second-line treatment of SCLC with significant improvement in ORR, and with survival trending in favor of amrubicin (HR 0.88), especially in the refractory group of patients (HR 0.77).
For combination therapy with cisplatin, the early results of a Phase III trial in a Japanese population were recently reported.Citation41 The study was designed to compare amrubicin and cisplatin (AP) with irinotecan and cisplatin (IP) for the treatment of ED-SCLC. The primary endpoint was OS and the secondary endpoints were RR, PFS adverse events, and quality of life. A total of 284 patients were randomized 1:1 to receive either IP or A P. Patients in the IP group received irinotecan (60 mg/m2, IV on days 1, 8, and 15), and cisplatin (60 mg/m2, IV on day 1, every 4 weeks). Patients in the AP group received amrubicin (40 mg/m2, IV on days 1–3, and cisplatin (60 mg/ m2, IV on day 1, every 3 weeks). One hundred ninety-one patients were enrolled, and more febrile neutropenia unexpectedly occurred in the AP group. To cope with the adverse events, the dose of amrubicin was then reduced to 35 mg/m2. The median OS of AP (15.0 months) was worse than that of IP (18.3 months). No improvement in the median PFS and ORR was found. AP showed more bone marrow suppression than expected although it caused fewer cases of diarrhea. Another multicenter Phase III trial of amrubicin with cisplatin versus etoposide-cisplatin for ED-SCLC is now being carried out in the People’s Republic of China (NCT00660504), but the results have not yet been released.
These results clearly indicate that improvement of current regimens is crucial for the success of amrubicin treatment. As part of these efforts, a triplet combination regimenCitation44 and a weekly regimenCitation28 have been studied for the treatment of patients with SCLC and NSCLC. Very recently, an alternating regimen with amrubicin plus cisplatin and weekly administration of irinotecan plus cisplatin for ED-SCLC has been reported with good outcomes.Citation43
Future directions for amrubicin therapy
During the last 10 years, amrubicin therapy has shown promise for the treatment of SCLC, but challenges still remain. First, finding the best regimen is the most important task. The prophylactic use of G-CSF to reduce myelosuppression and enhance the efficacy of amrubicin at a relatively low dose is a promising idea.Citation44 Second, several lines of evidence indicate that the genetic backgrounds of patients may be very important for achieving optimal outcomes. NADPH:NQO1 and NADPH-P450 reductase are enzymes involved in the metabolism of amrubicin.Citation25 The levels and gene polymorphisms of these enzymes in the Asian and Western population might explain the different effectiveness of the drug in patients of different ethnicities.Citation45 Clearly, finding predictive biomarkers for the selection of patients susceptible to amrubicin treatment is the basis of successful therapy. In the future, the design of personalized regimens by single-agent or combination therapy with amrubicin may achieve ideal effects. Finally, the profound elucidation of the signaling pathways related to carcinogenesis, proliferation, apoptosis, and metastasis in SCLC is crucial for the optimization of new therapeutic regimens. It may be worthwhile to design targeted therapy with amrubicin by combining it with monoclonal antibodies or with antibody drugs to reduce the toxicity and increase efficacy and safety.Citation46,Citation47 Antibody-drug conjugate as a new type of targeted therapy has been successfully used in cancer-targeted therapy.Citation48 As a rapidly expending drug modality, antibody-drug conjugates have also been actively studied for the treatment of lung cancer, including SCLC.Citation48,Citation49 The targeted therapy using antibody-amrubicin or amrubicinol may dramatically change therapeutic regimens and offer promise against the devastating cancer in the near future.
Acknowledgments
We thank Dr Iain Bruce, University of Hong Kong, for critical reading of the manuscript. This work was partially supported by Fundamental Research Funds for the Central Universities, People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ferlay J Shin HR Bray F Forman D Mathers C Parkin DM Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 Int J Cancer 2010 127 12 2893 2917 21351269
- Hann CL Rudin CM Management of small-cell lung cancer: incremental changes but hope for the future Oncology (Williston Park, NY) 2008 22 13 1486 1492
- Kalemkerian G P Akerley W Bogner P Small cell lung cancer J Natl Compr Canc Netw 2013 11 1 78 98 23307984
- Murray N Turrisi AT A review of first-line treatment for small-cell lung cancer J Thorac Oncol 2006 1 3 270 278 17409868
- Govindan R Page N Morgensztern D Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database J Clin Oncol 2006 24 28 4539 4544 17008692
- Stinchcombe TE Gore EM Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms Oncologist 2010 15 2 187 195 20145192
- Kurata T Okamoto I Tamura K Fukuoka M Amrubicin for non-small-cell lung cancer and small-cell lung cancer Invest New Drugs 2007 25 5 499 504 17628745
- Kimura T Kudoh S Hirata K Review of the management of relapsed small-cell lung cancer with amrubicin hydrochloride Clin Med Insights Oncol 2011 5 23 34 21499556
- Shah CM Amrubicin: a synthetic anthracyclin analogue in the treatment of extensive stage small cell lung cancer Recent Pat Anticancer Drug Discov 2009 4 3 241 245 19538159
- Hanada M Mizuno S Fukushima A Saito Y Noguchi T Yamaoka T A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex Jpn J Cancer Res 1998 89 11 1229 1238 9914793
- Morisada S Yanagi Y Noguchi T Kashiwazaki Y Fukui M Antitumor activities of a novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenografts Jpn J Cancer Res 1989 80 1 69 76 2496061
- Noda T Watanabe T Kohda A Hosokawa S Suzuki T Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs Invest New Drugs 1998 16 2 121 128 9848575
- Salvatorelli E Menna P Surapaneni S Pharmacokinetic characterization of amrubicin cardiac safety in an ex vivo human myocardial strip model. I. Amrubicin accumulates to a lower level than doxorubicin or epirubicin J Pharmacol Exp Ther 2012 341 2 464 473 22338033
- Salvatorelli E Menna P Gonzalez Paz O Pharmacokinetic characterization of amrubicin cardiac safety in an ex vivo human myocardial strip model. II. Amrubicin shows metabolic advantages over doxorubicin and epirubicin J Pharmacol Exp Ther 2012 341 2 474 483 22338034
- Yamaoka T Hanada M Ichii S Morisada S Noguchi T Yanagi Y Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells Jpn J Cancer Res 1998 89 10 1067 1073 9849587
- Frederick CA Williams LD Ughetto G Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin Biochemistry 1990 29 10 2538 2549 2334681
- Fornari FA Randolph JK Yalowich JC Ritke MK Gewirtz DA Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells Mol Pharmacol 1994 45 4 649 656 8183243
- Pigram WJ Fuller W Hamilton LD Stereochemistry of intercalation: interaction of daunomycin with DNA Nature New Biol 1972 235 53 17 19 4502404
- Minotti G Menna P Salvatorelli E Cairo G Gianni L Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity Pharmacol Rev 2004 56 2 185 229 15169927
- Okamoto I Hamada A Matsunaga Y Phase I and pharmacokinetic study of amrubicin, a synthetic 9-aminoanthracycline, in patients with refractory or relapsed lung cancer Cancer Chemother Pharmacol 2006 57 3 282 288 16028099
- Hamada A [Development of an individualized therapy for establishing the optimal dosage by the pharmacokinetics profiles of anticancer agents.] Yakugaku Zasshi 2005 125 8 631 637 Japanese 16079613
- Kimura T Kudoh S Mitsuoka S Plasma concentration of amrubicinol in plateau phase in patients treated for 3 days with amrubicin is correlated with hematological toxicities Anticancer Drugs 2009 20 6 513 518 19352172
- Matsunaga Y Hamada A Okamoto I Pharmacokinetics of amrubicin and its active metabolite amrubicinol in lung cancer patients Ther Drug Monit 2006 28 1 76 82 16418698
- Makino Y Yamamoto N Sato H Pharmacokinetic and pharmacodynamic study on amrubicin and amrubicinol in Japanese patients with lung cancer Cancer Chemother Pharmacol 2012 69 4 861 869 22042514
- Tani N Yabuki M Komuro S Kanamaru H Characterization of the enzymes involved in the in vitro metabolism of amrubicin hydrochloride Xenobiotica 2005 35 12 1121 1133 16418065
- Nagata M Kimura T Suzumura T C609T polymorphism of NADPH quinone oxidoreductase 1 correlates clinical hematological toxicities in lung cancer patients treated with amrubicin Clin Med Insights Oncol 2013 7 31 39 23467445
- Takakuwa O Oguri T Ozasa H C609T polymorphism of NAD(P) H quinone oxidoreductase 1 as a predictive biomarker for response to amrubicin J Thorac Oncol 2011 6 11 1826 1832 21964527
- Kitagawa C Saka H Kajikawa S Mori K Oki M Suzuki R Phase I and pharmacologic study of weekly amrubicin in patients with refractory or relapsed lung cancer: Central Japan Lung Study Group (CJLSG) 0601 trial Cancer Chemother Pharmacol 2012 69 5 1379 1385 22237957
- Ohe Y Negoro S Matsui K Phase I-II study of amrubicin and cisplatin in previously untreated patients with extensive-stage small-cell lung cancer Ann Oncol 2005 16 3 430 436 15653702
- Fukuda M Nakamura Y Kasai T Nagasaki Thoracic Oncology Group A phase I study of amrubicin and carboplatin for previously untreated patients with extensive-disease small cell lung cancer J Thorac Oncol 2009 4 6 741 745 19404211
- Inoue A Ishimoto O Fukumoto S A phase II study of amrubicin combined with carboplatin for elderly patients with small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0405 Ann Oncol 2010 21 4 800 803 19825887
- Asakuma M Yamamoto M Wada M Phase I trial of irinotecan and amrubicin with granulocyte colony-stimulating factor support in extensive-stage small-cell lung cancer Cancer Chemother Pharmacol 2012 69 6 1529 1536 22415148
- Onoda S Masuda N Seto T Thoracic Oncology Research Group Study 0301 Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301 J Clin Oncol 2006 24 34 5448 5453 17135647
- Inoue A Sugawara S Yamazaki K Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402 J Clin Oncol 2008 26 33 5401 5406 18854562
- Kaira K Sunaga N Tomizawa Y A phase II study of amrubicin, a synthetic 9-aminoanthracycline, in patients with previously treated lung cancer Lung Cancer 2010 69 1 99 104 19853960
- Ettinger DS Jotte R Lorigan P Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer J Clin Oncol 2010 28 15 2598 2603 20385980
- Jotte R Conkling P Reynolds C Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy J Clin Oncol 2011 29 3 287 293 21135284
- O’Brien ME Konopa K Lorigan P Randomised phase II study of amrubicin as single agent or in combination with cisplatin versus cisplatin etoposide as first-line treatment in patients with extensive stage small cell lung cancer – EORTC 08062 Eur J Cancer 2011 47 15 2322 2330 21684151
- Hirose T Nakashima M Shirai T Phase II trial of amrubicin and carboplatin in patients with sensitive or refractory relapsed small-cell lung cancer Lung Cancer 2011 73 3 345 350 21277039
- Jotte R von Pawel J Spigel DR Randomized phase III trial of amrubicin versus topotecan (Topo) as second-line treatment for small cell lung cancer (SCLC) 2011 American Society of Clinical Oncology Annual Meeting Proceedings (Post-Meeting Edition) June 3–7, 2011 Chicago, Illinois 29 No 15_suppl May 20 Supplement 2011 7000
- Kotani Y Satouchi M Ando A A phase III study comparing amrubicin and cisplatin (AP) with irinotecan and cisplatin (IP) for the treatment of extended-stage small cell lung cancer (ED-SCLC) 2012 American Society of Clinical Oncology Annual Meeting Proceedings (Post-Meeting Edition) June 1–5, 2012 Chicago, Illinois 30 No 15_suppl May 20 Supplement 2012 7003
- Kobayashi M Matsui K Iwamoto Y West Japan Oncology Group Phase II study of sequential triplet chemotherapy, irinotecan and cisplatin followed by amrubicin, in patients with extensive-stage small cell lung cancer: West Japan Thoracic Oncology Group Study 0301 JThorac Oncol 2010 5 7 1075 1080 20479694
- Noro R Yoshimura A Yamamoto K East Japan Chesters Group Alternating chemotherapy with amrubicin plus cisplatin and weekly administration of irinotecan plus cisplatin for extensive-stage small cell lung cancer Anticancer Res 2013 33 3 1117 1123 23482789
- Hata A Katakami N Fujita S Amrubicin at a lower-dose with routine prophylactic use of granulocyte-colony stimulating factor for relapsed small-cell lung cancer Lung Cancer 2011 72 2 224 228 20828862
- Ettinger DS Amrubicin for the treatment of small cell lung cancer: does effectiveness cross the Pacific? J Thorac Oncol 2007 2 2 160 165 17410034
- Abidin AZ Garassino MC Califano R Harle A Blackhall F Targeted therapies in small cell lung cancer: a review Ther Adv Med Oncol 2010 2 1 25 37 21789124
- Zhan J Han Q Wang K Development of antibody therapeutics for small cell lung cancer Expert Opin Investig Drugs 2013 22 2 235 244
- Scott AM Wolchok JD Old LJ Antibody therapy of cancer Nat Rev Cancer 2012 12 4 278 287 22437872
- Mullard A Maturing antibody-drug conjugate pipeline hits 30 Nat Rev Drug Discov 2013 12 5 329 332 23629491