Abstract
Purpose
To examine whether intraoperative dexmedetomidine reduces postoperative delirium (POD) in elderly patients who underwent a laryngectomy.
Methods
Patients were randomly assigned to receive dexmedetomidine or a saline placebo infused during surgery. The study period was July 2020 to January 2022. Participants were elderly individuals (≥65 years) who underwent a laryngectomy. Immediately after induction of anesthesia, a 0.5 μg.kg−1 bolus of study solution was administered for 10 min, followed by a maintenance infusion of 0.2 μg.kg−1.hr−1 until the end of surgery. Patients were assessed daily for POD (primary outcome). Plasma inflammatory factors were measured at baseline, on the first postoperative day, and on the third postoperative day.
Results
In total, 304 male patients were randomized; 299 patients [median (interquartile range) age, 69.0 (67.0–73.0) years] completed in-hospital delirium assessments. There was no difference in the incidence of POD between the dexmedetomidine and control groups (21.3% [32 of 150] vs 24.2% [36 of 149], P=0.560). However, dexmedetomidine reduced POD in patients with laryngeal cancer and a higher tumor stage (21.6% vs 38.5%; OR, 0.441; 95% CI, 0.209–0.979; P=0.039). Dexmedetomidine reduced levels of C-reactive protein (CRP) (P=0.0056) and interleukin 6 (IL-6) (P<0.001) on the first and third postoperative days, respectively. More patients had intraoperative hypotension in the dexmedetomidine group (29.3% [44 of 150] vs 17.4% [26 of 149], P=0.015).
Conclusion
Intraoperative dexmedetomidine administration did not prevent POD in patients with laryngeal cancer. Dexmedetomidine reduced serum CRP and IL-6 levels postoperatively but caused a higher occurrence of intraoperative hypotension in elderly patients after a laryngectomy.
Introduction
Populations are aging rapidly across the world,Citation1 and the number of older adults undergoing surgery is increasing.Citation2 Notably, postoperative delirium (POD) is a common postoperative complication, with an incidence of up to 54.4% especially in older patients after a major surgery.Citation3 POD is an independent predictor for postoperative mortality associated with adverse outcomes, such as a loss of independence, failure to return to work, substantial increase in healthcare costs, and death.Citation4–6 Delirium is also linked with cognitive declineCitation7,Citation8 and an increased risk of dementia.Citation9 Patients with head and neck tumors are a high-risk group for POD due to impairment, disfigurement, and psychosocial problems during surgery treatment.Citation10,Citation11 It is essential to develop effective strategies, both pharmacologic and nonpharmacologic interventions, to reduce POD.Citation12–14
Several studies have shown that dexmedetomidine, an α2-adrenergic agonist, may be a promising pharmacologic option for preventing POD,Citation15–18 as the neuroprotective effect of dexmedetomidine relates to its anti-inflammatory effect.Citation19,Citation20 Conversely, some studies do not support its neuroprotective effect in reducing POD.Citation21 In our previous observational study,Citation22 we found that intraoperative dexmedetomidine use had a preventive effect on POD in patients who underwent a laryngectomy. Due to limitations of our previous study and inconsistent findings of other studies, we conducted a prospective, single-center, randomized controlled trial to investigate whether intraoperative dexmedetomidine use prevents POD. We also aimed to evaluate perioperative changes in serum inflammatory factors, since inflammation contributes to the pathogenesis of delirium.Citation23,Citation24
Methods
Participants
This trial was approved by the Eye and ENT Hospital, Fudan University Review Board and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000032062). Written informed consent was obtained from all patients. This study was conducted in the accordance with the Declaration of Helsinki. During the period of July 2020 to January 2022, patients aged ≥65 years scheduled for a laryngectomy were recruited and randomly allocated (1:1 ratio) to receive dexmedetomidine or normal saline (NS).
Exclusion criteria consisted of patients who were unable to complete baseline cognitive assessments; factors that might affect cognitive assessment, such as language, visual, and auditory dysfunction; an unstable mental health or mental illness; sick sinus syndrome; second-degree or third-degree heart block or clinically significant sinus bradycardia; contraindication for use of an α2-adrenergic agonist; female sex; preoperative delirium or pre-existing cognitive impairment; and patients who declined to participate.
Anesthesia Protocol
Dexmedetomidine or NS was unknown to participants, investigators, and clinicians. The anesthesiologist was not blinded to the study. The concentration of dexmedetomidine (Jiangsu Enhua Medicine Co., Ltd., Jiangsu, China) was 2 μg.mL−1. Immediately after induction of anesthesia, a 0.5 μg.kg−1 bolus of study solution was administered for 10 min followed by a maintenance infusion of 0.2 μg.kg−1.hr−1 until the end of surgery. In case malignant arrhythmia or severe bradycardia during dexmedetomidine pumping occurs, dexmedetomidine use discontinues. All patients underwent standard intraoperative monitoring. In general, anesthetic management comprised propofol for induction, sevoflurane for maintenance (minimal alveolar concentration, MAC 0.8–1.0), remifentanil and sufentanil for intraoperative use, and nondepolarizing muscle relaxant use. Hydromorphone was administered at the end of surgery for pain control. The depth of anesthesia was maintained at bi-spectral indices of 40–60 as long as possible. Body temperature was maintained at ≥36 °C. Antiemetics were administered to prevent nausea and vomiting. Postoperatively, patients typically received patient-controlled analgesia (sufentanil), one intravenous bolus containing 0.025 μg.kg−1 sufentanil, and 2 mg of flurbiprofen axetil, with a lockout of 10 min and a background hourly infusion of 0.025 μg.kg−1 sufentanil and 2 mg of flurbiprofen axetil.
POD Assessment
Investigators with psychological training in POD assessments were blinded. The once-daily POD assessment was conducted on the first 5 postoperative days because evidence suggests that over 90% of delirium occurs within this period.Citation25 Patients were interviewed once daily between 4:00 pm and 6:00 pm. The presence of delirium was assessed using the confusion assessment method diagnostic algorithm.Citation26 Once the patients are diagnosed with POD, they will be evaluated continuously and be treated if necessary until patients’ cognitive functioning return to normal.
Inflammatory Factor Measurements
Blood samples were collected at three time points: shortly after anesthesia induction, and in the morning on postoperative day 1 and postoperative day 3. Samples were collected in tubes containing ethylenediaminetetraacetic acid and processed within 3 h of collection. Samples were centrifuged at 3000 × g for 10 min, and plasma was removed and stored at −80 °C until the assay. Plasma concentrations of C-reactive protein (CRP) and interleukin 6 (IL-6) were measured using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Human ELISA Kits, Biotech Well, Shanghai, China). All experiments were repeated three times. All coefficients of intra-assay and inter-assay variation for quantitative detection were less than 5%.
Other Data
Participants were interviewed prior to surgery to obtain baseline information, including level of education, as well as Mini-Mental State Examination (MMSE) and Cumulative Illness Rating Scale (CIRS) results. Additional perioperative information was obtained through a review of medical records. The earliest stage of laryngeal cancer is stage 0, also known as carcinoma in situ; the other main stages range from stage I to IV. We defined tumor stages 3 and 4 as higher tumor stages. Intraoperative hypotension was defined as a blood pressure 70% of baseline and lasting at least 30 min.Citation27,Citation28 Self-reported sleep quality was assessed using five dichotomous questions: (1) “Did you sleep well?” (2) “Did you sleep better than expected?” (3) “Did you sleep better than at home?” (4) “Were you awake for a long time before falling asleep?” and (5) “Do you feel sufficiently rested?” If the patient answered yes, they scored 1 point for these questions. The score for question 4 was reversed. A score <2 was categorized as poor sleep.Citation29 An unauthorized version of the Chinese MMSE was used by the study team without permission. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com).
Statistical Analysis
Based on our previous study,Citation22 we found that in patients aged ≥65 years, the overall incidence of POD was 15.0%, with 8.6% in patients who received dexmedetomidine and 20.3% in patients who did not receive dexmedetomidine. A sample size of 284 participants (142 per group) was calculated to have 80% power to detect differences at a statistical threshold of <0.05. We anticipated a 5% withdrawal and recruited 304 participants.
Continuous variables were compared using Student’s unpaired t-test or the Mann–Whitney U-test, as appropriate. Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test. Changes in the serum inflammatory factors of CRP and IL-6 were analyzed using generalized linear mixed modeling. We reported two-sided P-values and considered a value of p<0.05 to be statistically significant.
Results
Participant Characteristics
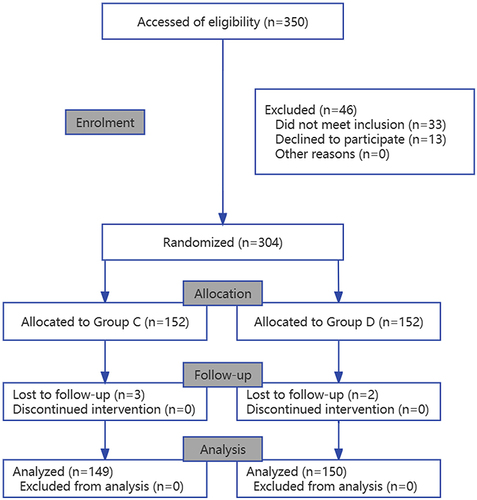
During the study period, 350 patients were included. Thirteen patients declined to participate preoperatively. Thirty-three patients switched to laser treatment after re-assessment by surgeons in the operating room. Therefore, 304 participants were included and categorized as follows: 152 to the dexmedetomidine group and 152 to the saline placebo group. Five patients dropped out during POD assessment, with two patients dropping out after receiving dexmedetomidine and three patients dropping out after receiving saline ().
Figure 1 Consolidated Standards of Reporting Trials diagram. A patient flow diagram is shown. Group C, Control group; Group D, Dexmedetomidine group.
At baseline, the median (interquartile range) age of patients was 69.0 (67.0–73.0) years. Characteristics of patients in the dexmedetomidine and placebo groups are shown in . There were more patients with a higher tumor stage in the dexmedetomidine group (74 vs 52, P=0.012). Intraoperative sufentanil use was less in the dexmedetomidine group (27.1 μg vs 30.6 μg, P<0.001).
Table 1 Patient Characteristics Between the Two Groups
Outcome of POD
A total of 299 patients completed the in-hospital POD assessments. Among these patients, 68 (22.7%) developed POD, including 32 in the dexmedetomidine group (21.3%) and 36 (24.2%) in the control group; the incidence of POD was comparable between the two groups (OR, 0.851; 95% CI, 0.493–1.45; P=0.560) ().
Table 2 Incidence of POD Between Two Groups
Participants were stratified by tumor stage into two strata (lower tumor stage and higher tumor stage). We found that in patients with laryngeal cancer and a lower tumor stage, the incidence of POD was similar between the control group (21.1%) and the dexmedetomidine group (16.5%) (OR, 1.35; 95% CI, 0.61–2.99; P=0.444). However, dexmedetomidine reduced POD in patients with laryngeal cancer and a higher tumor stage (21.6% vs 38.5%; OR, 0.441; 95% CI, 0.209–0.979; P=0.039) ().
Changes in Inflammatory Factors
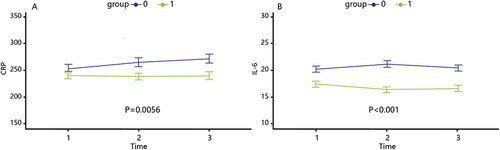
In total, 160 patients underwent a full course of blood sampling. Patient characteristics are presented in . Results of the mixed effect model showed that there were significant differences in CRP and IL-6 levels between the dexmedetomidine and control groups (P=0.0056 for CRP and P<0.001 for IL-6) (). illustrates that CRP levels in the control group show an upward trend, while those in the dexmedetomidine group are stable illustrates that IL-6 levels in the control group show an upward trend, whereas those in the dexmedetomidine group show a downward trend.
Table 3 Patient Characteristics Between the Two Groups of Blood Sampling
Other Outcomes
More patients in the dexmedetomidine group had intraoperative hypotension (42 vs 26, P=0.015). Hospital stay length (median:13 days vs 13 days, P=0.785) were comparable between the two groups. No patients experienced malignant arrhythmia or severe bradycardia during dexmedetomidine pumping.
Discussion
In the current study, we found that intraoperative dexmedetomidine did not reduce POD in elderly patients after a laryngectomy. Intraoperative dexmedetomidine use prevented the inflammatory factors of CRP and IL-6 from increasing postoperatively but was related to a higher occurrence of intraoperative hypotension.
The incidence of POD was 21.3% in patients who received dexmedetomidine. This is higher than a previously reported rate that was used to calculate the sample size (8.6%).Citation22 Possible reasons for this are as follows. First, the study design was different (a prospective, randomized study vs an observational study). Second, the sample size of patients aged ≥65 years in the current study was larger (150 vs 58 patients).
The findings of our study add to those of several studies that tested whether intraoperative dexmedetomidine use can reduce POD. Previous research involving patients under general anesthesia suggests that perioperative dexmedetomidine use can reduce POD after a major surgery.Citation15–19,Citation30 Though, consistent with a previous study,Citation21 we did not find a difference in delirium in patients randomized to perioperative dexmedetomidine use. However, subgroup analyses show that intraoperative dexmedetomidine use decreased the incidence of POD in patients with a higher tumor stage.
Previous studies concerning POD were usually carried out for severe traumatic surgery such as cardiac surgeryCitation16 and major non-cardiac surgery such as abdominal and thoracic surgery.Citation15 The surgical procedure for a laryngectomy is relatively less traumatic, especially for laryngeal tumors of stage I and II. However, the higher the stage, the more advanced the cancer, indicating more tissue trauma and a more severe inflammatory response caused by a laryngectomy. In addition, research on the association between inflammation and cancer pathogenesis reveals that inflammation can facilitate cancer angiogenesis, invasion, and metastasis.Citation31 Furthermore, CRP was significantly correlated with tumor size and stage in a previous study.Citation32 This may explain why one of our observations was that dexmedetomidine use reduced the incidence of POD in patients with a higher tumor stage.
Surgery elicits a systemic inflammatory responseCitation33 that involves the pathophysiology of delirium.Citation34 Dexmedetomidine has been reported to exert its neuroprotective effect by inhibiting the expression and release of inflammatory factors.Citation19,Citation20 CRP is associated with an increased risk for PODCitation35 and IL-6 has a clear association with severe postoperative complications in thoracic surgery.Citation36 In our study, we found that, compared to NS, dexmedetomidine reduced CRP and IL-6 postoperatively. This finding may provide evidence for the anti-inflammatory effects of dexmedetomidine.
We found that more patients in the dexmedetomidine group experienced intraoperative hypotension. Hypotension is one of the most common adverse reactions associated with dexmedetomidine.Citation37 As a result of temporarily insufficient cerebral perfusion, delirium may be a more sensitive manifestation of postoperative cerebral dysfunction. In terms of the negative result that dexmedetomidine use did not reduce the incidence of POD, an explanation may be that intraoperative hypotension outweighs the anti-inflammatory effect of dexmedetomidine. Our findings also support the optimization of intraoperative hemodynamics to lower the incidence of POD.
Limitations and Strengths
This study had several limitations. First, as this was a single-center study, only patients scheduled for a laryngectomy were enrolled. Therefore, the generalizability of our results may be limited. Second, we assessed POD only once daily and its incidence may have been underestimated. The single assessment was performed each day between 4:00 pm and 6:00 pm by investigators experienced in assessing POD, which means that POD may have been well detected only in the evening. In addition, we only included male patients, since there were few female patients with a laryngeal tumor and they tended to decline to participate in the study. Third, we did not collect data regarding the number of POD episodes, duration of POD, severity of POD, or potential consequences of POD, such as those affecting mortality and cognitive function at certain time points (eg, 6 months and 1 year). Finally, for pragmatic reasons, anesthesiologists were not blinded to group assignments. Furthermore, investigators who performed postoperative follow-ups and blood tests did not participate in perioperative care and had no knowledge of treatment assignments.
Strengths of this study include the following. We conducted our research in a homogeneous group for which we can make conclusive statements, and the observed changes in plasma levels of inflammatory factors over several days after surgery may improve our understanding of the correlation between postoperative inflammation and POD.
Conclusions
Based on these findings, we conclude that intraoperative dexmedetomidine use did not reduce POD in elderly patients after a laryngectomy. Dexmedetomidine use was associated with decreased postoperative CRP and IL-6 levels. The use of intraoperative dexmedetomidine resulted in a higher occurrence of intraoperative hypotension.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. Any information we share will be deidentified.
Ethical Statement
Ethics Approval: This trial was approved by the Eye and ENT Hospital, Fudan University Review Board and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000032062). Written informed consent was obtained from all patients.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors express their gratitude to the physicians and nurses of the departments that participated in this study. This study was funded by the Eye and ENT Hospital Double Priority Project A (SYA202010 to Dr. Shen).
References2
- Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
- McDermott KW, Freeman WJ, Elixhauser A. Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2014: statistical Brief #233; 2006.
- Scholz AF, Oldroyd C, McCarthy K, Quinn TJ, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21–8. doi:10.1002/bjs.10062
- Abelha FJ, Luís C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care. 2013;17(5):R257. doi:10.1186/cc13084
- Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150(12):1134–1140. doi:10.1001/jamasurg.2015.2606
- Maniar HS, Lindman BR, Escallier K, et al. Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality. J Thorac Cardiovasc Surg. 2016;151(3):815–823.e2. doi:10.1016/j.jtcvs.2015.10.114
- Gross AL, Jones RN, Habtemariam DA, et al. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med. 2012;172(17):1324–1331. doi:10.1001/archinternmed.2012.3203
- Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi:10.1056/NEJMoa1112923
- Olofsson B, Persson M, Bellelli G, et al. Development of dementia in patients with femoral neck fracture who experience postoperative delirium-A three-year follow-up study. Int J Geriatr Psychiatry. 2018;33(4):623–632. doi:10.1002/gps.4832
- De Boer MF, McCormick LK, Pruyn JF, Ryckman RM, van den Borne BW. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngol Head Neck Surg. 1999;120(3):427–436. doi:10.1016/S0194-5998(99)70287-1
- Singer S, Krauss O, Keszte J, et al. Predictors of emotional distress in patients with head and neck cancer. Head Neck. 2012;34(2):180–187. doi:10.1002/hed.21702
- Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in Older Persons: advances in Diagnosis and Treatment. JAMA. 2017;318(12):1161–1174. doi:10.1001/jama.2017.12067
- Wang YY, Yue JR, Xie DM, et al. Effect of the Tailored, Family-Involved Hospital Elder Life Program on Postoperative Delirium and Function in Older Adults: a Randomized Clinical Trial. JAMA Intern Med. 2020;180(1):17–25. doi:10.1001/jamainternmed.2019.4446
- Chen CC, Li HC, Liang JT, et al. Effect of a Modified Hospital Elder Life Program on Delirium and Length of Hospital Stay in Patients Undergoing Abdominal Surgery: a Cluster Randomized Clinical Trial. JAMA Surg. 2017;152(9):827–834. doi:10.1001/jamasurg.2017.1083
- Li CJ, Wang BJ, Mu DL, et al. Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br J Surg. 2020;107(2):e123–e132. doi:10.1002/bjs.11354
- Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus Propofol Sedation Reduces Delirium after Cardiac Surgery: a Randomized Controlled Trial. Anesthesiology. 2016;124(2):362–368. doi:10.1097/ALN.0000000000000951
- Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi:10.1016/S0140-6736(16)30580-3
- Brummel NE, Girard TD. Preventing delirium in the intensive care unit. Crit Care Clin. 2013;29(1):51–65. doi:10.1016/j.ccc.2012.10.007
- Li ZC, Jia YP, Wang Y, Qi JL, Han XP. Effects of dexmedetomidine post‑treatment on BDNF and VEGF expression following cerebral ischemia/reperfusion injury in rats. Mol Med Rep. 2018;17(4):6033–6037. doi:10.3892/mmr.2018.8597
- Yamanaka D, Kawano T, Nishigaki A, et al. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J Anesth. 2017;31(1):25–35. doi:10.1007/s00540-016-2264-4
- Deiner S, Luo X, Lin HM, et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: a Randomized Clinical Trial. JAMA Surg. 2017;152(8):e171505. doi:10.1001/jamasurg.2017.1505
- Wang Y, Yu H, Qiao H, et al. Risk Factors and Incidence of Postoperative Delirium in Patients Undergoing Laryngectomy. Otolaryngology–Head and Neck Surgery. 2019;161(5):807–813. doi:10.1177/0194599819864304
- Liu P, Li YW, Wang XS, et al. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: a prospective cohort study. Chin Med J. 2013;126(19):3621–3627.
- Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. doi:10.1111/j.1755-5949.2010.00173.x
- Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178. doi:10.1097/SLA.0b013e31818e4776
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
- Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. doi:10.1016/S0140-6736(97)07382-0
- Yang L, Sun DF, Han J, et al. Effects of Intraoperative Hemodynamics on Incidence of Postoperative Delirium in Elderly Patients: a Retrospective Study. Med Sci Monit. 2016;22:1093–1100. doi:10.12659/MSM.895520
- Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16(3):R73. doi:10.1186/cc11330
- Duan X, Coburn M, Rossaint R, et al. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
- Li Y, Liu X, Zhang J, Yao W. Prognostic role of elevated preoperative systemic inflammatory markers in localized soft tissue sarcoma. Cancer Biomark. 2016;16(3):333–342. doi:10.3233/CBM-160571
- Du Z, Wei SW, Zhang XY, Xiang Z, Qu SQ. The effect of dexmedetomidine premedication on postoperative systemic inflammatory response in children undergoing hernia repair surgery: a randomized controlled trial. Paediatr Anaesth. 2021;31(7):794–801. doi:10.1111/pan.14189
- Wang Y, Shen X. Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. 2018;30(11):1287–1295. doi:10.1007/s40520-018-1008-8
- Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-Reactive Protein Levels Predict Postoperative Delirium in Older Patients Undergoing Major Elective Surgery: a Longitudinal Nested Case-Control Study. Biol Psychiatry. 2017;81(2):145–153. doi:10.1016/j.biopsych.2016.03.2098
- Neff TA, Braun J, Rana D, et al. Interleukin-6 Is an Early Plasma Marker of Severe Postoperative Complications in Thoracic Surgery: exploratory Results From a Substudy of a Randomized Controlled Multicenter Trial. Anesth Analg. 2022;134(1):123–132. doi:10.1213/ANE.0000000000005639
- Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59(2):263–268. doi:10.2165/00003495-200059020-00012