Abstract
Objective
Chronic non-atrophic gastritis (CNAG) is a common clinical gastrointestinal disease with a long and recurrent course. In China, Wuzhuyu decoction (WZYD) has been used for centuries to treat gastrointestinal disorders. To unravel the efficacy and mechanism of WZYD for CNAG, a clinical study was conducted. And metabolomics was used to explore the mechanism of WZYD for CNAG patients.
Methods
Twenty patients in total were recruited in this study (Nos. ChiCTR2200062296) and the protocol was approved by the Ethics Committee (Approval number: KY-2022-2-6-1) and complied with the Declaration of Helsinki. The formula granule of WZYD were assessed by UHPLC-QQQ-TOF to discern the main potential active compounds. The endoscopy evaluation and histopathological changes were detected as effective indicators. Serum samples from patients were used for metabolomics. Inflammatory factors in patients’ serum were determined by ELISA. Metabolomics revealed a series of differential metabolites and signaling pathways.
Results
WZYD was capable to prevent CNAG by ameliorating score of endoscopy evaluation including erosion, hemorrhage, as well as chronic inflammation and active chronic inflammation score after treatment were decreased. The results indicated that 10 core metabolic components were associated with the treatment of WZYD. Moreover, these metabolic components proved that pyrimidine metabolism and thiamine metabolism were critically responsible for CNAG. In addition, WZYD treatment effectively reduced serum levels of TNF-α, IL-10, and COX-2.
Conclusion
Altogether, WZYD can effectively alleviate CNAG by inhibiting inflammation and regulating related metabolic processes, which might be the molecular mechanism of WZYD treatment of CNAG. More studies are warranted to be conducted in this area.
Trial Registration
ChiCTR, ChiCTR2200062296. Registered 1 August 2022, https://www.chictr.org.cn/com/25/showprojen.aspx?proj=174027.
Introduction
Efficacy and mechanism are two primary concerns in the field of traditional Chinese medicine (TCM).Citation1 Is TCM effective in treating specific diseases? What mechanisms are relevant to clinical efficacy? These challenges are inevitable in the development of TCM. With the emergence of bioinformatics, insights in this field have greatly advanced. Bioinformatics focuses on the comprehensive regulation of the whole organism, aligning closely with the ancient TCM theory of holism.Citation2,Citation3 Consequently, traditional theories and new concepts have converged in this new era. Researchers now recognize that diseases are characterized by complex and systematic pathology. As a result, therapy is correspondingly presented integrative signaling pathways or targets. Numerous therapies have already been explored for clinical applications. For example, a recent study conducted by a research team from China provided highly convincing evidence for the treatment of angina. The study involved a 31-center, randomized, double-blind clinical trial to evaluate the efficacy of Danhong injection (DHI) in patients with moderate symptomatic stable angina. The results showed that a short-term course of DHI, in combination with optimal medical therapy, effectively relieved angina symptoms and improved self-assessed health status. Additionally, a weighted gene co-expression network analysis suggested that anticoagulation and the regulation of cholesterol metabolism may be key processes contributing to the therapeutic effect.Citation4 Another study investigated the efficacy and mechanism of GeGen decoction on dysmenorrhea using a randomized, double-blinded, placebo-controlled trial. The study comprehensively explained that the therapeutic efficacy was closely linked to the reduction of 2-oxoisovaleric acid, lactate, and creatine, as well as the enhancement of isoleucine, methionine, valine, and tryptophan. Thus, the disturbed metabolic pathways exacerbate the disease, and GeGen decoction could alleviate this condition by modulating the network of metabolites.Citation5 However, there are still many therapies awaiting exploration, such as Wuzhuyu decoction (WZYD) for chronic non-atrophic gastritis (CNAG).
Wuzhuyu decoction is a classical Chinese formula that has been used for over 2,000 years to treat gastrointestinal diseases, hyperalgesia, and headaches.Citation6,Citation7 It was derived from the Shang Han Lun, a renowned treatise written in 219 AD by Zhongjing Zhang. The formula consists of four primary herbs: Euodia rutaecarpa (Juss.) Benth, Zingiber officinale Roscoe, Panax ginseng C. A. Meyer, and Ziziphus jujuba Mill. These herbs are believed to have an affinity for the spleen and stomach channels according to the principles of TCM, suggesting potential therapeutic effects on the digestive system. For example, numerous studies have investigated the regulatory function of Euodia rutaecarpa (Juss.) Benth in the context of chronic gastritis.Citation8,Citation9 Our research team has further explored its constituents, such as evodiamine, rutaecarpine, and dehydroevodiamine. Evodiamine has been found to inhibit the migration and invasion of gastric cancer cells by suppressing the PTEN-mediated EGF/PI3K signaling pathway.Citation10 Rutaecarpine has demonstrated a gastroprotective effect against ethanol-induced mucosal injury, likely through its integrative regulation of anti-inflammatory, antioxidant, and anti-apoptotic systems.Citation11 Additionally, dehydroevodiamine has shown the potential to alleviate gastric dyspepsia, reduce inflammatory infiltration, and mitigate tissue damage induced by indomethacin, with regulation of the ERK and p38 signaling pathways.Citation12 Collectively, these findings suggest that Fructus Evodiae (Euodia rutaecarpa) exhibits comprehensive actions against gastritis. Similar effects have also been observed with the other three herbs.Citation13–15 Given the previous research, the first question that this study aims to address is the efficacy and safety of Wuzhuyu decoction for chronic non-atrophic gastritis (CNAG).
The mechanism of action of WZYD in treating CNAG remains unclear, posing a second challenge. In the field of bioinformatics, metabolomics has emerged as an advanced analytical approach that has greatly contributed to the exploration of complex mechanisms. By combining multivariate analysis and network effect insights, metabolomics enables the identification of differential metabolites and signaling pathways, providing a comprehensive view of the organism’s response to treatment. In recent years, an increasing number of clinical studies on traditional Chinese medicine TCM have utilized metabolomics to investigate the direct regulatory effects of therapies at the metabolic level. For example, Shenlu Granule was found to be effective in treating aplastic anemia through the regulation of aminoacyl-tRNA biosynthesis and glycerophospholipid metabolism.Citation16 Similarly, a study focused on Taohong siwu decoction for coronary heart disease revealed that glycerophosphocholine, 8,9-DiHETrE, and 5’-methylthioadenosine were key regulators of its efficacy, while a decrease in glycerophospholipid metabolism and arachidonic acid metabolism played a pivotal role during treatment.Citation17 These studies highlight the potential application of metabolomics in investigating the therapeutic effects of WZYD for CNAG.
To comprehend the mechanisms and efficacy of WZYD for CNAG, a clinical trial incorporating serum metabolomics analysis was conducted. This study aims to establish the scientific foundation for the clinical application of WZYD and advance the exploration of TCM. Through the analysis of serum metabolomics data, we seek to uncover the underlying reasons behind the stable efficacy demonstrated by WZYD in treating CNAG. The findings of this study will not only contribute to the understanding of WZYD’s therapeutic effects but also provide valuable insights for future TCM research.
Material and Methods
Recruitment and Study Design
All patients with chronic non-atrophic gastritis (CNAG) were enrolled between August 2022 and January 2023 at the Fifth Medical Center of PLA General Hospital. The study protocol was approved by the Ethics Committee of the Fifth Medical Center of PLA General Hospital (Approval number: KY-2022-2-6-1). Prior to the start of the study, informed consent was obtained from all participants, and details are provided in the Supplementary Files. The data presented in this study are based on preliminary data from an ongoing clinical trial (Nos. ChiCTR2200062296). The procedures and purpose of the study were explained clearly to all participants. The diagnosis of CNAG was made according to the Chinese Consensus on Chronic Gastritis (Shanghai, 2017). The inclusion, exclusion, and rejection criteria were determined by consensus among physicians (Supplementary Table 1). A total of twenty patients were treated with WZYD for four weeks. WZYD was obtained from the Department of Pharmacy at the Fifth Medical Center of PLA General Hospital, with a dosage of 36 g/day. WZYD is a formula granule comprising Euodia rutaecarpa (Juss.) Benth, Zingiber officinale Roscoe, Panax ginseng C. A. Meyer, and Ziziphus jujuba Mill in a ratio of 6:12:6:12, respectively. Patients were advised to maintain a healthy lifestyle, including a light diet, regular work and rest, and appropriate exercise. Smoking, alcohol consumption, and spicy food were prohibited.
Quality Control of WZYD
The formula granule of WZYD was analyzed using UHPLC-QQQ-TOF to identify the key active compounds. The main compounds, namely evodiamine, rutaecarpine, limonin, dehydroevodiamine, 6-gingerol, and ginsenoside Rb1, were quantified using UHPLC. Supplementary Tables 2 and 3 provided the chromatography parameters for ensuring stability and reproducibility.
Clinical Outcome Measures
The outcomes measured in this study included endoscopy evaluation scores and histopathological changes. The endoscopy evaluation scores involved the assessment of red plaques, erosion, hemorrhage, and bile reflux (Supplementary Table 4). The histopathological changes were graded based on chronic inflammation (CI) and active chronic inflammation (ACI). CI reflected the severity of chronic inflammatory cells present in the mucosa, while ACI assessed the infiltration of neutrophils with abscess formation (Supplementary Table 5). Additionally, safety outcomes were monitored, which included the assessment of symptoms related to adverse reactions, as well as hepatic and renal function.
Serum Sample Collection and Metabolomics Performance
A 5 mL serum sample was collected from each group after a two-week treatment period, and a comparison was made with a group of 10 healthy volunteers. The serum samples were stored in a freezer at −80°C and were thawed and centrifuged at 3000×g for 15 minutes prior to the metabolomics procedure. For the extraction of metabolites, 50 μL of serum was mixed with 300 μL of pre-chilled 20% acetonitrile methanol at 12,000 rpm for 10 minutes at 4°C to obtain the supernatant. The supernatant was then dried in preparation for further analysis. Subsequently, 180 μL of the sample was centrifuged at 12,000 rpm for 3 minutes at 4°C. A pooled quality control (QC) sample solution was prepared using the same extraction method as the individual samples, which involved combining all the samples. The analysis of all samples was conducted using the Waters ACQUITY UPLC combined with Xevo TQ-S Micro mass spectrometer, as previously described.Citation18
ELISA Assay
The serum levels of interleukin-10 (IL-10), cyclooxygenase-2 (COX-2), and tumor necrosis factor-α (TNF-α) in the patients were measured using an ELISA Kit (Elabscience) following the manufacturer’s instructions.
Data Processing and Statistical Analysis
Statistical analysis for clinical efficacy and safety was performed using the Statistical Package for the Social Sciences (SPSS) version 26.0. The difference between two groups was explored using Student’s t-test. To present the values, Mean ± Standard Deviation was used. Statistical significance between groups was considered at P<0.05 and P<0.01. Metabolomics analysis involved unsupervised principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA). In OPLS-DA, significantly regulated metabolites between groups were determined based on VIP (Variable Importance in Projection) values and absolute Log2FC (fold change) not less than 1.0. VIP values were evaluated using score plots and permutation plots generated by the R package MetaboAnalystR. The data underwent log transformation (log2) and mean centering prior to OPLS-DA. Metabolites and related signaling pathways were identified and annotated using the KEGG database (http://www.kegg.jp/kegg/html). Significantly enriched pathways were confirmed using a hypergeometric test’s P-value with the provided list of metabolites.
Results
The Chemical Information About WZYD
Six representative compounds, namely evodiamine, rutaecarpine, limonin, dehydroevodiamine, 6-gingerol, and ginsenoside Rb1, were identified in WZYD. Furthermore, the content of these compounds was quantified in order to stabilize and control the experiment (Supplementary Figure 1 and Supplementary Table 6).
Characteristic of Participants
Twenty patients were diagnosed with CNAG through endoscopy evaluation and differentiation in TCM. The trial consisted of 12 men and 8 women with an average age of 58.20±7.11 years. No patients withdrew from the study after receiving WZYD treatment. WZYD demonstrated significant improvements in the endoscopy evaluation scores, including erosion, hemorrhage, bile reflux, and red plaques (P<0.05). Furthermore, the scores for chronic inflammation and active chronic inflammation were reduced after treatment (P=0.04, P<0.0001) (). Only one adverse event was reported, which involved slight dizziness in a 61-year-old woman. However, due to her long history of dizziness, it could not be conclusively attributed to the side effect of WZYD.
Table 1 Endoscopy and Histology Evaluation of WZYD Treatment (Mean±SD)
Classification of Serum Samples with Different Models
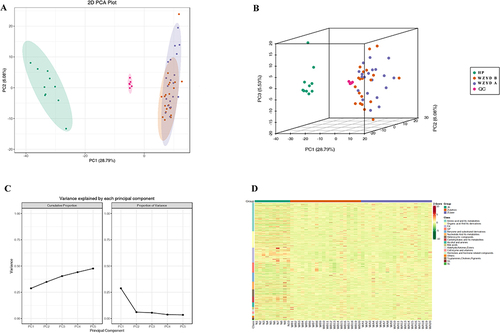
A total of 889 peaks were detected in 50 serum samples. The quality control assessment confirmed consistent repeatability throughout the entire procedure. To distinguish between metabolites in healthy individuals (HP), patients before WZYD treatment (WZYD B), and patients after WZYD treatment (WZYD A), we initially employed Principal Component Analysis (PCA). The PCA results successfully separated the HP group from both the WZYD B and WZYD A groups, but could not entirely differentiate between WZYD B and WZYD A in either the 2D or 3D plots (). Heatmap analysis using Hierarchical Clustering Analysis (HCA) further supported the distinct metabolic profiles among the three groups ().
Figure 1 Serum metabolites discrimination based on PCA and HCA. (A) 2D PCA plot for serum metabolites in participants; (B) 3D PCA plot for serum metabolites in participants; (C) PCA with variance explained by each principal component; (D) The heatmap of HCA for serum metabolites in participants.
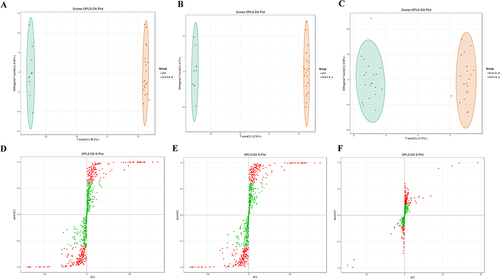
Orthogonal Partial Least-Squares Discriminant Analysis (OPLS-DA), a type of multivariate statistical analysis, was employed to achieve distinct separation among the three groups. The results presented in showed clear differentiation between HP, WZYD B, and WZYD A. The calculated values for R2X, R2Y, and Q2 were 0.407, 0.999, and 0.992, respectively, between HP and WZYD B. Similarly, between HP and WZYD A, the values were 0.423, 0.999, and 0.994 for R2X, R2Y, and Q2, respectively. Furthermore, for WZYD B and WZYD A, the values were 0.189, 0.987, and 0.565 for R2X, R2Y, and Q2, respectively. These findings indicate a robust model fitness and predictability. The S-plot in and highlighted significant biomarkers through the display of red dots.
Figure 2 Serum metabolites discrimination based on OPLS-DA. (A) Scores OPLS-DA plot for serum metabolites in patients between HP and WZYD B; (B) Scores OPLS-DA plot for serum metabolites in patients between HP and WZYD A; (C) Scores OPLS-DA plot for serum metabolites in patients between WZYD B and WZYD A; (D) S-plot for serum metabolites in patients between HP and WZYD B; (E) S-plot for serum metabolites in patients between HP and WZYD A; (F) S-plot for serum metabolites in patients between WZYD B and WZYD A.
Serum Metabolites Identification Based on OPLS-DA
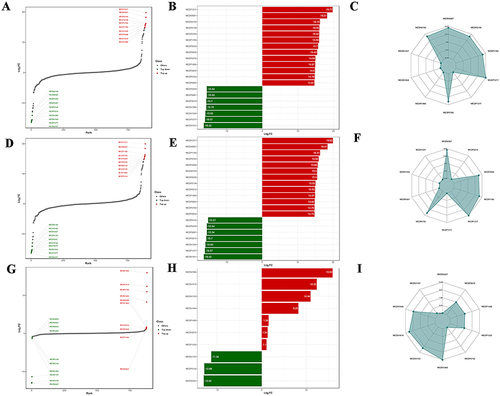
A total of 284 and 288 metabolites were identified between HP and WZYD B, HP and WZYD A, respectively. Importantly, 10 metabolites were identified as biomarkers for distinguishing between WZYD B and WZYD A (Supplementary Table 7). To elucidate the regulated characteristics of agent efficacy, the most up-regulated and down-regulated metabolites were highlighted. The top 10 up-regulated and down-regulated metabolites of HP and WZYD B included 3-methyl-4-pentenoic acid, Phe-Phe, Guanosine, 3-(3,4,5-trimethoxyphenyl)propionic acid, 3-(Methylthio)-1-propanol, Leu-Phe, 15-deoxy-δ-12,14-PGJ2, 2-Hydroxy-4-(methylthio)butyric acid, 4-Hydroxy-3-methoxyphenylacetic acid, Gly-Phe-Phe, and Citric Acid, L-Cysteine, Pro-Val, Ala-Lys, Lys-Ala, 5-Hydroxyindole, Pyrimidine-4-carboxylic acid sodium salt, 3-Hydroxycinnamic acid, 2’-O-methyladenosine, and 2-Methyl-d-erythritol 2,4-cyclodiphosphate (). Similarly, the top 10 up-regulated and down-regulated metabolites of HP and WZYD A were 3-methyl-4-pentenoic acid, Phe-Phe, Leu-Phe, 3-(Methylthio)-1-propanol, 2-Hydroxy-4-(methylthio)butyric acid, 15-deoxy-δ-12,14-PGJ2, Guanosine, 3-(3,4,5-trimethoxyphenyl)propionic acid, Gly-Phe-Phe, and 4-Hydroxy-3-methoxyphenylacetic acid, and Citric Acid, Pro-Val, L-Dihydroorotic Acid, 3’-Adenylic acid, Ala-Lys, Lys-Ala, 5-Hydroxyindole, 3-Hydroxycinnamic acid, 2’-O-methyladenosine, and 2-Methyl-d-erythritol 2,4-cyclodiphosphate (). Moreover, the top 10 up-regulated and down-regulated metabolites of WZYD B and WZYD A were Pyrimidine-4-carboxylic acid sodium salt, D-Arabinose 5-phosphate, 3-Hydroxy-4-methoxybenzoic acid, 16-HDoHE, 3,4,5-Trimethoxycinnamic Acid, Pantothenol, Chaps, 3-aminobenzamide, Allopurinol, 6-Methylnicotinamide, and 3-Amino-4-Hydroxybenzoic Acid, 3-Amino-5-hydroxybenzoic acid, 3-Hydroxyanthranilic Acid, ST-638, 1-Methylxanthine, 3-(3,4,5-trimethoxyphenyl)propionic acid, 15®-Prostaglandin E1, 8-Methoxyalkynurate, 3’-Adenylic acid, and L-Dihydroorotic Acid (). Furthermore, the Venn diagram highlighted 315 potential metabolites (Supplementary Table 8, Supplementary Figure 2). These metabolites primarily belonged to amino acids and their metabolites, organic acids and their derivatives, nucleotides and their metabolites.
Figure 3 Serum metabolites identification and its characteristics. (A–C) Dynamic distribution of upregulated and downregulated metabolites between HP and WZYD B; (D–F) Dynamic distribution of upregulated and downregulated metabolites between HP and WZYD A; (G–I) Dynamic distribution of upregulated and downregulated metabolites between WZYD B and WZYD.
Metabolites Pathways Analysis
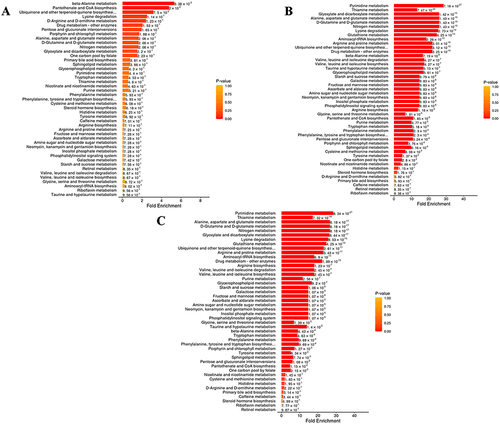
The signals based on KEGG were utilized to determine the functional roles of metabolites in the entire body. These distinct metabolites interact with each other, forming various pathways in CNAG patients. Furthermore, treatment with WZYD impacts several signaling pathways, including pantothenate and CoA biosynthesis, porphyrin metabolism, and biosynthesis of nucleotide sugars (). Additionally, the differential abundance score reflects the overall changes observed in all metabolites within a specific pathway. Moreover, the signaling pathways for purine metabolism, arginine biosynthesis, thiamine metabolism, and pyrimidine metabolism were upregulated when comparing HP and WZYD A (). These results indicate that thiamine metabolism, tyrosine metabolism, arginine and proline metabolism, and purine metabolism signaling pathways are the major regulated signals when comparing HP and WZYD B ().
Figure 4 KEGG enrichment analyses of differential metabolites in CNAG patients. (A) WZYD B vs WZYD A; (B) HP vs WZYD A; (C) HP vs WZYD B.
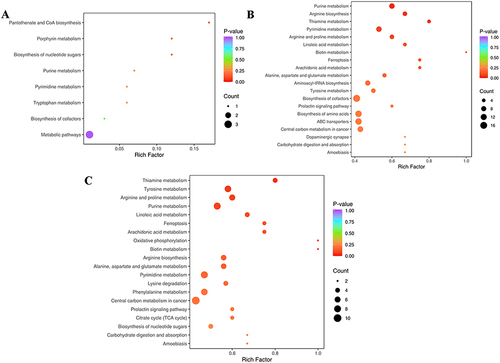
The Metabolic Set Enrichment Analysis (MSEA) does not require a specific threshold for differential metabolites. Its objective is to establish a range of metabolic sets and enrich the metabolomic data with significant metabolic sets. The results indicate that beta-alanine metabolism, pantothenate and CoA metabolism, and lysine degradation are responsible for the differences between WZYD B and WZYD A (). Additionally, pyrimidine metabolism, thiamine metabolism, and glyoxylate and dicarboxylate metabolism are of great importance in the treatment of WZYD when comparing HP and WZYD A (). Furthermore, specific emphasis is placed on signaling pathways such as pyrimidine metabolism, thiamine metabolism, and alanine, aspartate, and glutamate metabolism between HP and WZYD B (). These outcomes suggest that pyrimidine metabolism and thiamine metabolism play a crucial role in the development and progression of CNAG.
WZYD Reduced Inflammation in CNAG Patients
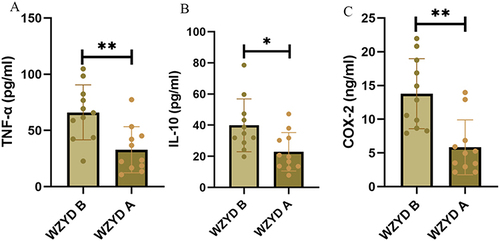
To confirm the effect of WZYD on inflammatory factors in patients with CNAG, patient serum was examined, and ELISA results demonstrated that WZYD treatment effectively reduced serum levels of TNF-α(P<0.01), IL-10(P<0.05), and COX-2(P<0.01), implying that WZYD may alleviate CNAG by suppressing the inflammatory response ().
Figure 6 ELISA detection of inflammatory factors before and after WZYD treatment in patients’ serum (* P<0.05, **P<0.01). (A) Expression of TNF-α in patients’ serum; (B) Expression of IL-10 in patients’ serum; (C) Expression of COX-2 in patients’ serum.
Discussion
CNAG is a recurrent and prevalent gastrointestinal disease.Citation19 CNAG is widely regarded as the initial step leading to gastric cancer, underlining the profound significance of its prevention and treatment.Citation20,Citation21 In recent decades, numerous studies have focused on the therapeutic potential of Chinese herbal remedies due to their multi-targeted network modulation.Citation18,Citation22,Citation23 As a representative TCM formula, WZYD has been extensively used in China for centuries to treat gastrointestinal disorders. It consists of Euodia rutaecarpa (Juss.) Benth, Zingiber officinale Roscoe, Panax ginseng C. A. Meyer, and Ziziphus jujuba Mill in a ratio of 6:12:6:12. In our preclinical studies, we identified active ingredients derived from Euodia rutaecarpa (Juss.) Benth, the primary herb in WZYD, which demonstrated the potential to repair gastric damage. Wen et al found that dehydroevodiamine alleviated chronic atrophic gastritis in rats by inhibiting the HIF-1α/VEGF signaling pathway.Citation24 In addition, dehydroevodiamine was reported to alleviate indomethacin-induced gastric injury by inhibiting MAPK signaling pathway.Citation12 Moreover, rutaecarpine was found to prevent ethanol-induced acute gastric injury by activating the PI3K/AKT signaling pathway.Citation11 Therefore, to unravel the efficacy and mechanism of WZYD for CNAG, a total of 20 patients were recruited for this study. Our results suggested that WZYD ameliorated score of endoscopy evaluation including erosion, hemorrhage, bile reflux and red plaques. In addition, chronic inflammation and active chronic inflammation score after treatment were decreased. These findings provide convincing evidence for the effectiveness of WZYD in the treatment of CNAG in the clinical setting.
Subsequently, we investigated the direct mechanism of WZYD for CNAG using metabolomics. The results indicated that 10 core metabolic components were associated with the treatment of WZYD, including 16-HDoHE, 3’-Adenylic acid, 3-Hydroxy-4-methoxybenzoic acid, 8-Methoxyalkynurate, Chaps, D-Arabinose 5- phosphate, L-Dihydroorotic Acid, Pantothenol, Pyrimidine-4-carboxylic acid sodium salt, and Urobilin. Moreover, these metabolic components proved that pyrimidine metabolism and thiamine metabolism were critically responsible for the development and progression of CNAG. Wu et al found that pyrimidine metabolism was downregulated in gastric cancer cells and in clinical samples, suggesting that WZYD may prevent carcinogenesis by regulating pyrimidine metabolism in CNAG patients.Citation25 More, the modulation of thiamine metabolism inhibits the development of inflammation in a variety of diseases.Citation26,Citation27 These findings provide convincing evidence for the effectiveness of WZYD in the treatment of CNAG in the clinical setting by suppressing inflammation and modulating core metabolism process. To confirm the effect of WZYD on inflammatory factors in patients with CNAG, patient serum was examined, and ELISA results demonstrated that WZYD treatment effectively reduced serum levels of TNF-α, IL-10, and COX-2.
However, the direction of improvement of this study needs to be pointed out. First, Many difficulties prevent large multicenter clinical trials from being conducted, such as differences in prescription sources. Despite these difficulties, we are pursuing a multi-center large-scale clinical trial to verify the effect of WZYD for CNAG, which could provide a more reliable chain of evidence for the clinical application of WZYD. Second, the molecular mechanism of WZYD for CNAG should be investigated in greater depth. Therefore, some methods such as transcriptomics, proteomics, and single cell transcriptomics can be applied to investigate the molecular mechanism of WZYD for CNAG. Third, the study primarily focuses on short-term improvements, leaving questions about the treatment’s long-term sustainability. We will explore the long-term sustainability of WZYD for the treatment of CNAG in subsequent studies. In addition, despite the fact that no side effects were reported in the current study, potential side effects of WZYD should be noted. In a 2018 clinical trial, patients in the WZYD treatment group were reported to develop oral ulcers, facial acne, and constipation.Citation28 This suggests that we need to thoroughly analyze the potential side effects of WZYD and assess its safety in future studies. Moreover, future research could explore tailored treatments based on disease subtypes or individual characteristics.
Conclusion
Altogether, WZYD can effectively alleviate CNAG by inhibiting inflammation and regulating related metabolic processes, which might be the molecular mechanism of WZYD treatment of CNAG. More studies are warranted to be conducted in this area.
Data Sharing Statement
All data are available in the manuscript and they are showed in figures and tables.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the reviewers and also the authors of all references. The reviewer’s advice really makes the great improvement of this paper.
Additional information
Funding
References
- Huang K, Zhang P, Zhang Z, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi:10.1016/j.pharmthera.2021.107843
- Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi:10.1016/j.jep.2023.116306
- Wang S, Sun S-T, Zhang X-Y, et al. The evolution of single-cell RNA sequencing technology and application: progress and perspectives. Int J Mol Sci. 2023;24. doi:10.3390/ijms24032943
- Liu J, Li -D-D, Dong W, et al. Detection of an Anti-angina therapeutic module in the effective population treated by a multi-target drug danhong injection: a randomized trial. Signal Transduct Target Ther. 2021;6(1):329. doi:10.1038/s41392-021-00741-x
- Chai C, Hong F, Yan Y, et al. Effect of Traditional Chinese medicine formula gegen decoction on primary dysmenorrhea: a randomized controlled trial study. J Ethnopharmacol. 2020;261:113053. doi:10.1016/j.jep.2020.113053
- Nan N, Gong M-X, Wang Q, et al. Wuzhuyu decoction relieves hyperalgesia by regulating central and peripheral 5-HT in chronic migraine model rats. Phytomedicine. 2022;96:153905. doi:10.1016/j.phymed.2021.153905
- Xu Y, Wu S, Wu Y, Gong M, Wang Z. Recognition and optimization of ingredients treating nitroglycerin-induced migraine rats from Wuzhuyu decoction. Evid Based Complement Alternat Med. 2019;2019:6156754. doi:10.1155/2019/6156754
- Li -Y-Y, Feng J-L, Li Z, Zang X-Y, Yang X-W. Separation and enrichment of alkaloids from coptidis rhizoma and euodiae fructus by macroporous resin and evaluation of the effect on Bile Reflux Gastritis rats. Molecules. 2022;27. doi:10.3390/molecules27030724
- Yang Y-F, Zhou Q-L, Yang X-W. Elucidation of compatibility interactions of Traditional Chinese Medicines: in vitro absorptions across Caco-2 Monolayer of Coptidis Rhizoma and Euodiae Fructus in Zuojin and Fanzuojin formulas as a case. Phytother Res. 2017;31(8):1220–1229. doi:10.1002/ptr.5843
- Yang R, Wen J, Yang T, Dai C, Zhao Y, Souza P. Evodiamine inhibits gastric cancer cell proliferation via PTEN-mediated EGF/PI3K signaling pathway. Evid Based Complement Alternat Med. 2021;2021:5570831. doi:10.1155/2021/5570831
- Ren S, Wei Y, Wang R, et al. Rutaecarpine ameliorates ethanol-induced gastric mucosal injury in mice by modulating genes related to inflammation, oxidative stress and apoptosis. Front Pharmacol. 2020;11:600295. doi:10.3389/fphar.2020.600295
- Wei Y, Ren S, Wang J, et al. Dehydroevodiamine ameliorates indomethacin-induced gastric injury via inhibition of ERK and P38 signaling pathway. Phytomedicine. 2021;93:153764. doi:10.1016/j.phymed.2021.153764
- Haniadka R, Saldanha E, Sunita V, Palatty PL, Fayad R, Baliga MS. A review of the gastroprotective effects of ginger (Zingiber Officinale Roscoe). Food Funct. 2013;4(6):845–855. doi:10.1039/c3fo30337c
- Yu T, Rhee MH, Lee J, et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am J Chin Med. 2016;44(03):595–615. doi:10.1142/S0192415X16500336
- Hamedi S, Arian AA, Farzaei MH. Gastroprotective effect of aqueous stem bark extract of Ziziphus Jujuba L. against HCl/Ethanol-Induced gastric mucosal injury in rats. J Tradit Chin Med. 2015;35(6):666–670. doi:10.1016/s0254-6272(15)30157-6
- Feng Z, Hu X, Qu W, et al. Metabolomics-based clinical efficacy of compound shenlu granule, a Chinese Patent Medicine, in the supportive management of aplastic anemia patients: a randomized controlled pilot trial. Evid Based Complement Alternat Med. 2021;2021:6655848. doi:10.1155/2021/6655848
- Tao T, He T, Mao H, Wu X, Liu X. Non-targeted metabolomic profiling of coronary heart disease patients with Taohong Siwu Decoction treatment. Front Pharmacol. 2020;11:651. doi:10.3389/fphar.2020.00651
- Ma X, Xie S, Wang R, et al. Metabolomics profiles associated with the treatment of Zuojin Pill on patients with chronic nonatrophic gastritis. Front Pharmacol. 2022;13:898680. doi:10.3389/fphar.2022.898680
- Sipponen P. Prevalence rates of heathy stomach mucosa, chronic non-atrophic and atrophic gastritis in endoscopic biopsies in adults born in Finland in 1890–1977. Scand J Gastroenterol. 2021;56(8):874–881. doi:10.1080/00365521.2021.1929451
- Robles-Medranda C, Puga-Tejada M, Oleas R, et al. Newly proposed quantitative criteria can assess chronic atrophic gastritis via Probe-Based Confocal Laser Endomicroscopy (PCLE): a Pilot Study. Endosc Int Open. 2022;10(04):E297–E306. doi:10.1055/a-1662-5150
- Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325–1332.e7. doi:10.1053/j.gastro.2021.06.078
- Hu Q, Wei S, Wen J, et al. Network pharmacology reveals the multiple mechanisms of xiaochaihu decoction in the treatment of non-alcoholic fatty liver disease. BioData Min. 2020;13(1):11. doi:10.1186/s13040-020-00224-9
- Hu Q, Jiang L, Yan Q, Zeng J, Ma X, Zhao Y. A natural products solution to diabetic nephropathy therapy. Pharmacol Ther. 2023;241:108314. doi:10.1016/j.pharmthera.2022.108314
- Wen J-X, Tong Y-L, Ma X, et al. Therapeutic effects and potential mechanism of dehydroevodiamine on N-Methyl-N’-Nitro-N-Nitrosoguanidine-induced chronic atrophic gastritis. Phytomedicine. 2021;91:153619. doi:10.1016/j.phymed.2021.153619
- Wu Z, Tan J, Zhuang Y, et al. Identification of crucial genes of pyrimidine metabolism as biomarkers for gastric cancer prognosis. Cancer Cell Int. 2021;21(1):668. doi:10.1186/s12935-021-02385-x
- Anthonymuthu S, Sabui S, Sheikh A, Fleckenstein JM, Said HM. Tumor necrosis factor α impedes colonic thiamin pyrophosphate and free thiamin uptake: involvement of JNK/ERK 1/2 -mediated pathways. Am J Physiol Cell Physiol. 2022;323(6):C1664–C1680. doi:10.1152/ajpcell.00458.2022
- Ma Y, Elmhadi M, Wang C, et al. Thiamine supplementation alleviates lipopolysaccharide-triggered adaptive inflammatory response and modulates energy state via suppression of NFκB/P38 MAPK/AMPK signaling in rumen epithelial cells of goats. Antioxidants. 2022;11(10):2048. doi:10.3390/antiox11102048
- Liu H-W, Zou Y-H, Cao K-G, et al. Efficacy of Modified Wuzhuyu Decoction Granule () for migraine patients with cold and stasis obstructing meridian syndrome: a randomized, double-blind, placebo-controlled trial. Chin J Integr Med. 2018;24(6):409–414. doi:10.1007/s11655-017-2547-5