Abstract
Background
Uncontrolled asthma is characterized by considerable variability. Well controlled asthma is associated with less unplanned use of health care resources and fewer acute exacerbations. In this study, we attempted to increase inhaled corticosteroid (ICS) doses initially in suboptimally controlled asthmatics, hypothesizing that early achievement of asthma control using this strategy would be associated positively with a higher level of stability.
Methods
This was a randomized, open-label, prospective study including patients with uncontrolled asthma who were randomized to receive higher-dose (HD) ICS in combination with a long-acting beta-agonist (LABA) for one month and then shifted to doses suggested in the practice guidelines (GD) or to receive GD therapy alone. Lung function, ie, forced expiratory volume in one second (FEV1), peak expiratory flow, Asthma Control Test scores, and frequency of acute exacerbations, was followed up for one year.
Results
Seventy-six patients were treated with the HD strategy and 80 with the GD strategy. The increase in FEV1 from baseline was greater in the HD group than in the GD group, especially during the first month of treatment (304 ± 49 mL versus 148 ± 39 mL, respectively, P = 0.01). Numbers of patients with completely or well controlled asthma were higher in the HD group than in the GD group (92.1% versus 81.1%, respectively, P = 0.03). Further, there was a significant difference between the groups with regard to frequency of acute exacerbations (9.2% in the HD group versus 21.3% in the GD group, P = 0.02); this effect was more pronounced for patients in the HD group with partially controlled or uncontrolled asthma.
Conclusion
Patients receiving HD therapy achieved asthma control more rapidly and maintained greater stability than those receiving GD therapy. This represents a novel strategy for gaining disease control in patients with uncontrolled asthma.
Introduction
The aim of treatment for patients with asthma is to achieve and maintain good control of the disease without side effects from treatment.Citation1–Citation3 The GINA (Global INitiative for Asthma) guideline recommends regular assessment, with adjustments made to maintenance therapy based on disease control.Citation1 Further medications are added if asthma is uncontrolled or only partially controlled, and once control is achieved, the doses are maintained or stepped down to the lowest dose that maintains stability. However, Rabe et al have reported that the level of asthma control worldwide falls short of the treatment aims outlined in the international guideline and that the use of anti-inflammatory agents is low, even in patients with severe persistent asthma, ranging from 26% in Western Europe to 9% in Japan.Citation4 Daytime symptoms and hospital emergency visits are still high, and guideline-based asthma control was achieved in only 1.3% of participants in the AIRET (Asthma Insights and REality in Turkey) study.Citation5 Further, there is a trend towards an increasing incidence of asthma and growing drug costs.Citation6 Clearly, more practical and proactive treatment strategies and education programs are needed to meet the goals set out in the guideline for asthma control.
It is now well recognized that adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy is more effective than ICS alone, even if the ICS dose is doubled, in improving asthma stability and control in symptomatic patients.Citation7–Citation9 Combined use of an ICS and LABA is an integral part of the asthma treatment guideline.Citation1 Theophylline may also be useful as an add-on therapy for patients who do not gain control of their asthma on an ICS alone or on a combination of an ICS and a LABA.Citation10,Citation11 Leukotriene modifiers can also help to improve poor asthma control.Citation12,Citation13
Uncontrolled asthma is characterized by wide disease variability. The current asthma guideline focuses on achievement and maintenance of disease control, but few studies have addressed stability and potential duration of control once achieved using composite measures of control. In a post hoc analysis of the results of the GOAL (Gaining Optimal Asthma controL) study,Citation14 Bateman et al identified an association between the level of asthma control achieved during the step-up phase and the stability of control during the maintenance phase. Further, patients achieving the most control are more likely to stabilize at a high level of control, and conversely, patients who were not well controlled initially, and therefore have variable asthma control, are those most likely to consume unscheduled health care resources.Citation15 Therefore, it is important that physicians achieve asthma control as soon as possible, and then utilize strategies that minimize the risk of further instability and exacerbations.
The dose-response curve for ICS varies widely in different asthma populations. A previous review concluded that commencing ICS at double or quadruple at moderate or low dose has no greater effect than commencing with a high dose.Citation16 However, our strategy involves prescribing higher doses of controller medications, especially the dose of ICS (fluticasone 500–1000 μg per day) when combined with salmeterol in the initial one-month period, in the hope of achieving asthma control sooner and maintaining future disease stability in patients with uncontrolled asthma.
Materials and methods
Inclusion and exclusion criteria
This prospective, randomized 52-week study was conducted at the Far Eastern Memorial Hospital, a tertiary medical center in Taiwan. Men and women aged >18 years with poorly controlled asthma diagnosed at least 6 months prior to screening were included, and classified as having asthma which was partly controlled, uncontrolled, and uncontrolled with exacerbation.Citation1 Patients were identified as having uncontrolled asthma with exacerbation if they had a peak expiratory flow <60% of predicted, an Asthma Control Test score <14, and wheezing on auscultation. Subjects were also required to show a reversibility of ≥12% in forced expiratory volume in one second (FEV1) after inhalation of salbutamol (two actuations, 100 μg per actuation) to be eligible for randomization. Patients also needed to demonstrate correct inhaler technique to be able to participate in the study. Any patient who had been treated with theophylline or leukotriene modifiers before randomization was allowed to continue this medication during the study.
Exclusion criteria included the following: life-threatening asthma within the past year; a hospitalization or emergency department visit for asthma in the 4 weeks before screening; use of systemic corticosteroids in the month before screening; treatment with omalizumab in the previous 6 months; use of a leukotriene receptor antagonist in the week before screening; a history of smoking that was either recent (in the previous 12 months) or equivalent to ≥10 pack years; significant nonreversible active pulmonary disease; and clinically significant respiratory tract infection in the 4 weeks before screening. Current use of medications with an effect on bronchospasm and/or lung function was also an exclusion criterion.
A full medical history, including of asthma and allergic rhinitis, was obtained at baseline. Patients with a history of clinically significant medical illness that, in the opinion of the investigator, could interfere with the study results or require treatment that might interfere with the conduct of the study were not enrolled.
Interventions and randomization
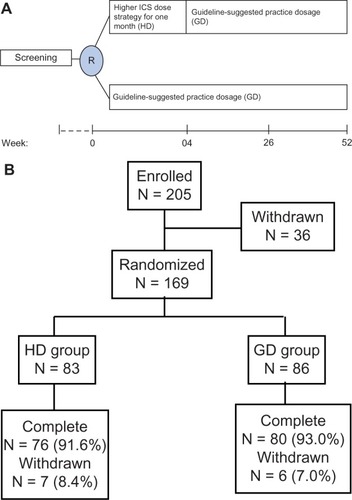
Patients were randomized 1:1 to receive higher doses of ICS (HD group) or to receive the dose suggested by the practice guidelines (GD group). The starting dose of ICS was based on the severity of asthma in each patient. Both study treatments were administered via a hydrofluoroalkane pressurized metered-dose inhaler using a space device (GlaxoSmithKline, Uxbridge, UK). The strategy for HD was to use medication that was one step higher than that recommended by GINA, specifically a higher ICS dose and prescription of salmeterol for the first 4 weeks, which was then stepped down to the GD dose for the continuous phase lasting 48 weeks. For example, if the patient required step 3 therapy according to the practice guideline, we would step up to a step 4 medication (ie, higher-dose ICS); however, patients in the GD group, therapies for poorly controlled asthma status based on guideline’s suggested practice strategy (52 weeks, ).
Assessment of efficacy and safety
During the 52 weeks of treatment, the patients underwent clinical evaluation, including lung function tests by spirometry (FEV1 and forced vital capacity) and peak flow measurements (morning and evening peak expiratory flow), and asthma symptom scoring with the Asthma Control Test.Citation17,Citation18 Asthma was defined as totally controlled, well controlled, or not well controlled according to the GOAL criteria.Citation15 The primary endpoint was asthma control status at weeks 4 and 52. Secondary endpoints included the proportion of asthma-free days (ie, no use of a short-acting beta-agonist, no asthma symptoms, 24-hour peak expiratory flow variability <20% of baseline, no unscheduled visits to a medical facility, and no nocturnal awakening), time to first acute exacerbation (asthma deterioration resulting in emergency treatment, hospitalization, or treatment with asthma medication other than a short-acting beta-agonist). Spirometry was performed at baseline, at each study visit (at eight-week intervals), and at the end of the study. Adverse events were documented based on spontaneous reporting, patient interview, and diary entries. Daily ICS consumption was also calculated. Ethical approval from the hospital and informed consent from all study subjects were obtained. The study is registered with ClinicalTrials.gov (NCT01647646).
Statistical analysis
Categorical variables were compared using the chi-squared test and continuous variables were compared using the Student’s t-test. P < 0.05 was considered to be statistically significant. Fisher’s exact test (qualitative data) or analysis of variance (quantitative data) were used to test homogeneity between the treatment groups. Comparability of the two treatment groups at baseline with respect to FEV1 and peak expiratory flow was done using analysis of variance. All statistical analyses were performed using the Statistical Package for Social Sciences version 9 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Recruitment and patient disposition
The study was initiated (first patient, first visit) in March 2009. Of the 169 patients randomized (83 patients in the HD group and 86 in the GD group), 156 (92.3%) completed the study and 13 (7.7%) discontinued prematurely (). Of the 13 patients who did not complete the study, six withdrew because of lack of therapeutic effect (three in each group), four were lost to follow-up, and three dropped out for personal reasons.
Baseline demographics and clinical characteristics
The demographics and clinical characteristics of patients in the HD and GD groups are summarized in . There were no significant differences between the two groups before randomization. Prescriptions for ICS and other medications, including theophylline and leukotriene modifiers, were similar. Most patients (82%–84%) were classified as having partly controlled or uncontrolled asthma at the time of screening, and a minority (15%–17%) were classified as having uncontrolled asthma with exacerbation. The mean baseline Asthma Control Test score was 16 ± 4 in the HD group and 17 ± 5 in the GD group.
Table 1 Demographic characteristics, asthma status, and treatment at screening
Assessment and measurement of outcomes
Efficacy
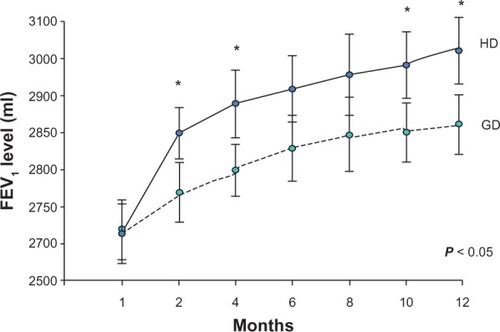
The majority of patients in each group completed the 52-week treatment period, thereby having sufficient exposure to ICS doses to characterize their response profiles. The proportion of patients using fluticasone 1000 μg/day in the HD group was higher than that in the GD group (85.5% versus 27.5%, respectively, P < 0.001) in the initial phase after randomization. No ICS (step 2) was prescribed in the HD group, and only 6.2% patients in GD group used an ICS. The proportion of patients with completely or well controlled asthma at week 4 in the HD group was significantly higher than that in the GD group (64.5% versus 41.3%, respectively, P = 0.01) and this advantage was maintained until the end of the study (92.1% versus 81.1%, P = 0.03). Patients in the HD group achieved sustained control of their asthma earlier than those in the GD group. Analysis of pulmonary function tests according to FEV1 and forced vital capacity showed that lung function improved in both treatment groups during the study. However, greater improvement in FEV1 was detected by week 4 in more patients in the HD group than in the GD group (141 ± 33 mL versus 66 ± 18 mL, respectively, P = 0.02) and this effect was observed at every assessment point throughout the study (HD 304 ± 49 mL versus GD 148 ± 39 mL, P = 0.01, ). Moreover, peak expiratory flow, recorded twice daily by patients before taking their study medication, showed an increase in mean values between baseline and week 52 in both treatment groups, with significantly more improvement in the HD group than in the GD group (248 ± 47 L per minute versus 129 ± 36 L per minute, respectively, P < 0.01), with the difference becoming apparent in the first month of the study.
Figure 2 Lung function showing improvement in FEV1 during the study period.
Abbreviations: FEV1, forced expiratory volume in one second; HD, initially higher-dose inhaled corticosteroids; GD, inhaled corticosteroid dose recommended by practice guidelines.
Mean patient-assessed asthma symptom scores on the Asthma Control Test were also higher in the HD group (21 ± 4) than in the GD group (19 ± 5) at the initial week 4 visit. Overall, Asthma Control Test scores improved in both groups during the study, with a statistically significant difference in favor of the HD group compared with the GD group (24 ± 4 versus 20 ± 5, respectively, P = 0.02) by the end of the study. The proportion of patients with totally controlled and well controlled asthma was higher in the HD group than in the GD group (92.1% versus 81.1%, respectively, P = 0.03). There was a trend towards better quality of life in patients who gained control of their asthma. Absolute mean changes from baseline in mean Asthma Control Test scores were significantly different between the HD and GD groups (8 ± 2 versus 4 ± 2, respectively, P = 0.04) at the end of study; further, the mean number of asthma-free days during the study was greater in the HD group than in the GD group (82.4 ± 26.5 days versus 69.2 ± 24.3 days, P = 0.02).
There were seven episodes (9.2%) of acute exacerbation in the HD group, comprising emergency department visits by four patients and a need for additional medication in three. However, 17 episodes (21.3%) of acute exacerbation were documented in the GD group, comprising emergency department visits by eleven patients, a need for additional medication in four, and hospitalization in two. There was a significant difference in the rate of acute exacerbations between the HD and GD groups (9.2% versus 21.3%, respectively, P = 0.02, ). No episodes of life-threatening asthma occurred during the study. Time to first exacerbation was significantly (P = 0.01) longer in the HD group (105 ± 26 days) than in the GD group (67 ± 28 days), indicating more rapid improvement in lung function and control of asthma symptoms using the HD treatment strategy.
Table 2 Efficacy of treatment in patients with asthma using HD versus GD therapy
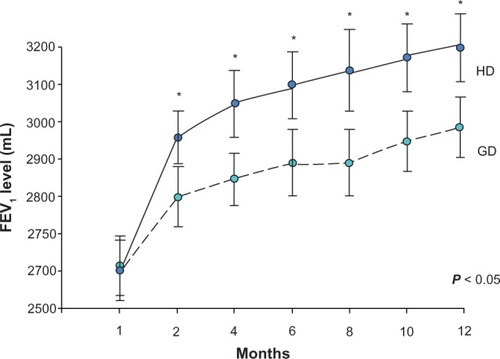
In a subgroup analysis, the effect of treatment was more marked in patients with partly or uncontrolled asthma, for whom the increase in FEV1 was significantly higher in the HD group than in the GD group (239 ± 52 mL versus 98 ± 49 mL, respectively, P < 0.01) at the initial week 4 visit, and this difference was also significant at each subsequent follow-up visit (). Meanwhile, the proportion of patients with uncontrolled asthma was significantly lower in the HD group than in the GD group (9.5% versus 19.1%, respectively, P = 0.02). There was also a significantly greater improvement from baseline in mean scores on the Asthma Control Test in the HD group compared with the GD group (9 ± 4 versus 5 ± 3, respectively, P = 0.03) by the end of study, and the rate of acute exacerbations was also lower in the HD group than in the GD group (4.8% versus 13.2%, P = 0.04, ).
Table 3 Treatment responses using HD and GD therapy in patients with partially or uncontrolled asthma
Figure 3 Lung function showing improvement in FEV1 in patients with partially or uncontrolled asthma during the study period.
Abbreviations: HD, initially higher-dose inhaled corticosteroids; GD, inhaled corticosteroid dose recommended by practice guidelines; FEV1, forced expiratory volume in one second.
Safety
The proportion of patients reporting potentially treatment-related side effects was similar between the groups. Daily ICS consumption in the HD and GD groups was not significantly different (736 ± 219.7 μg versus 663.1 ± 267.2 μg, respectively P = 0.17). Frequently reported side effects were dysphonia (3.2% in the HD group versus 2.8% in the GD group, P = 0.63), headache (2.1% versus 2.4%, respectively, P = 0.59), tremor (3.4% versus 3.7%, P = 0.71), and hoarseness of voice (4.2% versus 3.8%, P = 0.51). There were no reports of oral candidiasis or pharyngitis in either treatment group.
Discussion
This study assessed the long-term efficacy of a novel treatment strategy using a higher initial dose of ICS combined with a LABA. We increased the fluticasone dose used in combination therapy for one month, with significant improvements in lung function and symptom control seen in HD patients compared with GD patients. These effects were sustained throughout the duration of the study, and accompanied by a significant decrease in frequency of acute exacerbations in the HD group. This study indicates that a higher initial ICS dose strategy may result in better asthma control than that achieved following the currently recommended practice guideline.
It is reasonable to assume that patients who do not have controlled asthma have persistent ongoing inflammation and are at high risk of deterioration. If more effective controllers could be prescribed to halt this vicious cycle, control of asthma could be achieved sooner. In this study, we attempted to increase the amount of controller medication, in particular the ICS dose, to suppress the cascade of events initially leading to inflammation and achieve disease stability. Combination of an ICS with a LABA has synergistic anti-inflammatory activity, including inhibition of the acute inflammatory changes that occur during a severe exacerbation, increased fluticasone-induced MKP-1 and modulated airway smooth muscle synthetic function.Citation19 Several long-term, short-term, and intermittent step-up dosing strategies have been investigatedCitation20 for asthma that is not well controlled, given that variability in symptom control is a particularly challenging feature of asthma that necessitates careful monitoring and an individualized therapeutic regimen over time. Our strategy may be as same as the step-up short-term strategy.
The results of this study highlight the advantages of early achievement of asthma control that are not mentioned in the current GINA guidelines.Citation1 First, control can be achieved earlier and greater stability maintained using a higher initial ICS dose. Our patients achieved near-maximal health status scores and improvement in lung function, and continued to improve using this treatment strategy. Second, rapid achievement of asthma control is associated with a lower risk of acute exacerbations and unscheduled use of health care resources. Therefore, further instability and exacerbations prevented by prescribing higher initial ICS doses are indeed beneficial. Third, there was no significant increase in side effects when using the higher ICS dose in this study.
Stable asthma control means sustained prevention of symptoms with low or no reliever use and few exacerbations over a long period of time, which is the stated goal in the current treatment guideline. Treatment according to this guideline entails a stepwise increase in treatment until control is achieved, and then stepping medication down if control has been maintained for at least 3 months. The first step is always to ensure that standard therapies are optimized when initially treating poorly controlled asthma. However, using a titrated treatment strategy, suboptimal therapy and dose adjustments may hinder drug efficacy and delay achievement of disease control. This might be one of the reasons why surveys continue to report unsatisfactory results in the current management of asthma.Citation21–Citation23 In addition to heightening patient awarenessCitation24,Citation25 and increasing educational asthma programs,Citation26–Citation28 we may also need to adjust the therapeutic strategy and treat proactively in different ways. For example, the GOAL studyCitation14 has introduced the novel concept of step-up treatment even in patients with well controlled asthma. Our strategy is different from that used in the GOAL study, which increased doses in a continuous manner. However, both these treatment strategies seem to be beneficial for establishing and maintaining disease control in patients with asthma.
Several meta-analysesCitation29–Citation32 have reported no clinical difference in outcome measures, including spirometric lung volumes, symptom control, β-agonist use, and frequency of exacerbations, using daily fluticasone doses of 200–500 μg. These findings do not support the “start high, step down” approach, and may result in inappropriately high doses of ICS being used in the long term. The initial impressive and sustained response in terms of spirometric lung volumes in our HD group is greater than would be expected from the existing literature. We believe that the major limitation of the meta-analyses published to date is the small number of published studies available using ICS doses higher than 500 μg/day and did not combine with LABA.Citation29 In this study, we have demonstrated the positive effects of starting with a higher ICS dose in combination with LABA therapy to achieve early asthma control and to decrease the frequency of exacerbations, and our findings are similar to those of the FACET (Formoterol and Corticosteroids Establishing Therapy) study that used a strategy of high-dose budesonide (800 μg) in combination with formoterol.Citation33 A larger prospective study should now be performed to investigate the effects of initial higher-dose ICS therapy in more detail.
Our present study should be viewed in light of some limitations. First, this was a single-center study conducted in a teaching hospital and included a relatively small patient population, so there is the possibility of patient selection bias such that our sample may not be representative of the overall population of individuals in the community with asthma. Further, the study was not randomized, double-blind, or prospective in design, so potential bias on the part of the investigators and/or patients cannot be excluded. Second, with regard to the safety of higher-dose ICS therapy, we did not check for adrenal suppression, dermatological effects, effects on bone mineral density, or ocular changes. Third, no inflammatory or molecular parameters, such as eosinophil count, exhaled nitric oxide, or bronchial biopsy, were included as further objective measures of efficacy.
In conclusion, initial step-up ICS therapy in combination with a LABA, as opposed to the current treatment strategy, can increase the chances of achieving disease control, decreasing the frequency of acute exacerbations, and maintaining stability in patients with poorly controlled asthma. This is also a novel way to make advances in asthma therapy in comparison with the conventional “ladder” strategy. Further studies should be performed in larger patient populations, using the present study as a benchmark, to confirm the effectiveness and superiority of initially higher doses of ICS.
Acknowledgment
This study was supported by grants from the Far Eastern Memorial Hospital (FEMH-96-C-014, FEMH-97-C-007).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention 2011 Update. Available from: http://www.ginasthma.org/GINA-Report,-Global-Strategy-for-Asthma-Management-and-Prevention Accessed May 19, 2013
- British Thoracic Society Scottish Inter-collegiate Guideline on Asthma Management Revised May 2011. Available from: http://www.brit-horacic.org.uk/Portals/0/Guidelines/AsthmaGuidelines/qrg101%202011pdf Accessed May 19, 2013
- Bousquet J Clark TJ Hurd S GINA guidelines on asthma and beyond Allergy 2007 62 102 112 17298416
- Rabe KF Adachi M Lai CK Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys J Allergy Clin Immunol 2004 114 40 47 15241342
- Sekerel BE Gemicioglu B Soriano JB Asthma insights and reality in Turkey (AIRET) study Respir Med 2006 100 1850 1854 16564685
- Haahtela T Tuomisto LE Pietinalho A A 10 year asthma programme in Finland: major change for the better Thorax 2006 61 663 670 16877690
- Woolcock A Lundback B Ringdal N Jacques LA Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids Am J Respir Crit Care Med 1996 153 1481 1488 8630590
- Condemi JJ Goldstein S Kalberg C Yancey S Emmett A Rickard K The addition of salmeterol to fluticasone propionate versus increasing the dose of fluticasone propionate in patients with persistent asthma. Salmeterol Study Group Ann Allergy Asthma Immunol 1999 82 383 389 10227337
- Greening AP Ind PW Northfield M Shaw G Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid Lancet 1994 344 219 224 7913155
- Evans DJ Taylor DA Zetterstrom O Chung KF O’Connor BJ Barnes PJ A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma N Engl J Med 1997 337 1412 1418 9358138
- Brenner M Berkowit R Marshall N Strunk RC Need for theophylline in severe steroid-requiring asthmatics Clin Allergy 1988 18 143 150 3365858
- Vaquerizo MJ Casan P Castillo J CASIOPEA Study Group Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma Thorax 2003 58 204 210 12612294
- Virchow JCJr Prasse A Naya I Summerton L Harris A Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids Am J Respir Crit Care Med 2000 162 2 Pt 1 578 585 10934090
- Bateman ED Boushey HA Bousquet J GOAL Investigators Group Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL Study Am J Respir Crit Care Med 2004 170 836 844 15256389
- Bateman ED Bousquet J Busse WW GOAL Steering Committee and Investigators Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study Allergy 2008 63 932 938 18588561
- Adams NP Bestall JC Jones P Lasserson TJ Griffiths B Cates CJ Fluticasone at different doses for chronic asthma in adults and children Cochrane Database Syst Rev 2008 4 CD003534 18843646
- Nathan RA Sorkness CA Kosinski M Development of the Asthma Control Test: a survey for assessing asthma control J Allergy Clin Immunol 2004 113 59 65 14713908
- Schatz M Sorkness CA Li JT Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists J Allergy Clin Immunol 2006 117 549 556 16522452
- Manetsch M Rahman MM Patel BS Long-acting β2-agonists increase fluticasone propionate-induced mitogen-activated protein kinase phosphatase 1 (MKP-1) in airway smooth muscle cells PLoS One 2013 8 e59635 23533638
- Thomas A Lemanske RFJr Jackson DJ Approaches to stepping up and stepping down care in asthmatic patients J Allergy Clin Immunol 2011 128 915 924 21855125
- Murphy KR Meltzer EO Biaiss MS Nathan RA Stoloff SW Doherty DE Asthma management and control in the Unites States: results of the 2009 Asthma Insight and Management survey Allergy Asthma Proc 2012 33 54 64 22309716
- Nathan RA Meltzer EO Blaiss MS Murphy KR Doherty DE Stoloff SW Comparison of Asthma in America and Asthma Insight and Management surveys: did asthma burden and care improve in the Unites States between 1998 and 2009? Allergy Asthma Proc 2012 33 65 76 22309828
- Demoly P Gueron B Annunziata K Adamek L Walters RD Update on asthma control in five European countries: results of a 2008 survey Eur Respir Rev 2010 19 150 157 20956184
- Haughney J Barnes G Partridge M Cleland J The Living and Breathing Study: a study of patients’ views of asthma and its treatment Prim Care Respir J 2004 13 28 35 16701634
- Janssens T Verleden G Van den Bergh O Symptoms, lung function and perception of asthma control: an exploration into the heterogeneity of the asthma control construct J Asthma 2012 49 63 69 22121947
- Pbert L Madison JM Druker S Effect of mindfulness training on asthma quality of life and lung function: a randomized controlled trial Thorax 2012 67 769 776 22544892
- Kaferle JE Wimsatt LA A team-based approach to providing asthma action plans J Am Board Fam Med 2012 25 247 249 22403208
- Ring N Malcolm C Wyke S Promoting the use of personal asthma action plans: a systematic review Prim Care Respir J 2007 16 271 283 17710351
- Holt S Suder A Weatherall M Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis BMJ 2001 323 253 256 11485952
- Masoli M Weatherall M Holt S Clinical dose-response relationship of fluticasone propionate in adults with asthma Thorax 2004 59 16 20 14694240
- Masoli M Holt S Weatherall M Dose-response relationship of inhaled budesonide in adult asthma: a meta-analysis Eur Respir J 2004 23 552 559 15083753
- Bousquet J Ben-Joseph R Messonnier M A meta-analysis of the dose-response relationship of inhaled corticosteroids in adolescents and adults with mild to moderate persistent asthma Clin Ther 2002 24 1 20 11833824
- Pauwels RA Löfdahl CG Postma DS Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group N Engl J Med 1997 337 1405 1411 9358137