Abstract
Mouse embryonic stem cells were previously observed along with mesenchymal stem cells from different sources, after being treated with a mixed ester of hyaluronan with butyric and retinoic acids, to show a significant increase in the yield of cardiogenic and vascular differentiated elements. The aim of the present study was to determine if stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) and cultured in the presence of a mixture of hyaluronic (HA), butyric (BU), and retinoic (RA) acids show a higher yield of differentiation toward the cardiovascular phenotype as compared with untreated cells. During the differentiation process elicited by exposure to HA + BU + RA, genes controlling pluripotency and plasticity of stem cells, such as Sox2, Nanog, and Oct4, were significantly downregulated at the transcriptional level. At this point, a significant increase in expression of genes controlling the appearance of cardiogenic and vascular lineages in HA + BU + RA-treated cells was observed. The protein expression levels typical of cardiac and vascular phenotypes, evaluated by Western blotting, immunofluorescence, and flow cytometry, were higher in hAFSCs cultured in the presence of HA + BU + RA, as compared with untreated control cells. Appearance of the cardiac phenotype was further inferred by ultrastructural analysis using transmission and scanning electron microscopy. These results demonstrate that a mixture of HA + BU + RA significantly increased the yield of elements committed toward cardiac and vascular phenotypes, confirming what we have previously observed in other cellular types.
Introduction
Mesenchymal stem cells have generated a great deal of excitement as a potential source of cells for cell-based therapeutic strategies to promote regeneration of injured heart muscle.Citation1–Citation3 Their most interesting characteristic is that they may be directly obtained from individual patients, avoiding the complications associated with immune rejection of allogeneic cells. Tissue engineering-based therapies using autologous stem cells can represent a tool to create cardiac constructs for repairing injured hearts, although identification of an ideal source of immature cells capable of differentiating into cell lineages still represents a challenge. In this regard, stem cells derived from primitive fetal cells present in human and mouse amniotic fluid (hAFSCs) appear to have many of the key therapeutic benefits of embryonic stem cells while avoiding their ethical, medical, and logistical drawbacks.Citation4 Some authors have previously described specific cardiomyogenic and vascular commitment of hAFSCs in vitro and cardiovascular structures when transplanted in models of heart injury in different species.Citation5–Citation8 However, although mesenchymal stem cells may be committable to cardiovascular lineages, it is well established that this differentiation process occurs with a very low yield. Hence, enabling high-throughput of cardiogenesis and vasculogenesis in hAFSCs would have obvious therapeutic potential.
In previous work, we used a mixed ester of hyaluronan with butyric and retinoic acids (HBR) acting as a differentiating agent.Citation9,Citation10 This compound was able to increase the yield of cardiomyocytes derived from mouse GTR1 embryonic stem cellsCitation9 and human mesenchymal stem cells isolated from bone marrow and alternative sources, including the dental pulp and fetal membranes of term placenta (FMhMSCs),Citation10 the latter showing a higher yield of cardiovascular differentiation. More recently, it was observed that a mixture of hyaluronic acid (HA), butyric acid (BU), and retinoic acid (RA) resulted in optimization of graft revascularization after islet/stem cell intrahepatic cotransplantation in syngeneic diabetic rats, with better survival of transplanted tissue.Citation11 The aim of the present study was to evaluate the effects of a combination of HA, BU, and RA on cardiogenic and angiogenic differentiation of hAFSCs as we had previously observedCitation9,Citation10 in other types of mouse and human stem cells, and if these processes occur with a high yield.
Results obtained from immunofluorescence showed the mixture of HA + BU + RA to be most effective in inducing the appearance of a cardiovascular phenotype compared with combinations of HA + BU, HA + RA, or BU + RA.
Materials and methods
Preparation of amniotic fluid-derived stem cells
Amniotic fluid-derived stem cells were obtained from amniocenteses performed during six consecutive pregnancies for routine prenatal diagnosis at 15–18 weeks’ gestation at Clinica Ginecologica, Università di Sassari. All the pregnant women, aged 30–40 years, gave their written consent for the study, which was accepted by the local ethics committee (Comitato di Bioetica, Sassari, Italy N°1098/L). None of the participants were affected by chronic disease and were not taking any medications. The technique described by De Coppi et alCitation4 was used to isolate hAFSCs c/kit+. Briefly, confluent amniotic fluid stem cell cultures were expanded for obtaining single cell suspensions by gentle trypsinization (trypsin-NA-EDTA solution, Invitrogen, Carlsbad, CA, USA). The hAFSCs were immunomagnetically sorted for c/kit using a monoclonal anti-c/kit (CD117) antibody (Miltenyi Biotech, Minneapolis, MN, USA) directly conjugated to microBeads (Miltenyi Biotech) and then expanded in subconfluent conditions in Alpha Mem medium (Invitrogen) containing 15% fetal bovine serum (Invitrogen), 20% Chang medium C (Chang B plus Chang C; Irvine Scientific, Santa Ana, CA, USA), 1% glutamine, and 1% penicillin/streptomycin. Single-cell derived amniotic fluid stem cell clones were established by limiting dilution as previously described.Citation12 At least four different clones obtained from the same sample were tested. When the cells reached confluence, they were subcultured in T25 cm2 flasks (BD Biosciences, San Jose, CA, USA) in the same medium. All cells used for experimentation were not frozen and were treated between passages 4 and 8.
Flow cytometry analysis
Flow cytometry analysis was used to characterize the isolated hAFSCs and to evaluate specific antigen markers. Cells were harvested by treatment with 0.08% trypsin-EDTA and incubated with 1 μg/106 cells of fluorophor-conjugated antibodies for 40 minutes at 4°C in the dark. The antibodies used were CD29 (PE-Cy5-conjugated), CD31 (PE-conjugated), CD34 (PE-conjugated), CD44 (fluorescein isothiocyanate [FITC]-conjugated), and CD45 (FITC-conjugated), all sourced from BD Pharmingen (San Jose, CA, USA), CD90 (FITC-conjugated) from BD Biosciences (San Jose, CA, USA), CD105 (PE-conjugated) from R&D (Minneapolis, MN, USA), and CD133 (PE-conjugated) from Miltenyi Biotec. After washing, the cells were analyzed on a flow cytometer (FACSAria, BD Biosciences) by collecting 10,000 events, and data were analyzed using FACSDiva software (BD Biosciences). The hAFSCs used in this study were stained positive for CD90, CD105, CD44, and CD29, Oct4, Sox2, and Nanog, antigens typical of mesenchymal stem cells, and negative for CD34, CD133, and CD45.
Flow cytometry was also used to assess the percentage of hAFSCs expressing specific cell phenotype markers. For this reason, cells (1 μg/106 cells) were cultured in the absence or presence of HA + BU + RA for 10 days. These cells were also cultured in the absence or presence of HA 2 mg/mL + BU 5 mM, or HA 2 mg/mL + RA 1 μM, or BU 5 mM + RA 1 μM; after a fixation/permeabilization step, the cells were incubated with a primary antibody directed against α-sarcomeric actinin (Sigma-Aldrich, St Louis, MO, USA), α-myosin heavy chain (Santa Cruz Biotechnologies Inc, Santa Cruz, CA, USA), or von Willebrand factor (Abcam, Cambridge, MA, USA) for one hour at 4°C, and with 1 μg of FITC-conjugated secondary antibody (Santa Cruz Biotechnologies Inc) for one hour at 4°C in the dark. After washing, the cells were analyzed on the FACSAria flow cytometer by collecting 10,000 events, and the data were analyzed using the FACSDiva software.
Differentiation of hAFSCs towards a cardiovascular phenotype
To induce cardiogenesis, human hAFSCs isolated from six donors were separately cultured in ultralow attachment plates for 2 days. On day 3, the cells were transferred to six-well tissue culture plates for an additional 7 days. hAFSCs were seeded at 8,000 cells/cm2 in Alpha Mem medium containing 15% fetal bovine serum, 20% Chang medium containing HA 2 mg/mL, BU 5 mM, and RA 1 μM (all purchased from Sigma-Aldrich). The cells were also treated with HA + BU, HA + RA, or BU + RA. Control cells were cultured in Alpha Mem medium containing 15% fetal bovine serum and 20% Chang medium C. HA, BU, and RA were dissolved in water and added every 24 hours.
Gene expression analysis
Total RNA was isolated from cells cultured for 24, 48, and 72 hours, and at 7 and 10 days in the absence or presence of HA + BU + RA, using Trizol reagent according to the manufacturer’s instructions (Invitrogen). Total RNA was dissolved in RNase-free water and, for real-time polymerase chain reaction (PCR), cDNA was synthesized in a 50 μL reaction volume with 1 μg of total RNA and M-MLV reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Quantitative real-time PCR was performed using an iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA). Two microliters of cDNA were amplified in 50 μL reactions using Platinum Supermix UDG (Invitrogen), 200 nM of each primer, 10 nM fluorescein (BioRad), and Sybr Green. After an initial denaturation step at 94°C for 10 minutes, temperature cycling was initiated. Each cycle consisted of 94°C for 15 seconds, 55°C–59°C for 30 seconds, and 60°C for 30 seconds, the fluorescence being read at the end of this step. Primers used for analysis of cardiogenesis (Prodynorphin, GATA-4, NKX-2.5, cardiac troponin T, alpha myosin heavy chain, Tbx-5, Mef2C α-sarcomeric actinin), vascular phenotype (vascular endothelial growth factor, hepatocyte growth factor, von Willebrand factor), and smooth muscle differentiation (smooth muscle actin, calponin) were specific and spanning exons, and are reported in . To evaluate the quality of the product of real-time PCR assays, melting curve analysis was performed after each assay. Relative expression was determined using the delta-CT method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene.
Table 1 Description of primers used in real-time polymerase chain reaction
Immunostaining
Cells were cultured for a period of 10 days with and without HA + BU + RA. After 10 days, the cells were treated with trypsin, and the resulting suspension was cultured at low density to allow visualization of individual cells. The cultures were fixed with 4% paraformaldehyde. Cells were exposed overnight at 4°C to mouse monoclonal antibodies against α-sarcomeric actinin, cardiac troponin T, von Willebrand factor, or vascular endothelial growth factor, smooth muscle actin, and calponin (Thermo Scientific) or with rabbit polyclonal antibodies against α-myosin heavy chain (Santa Cruz Biotechnology Inc.), and stained at 37°C for one hour with fluorescein-conjugated goat IgG. All microscopy analysis was performed with a confocal microscope (TCSSP5, Leica, Nussloch, Germany). DNA was visualized with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI).
Immunoblotting analysis
Cells were cultured in the presence or absence of HA + BU + RA for 24, 48, or 72 hours and for 7 or 10 days. Total cell lysates from hAFSCs were electrophoresed on 10% Novex Tris-glycine polyacrylamide gels (Invitrogen) in MOPS sodium dodecyl sulfate running buffer using an XCell SureLock™ Mini-Cell (Invitrogen) according to the instructions provided by the manufacturer. After protein transfer to polyvinylidene difluoride membranes (Invitrogen), and membrane saturation and washing, immunoreaction was carried out for one hour at room temperature in the presence of the primary antibody, antisera against GATA-4 (Santa Cruz Biotechnology, Inc.), Nkx-2.5 (Santa Cruz), Tbx5 (Thermo Scientific), Mef2C (Thermo Scientific), α-sarcomeric actinin (Sigma-Aldrich), α-myosin heavy chain (Santa Cruz Biotechnology, Inc.), cardiac troponin T (Thermo Scientific), vascular endothelial growth factor (Thermo Scientific), hepatocyte growth factor (Thermo Scientific), von Willebrand Factor (Santa Cruz Biotechnology, Inc.), smooth muscle actin (Thermo Scientific), calponin (Thermo Scientific), Oct4 (Santa Cruz Biotechnology, Inc.), Sox2 (Sigma-Aldrich), Nanog (Santa Cruz Biotechnology, Inc.), and GAPDH (Santa Cruz Biotechnology, Inc.) diluted to 1:1000. After additional washing, membranes were incubated with antirabbit (Nkx-2.5, Tbx5, Mef2C, myosin heavy chain, vascular endothelial growth factor, Oct4, Sox2, Nanog, GAPDH) or antimouse (GATA4, cardiac troponin T, α-sarcomeric actinin, hepatocyte growth factor, von Willebrand factor, smooth muscle actin, calponin), and horseradish peroxidase-conjugated secondary antibody (Pierce Biotechnology, Rockford IL, USA). Targeted protein expression was assessed using a chemoluminescence detection system (ECL Western blotting detection reagents were from Amersham Biosciences Corporation, Piscataway, NJ, USA).
Ultrastructural analysis of HA + BU + RA-treated cells
Ultrastructural features of the cultured cells were determined using transmission (TEM) and scanning electron (SEM) microscopy. Cells cultured in the absence and presence of HA + BU + RA for 10 days were washed with phosphate-buffered saline and then fixed in 0.1 M pH 7.4 phosphate-buffered saline containing 2.5% glutaraldehyde for 2 hours and post-fixed in osmium tetroxide. For TEM study, the specimens were embedded in Durcupan™ ACM (Sigma), ultrathin sections were collected in copper grids, stained with uranyl acetate and lead citrate, and then observed and photographed using a 902A electron microscope (Zeiss, Oberkochen, Germany). For SEM, the cells were dehydrated with acetone, followed by hexamethyldisilazane, then coated with gold and observed by SEM (Inspect S and Quanta 200, FEI Quanta, Eindhoven, The Netherlands).
Data analysis
The statistical data analysis was performed using Statistical Package for the Social Sciences version 13 software (SPSS Inc, Chicago, IL, USA). For this study, nonparametric statistical tests were used ie, the Kruskal–Wallis rank sum and Wilcoxon signed-rank test. The former was used to evaluate the distributions and homogeneity of each group variance at different times of observation; the latter was used to evaluate, in the same group, differences (delta-CT) between the data collected over a period of observation and the reference value at baseline. All results were considered to be statistically significant at P < 0.05.
Results
Culturing hAFSCs with a mixture of HA, BU, and RA enhances expression of cardiogenic and angiogenic genes
shows that all cells used in this study stained positive for CD90, CD105, CD44, CD29, Oct4, Sox2, and Nanog, and negative for CD34, CD133, and CD45 before HA + BU + RA treatment. In hAFSCs, a mixture of HA + BU + RA significantly increased (mean ± standard error of the mean; n = 6; P < 0.05) the expression of GATA-4 and Nkx-2.5, encoding a zinc finger and a homeodomain essential for cardiogenesis.Citation13,Citation14 As shown by real-time PCR analysis (), the transcriptional effect of HA + BU + RA on these genes was evident just 24 hours after exposure. Similarly, even expression of the prodynorphin gene, a known orchestrator of cardiogenesis,Citation15–Citation17 and of Tbx5 and Mef2C, both having an essential role in the cardiac commitment, was significantly enhanced (P < 0.05) in HA + BU + RA-treated cells. The cardiac-specific genes for α-sarcomeric actinin, α-myosin heavy chain, and cardiac troponin T showed the same trend (). shows also that the expression of a set of vasculogenic genes, including for vascular endothelial growth factor, hepatocyte growth factor, and von Willebrand factor (–), was markedly increased (mean ± standard error of the mean; n = 6; P < 0.05) in cells exposed to HA + BU + RA, even after 10 days of treatment. These genes have been shown to play a pivotal role in both endothelial tissue formation and neoangiogenesis.Citation18,Citation19 Moreover, in cells treated with HA + BU + RA, expression of smooth muscle actin and calponin, two smooth muscle-related genes, was superimposable on what was detected in untreated control cells ().
Figure 1 Flow cytometric immunophenotype analysis of stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs). Undifferentiated hAFSCs were stained with primary antibodies specific for stemness markers (Oct4, Sox2, Nanog), MSC markers (CD44, CD29, CD105, CD90), or hemopoietic markers (CD45, CD133, CD34) and with fluorescein isothiocyanate-conjugated secondary antibody. The graphics show the immunophenotype analysis (mean ± standard error of the mean; n = 6; P < 0.05).
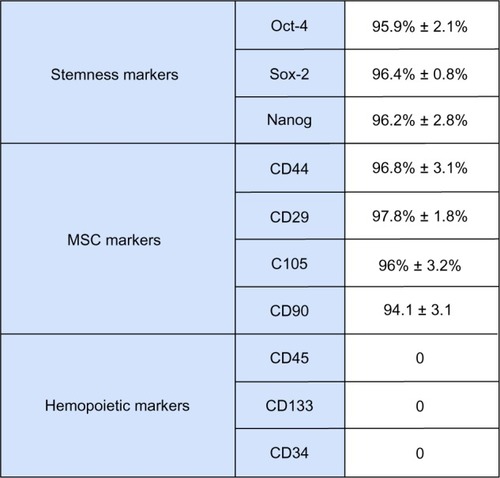
Figure 2 Effect of HA + BU + RA on expression of genes specific for cardiogenic, angiogenic, and smooth muscle in stem cells derived from primitive fetal cells present in human amniotic fluid. Cells were exposed for 1, 2, 3, 7, and 10 days in the absence or presence of HA 2 mg/mL + BU 5 mM + RA 1 μM. The mRNA amounts of prodynorphin, Nkx-2.5, GATA-4, Tbx-5, Mef2C, α-sarcomeric actinin, α-myosin heavy chain, cTNT, VEGF, HGF, vWF, smooth muscle actin, and calponin from HA + BU + RA-treated or untreated cells were normalized to GAPDH, and the mRNA expression levels in HA + BU + RA-treated cells was plotted at each time point as fold change relative to expression in untreated control cells, defined as 1. All the HA + BU + RA-treated cells at each time point were significantly different from untreated control cells (mean ± standard error of the mean; n = 6; P < 0.05).
Exposure of hAFSCs to a mixture of HA, BU, and RA downregulates expression of stemness marker genes
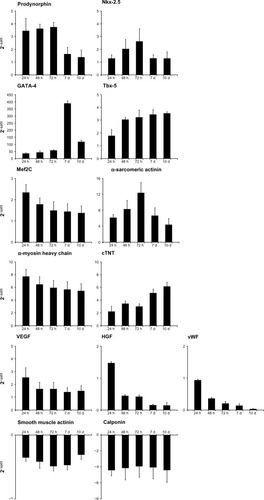
shows that treatment with HA + BU + RA influenced the expression of Nanog, Sox2, and the octamer binding protein, Oct4; all of these genes code for specific factors controlling self-renewal and differentiation of stem cells.Citation20 As expected during commitment toward a differentiated phenotype, Nanog, Sox2, and Oct4 were downregulated (mean ± standard error of the mean; n = 6; P < 0.05) in cells exposed to HA + BU + R A (). This effect was evident after 24 hours, and was still clear after 10 days in culture.
Figure 3 Effects on stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) of exposure to a mixture of HA + BU + RA on pluripotency gene transcription. hAFSCs were exposed for 1, 2, 3, 7, or 10 days in the absence or presence of HA 2 mg/mL + BU 5 mM + RA 1 μM. The mRNA amounts of Sox2, Oct4, and Nanog from HA + BU + RA-treated or untreated cells were normalized to GAPDH, and the mRNA expression levels in HA + BU + RA-treated cells were plotted at each time point as fold change relative to expression in untreated control cells, defined as 1. All the HA + BU + RA-treated cells at each time point were significantly different from untreated control cells (mean ± standard error of the mean; n = 6; P < 0.05).
Exposure of hAFSCs to a mixture of HA, BU, and RA modulates expression of cardiac, vascular, and stemness-related proteins
shows the effect of treatment with HA + BU + RA on the expression of cardiac and vascular-restricted marker proteins. Western blot analysis revealed that GATA-4, Nkx-2.5, Mef2C, Tbx5, α-sarcomeric actinin, and α-myosin heavy chain, representative of cardiac specification, and vascular endothelial growth factor, hepatocyte growth factor, and von Willebrand factor, representative of vascular specification, were significantly more expressed (P < 0.05) in hAFSCs cultured in the presence of HA + BU + RA than in untreated control cells (). Such an increase was evident after 2 days of treatment and persisted during the following 8 days in culture (). On the other hand, no significant expression was detected for smooth muscle actin and calponin, two specific markers of smooth muscle lineage (). In hAFSCs exposed to HA + BU + RA, expression of Sox2, Nanog, and Oct4 proteins mirrored the transcriptional responses elicited by the mixture of these compounds; in fact all of these proteins were expressed with lower levels in treated as compared with untreated cells.
Figure 4 Exposure of stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) to HA + BU + RA modulates expression of selected, tissue-restricted, and stemness-related proteins. Total lysates were isolated from hAFSCs cultured for different periods of time, ie, 24, 48, and 72 hours, and 7 and 10 days, in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM. Samples were analyzed by Western blot, using polyclonal antisera against GATA4, Nkx-2.5, Tbx-5, Mef2C, α-sarcomeric actinin, MHC, cTNT, VEGF, HGF, vWF, SMA, calponin, Sox2, Oct4, and Nanog and GAPDH. The sizes of the bands were determined using prestained marker proteins. The data presented are representative of five separate experiments.
![Figure 4 Exposure of stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) to HA + BU + RA modulates expression of selected, tissue-restricted, and stemness-related proteins. Total lysates were isolated from hAFSCs cultured for different periods of time, ie, 24, 48, and 72 hours, and 7 and 10 days, in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM. Samples were analyzed by Western blot, using polyclonal antisera against GATA4, Nkx-2.5, Tbx-5, Mef2C, α-sarcomeric actinin, MHC, cTNT, VEGF, HGF, vWF, SMA, calponin, Sox2, Oct4, and Nanog and GAPDH. The sizes of the bands were determined using prestained marker proteins. The data presented are representative of five separate experiments.](/cms/asset/53f9a785-c51b-45f1-a8c4-cb45cf586b86/dddt_a_44706_f0004_b.jpg)
hAFSCs treated with a mixture of HA, BU, and RA exhibit a high differentiation rate toward cardiogenic and vascular phenotypes
The overexpression of cardiovascular-restricted marker proteins in HA + BU + RA-treated cells was also confirmed by confocal microscopy analysis. The cardiac specific markers α-sarcomeric actinin, the myosin heavy chain, and the vascular specific marker, von Willebrand factor, were expressed in a limited number (1%–5%) of untreated hAFSCs (). Interestingly, treatment with HA + BU + RA markedly increased the yield of cells expressing both cardiac and vascular marker proteins (). In this regard, we have previously describedCitation11,Citation21 that adipose-derived mesenchymal stem cells spontaneously express vasculogenic markers (vascular endothelial growth factor, hepatocyte growth factor). Moreover, shows that smooth muscle actin and calponin, specific for smooth muscle differentiation, are not influenced by HA + BU + RA treatment.
Figure 5 HA + BU + RA treatment mediated differentiation of stem cells derived from primitive fetal cells present in human amniotic fluid. Expression of α-sarcomeric actinin, α-myosin heavy chain, cardiac troponin T, von Willebrand factor, and vascular endothelial growth factor was assessed in cells cultured in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM for 10 days, and cultured for an additional one day in tissue chamber slides suitable for immunofluorescence staining. Images were obtained using a confocal microscope (TCSSP5, Leica, Nussloch, Germany) using a 20× objective. Nuclei are labeled with 4′,6-diamidino-2-phenylindole (DAPI, blue). Data shown are representative of five separate experiments.
![Figure 5 HA + BU + RA treatment mediated differentiation of stem cells derived from primitive fetal cells present in human amniotic fluid. Expression of α-sarcomeric actinin, α-myosin heavy chain, cardiac troponin T, von Willebrand factor, and vascular endothelial growth factor was assessed in cells cultured in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM for 10 days, and cultured for an additional one day in tissue chamber slides suitable for immunofluorescence staining. Images were obtained using a confocal microscope (TCSSP5, Leica, Nussloch, Germany) using a 20× objective. Nuclei are labeled with 4′,6-diamidino-2-phenylindole (DAPI, blue). Data shown are representative of five separate experiments.](/cms/asset/f766ea04-c476-4fda-a086-9c6594527c00/dddt_a_44706_f0005_c.jpg)
Mixture of HA, BU, and RA shows a higher differentiation capability toward cardiogenic and vascular phenotypes as compared with HA, BU, and RA alone
To evaluate the effect of HA, BU, and RA on cardiovascular differentiation, hAFSCs were exposed to a mixture of HA + BU + RA, HA + BU, HA + RA, or RA + BU, for 10 days. Using flow cytometric analysis, we then evaluated the expression of α-sarcomeric actinin, α-myosin heavy chain, and von Willebrand factor. shows that the mixture containing HA + BU + RA was significantly (P < 0.05) more effective in inducing the appearance of the cardiac and vascular phenotype as compared with the mixtures containing only two compounds (HA + BU, HA + RA, BU + RA).
Figure 6 Flow cytometry analysis of cell lineage marker expression in stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs). The hAFSCs were cultured in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM for 10 days. Cells were stained with primary antibodies specific for the marker of interest, in particular, α-sarcomeric actinin, α-MHC, and vWF, and with fluorescein isothiocyanate-conjugated secondary antibody. In order to validate the results, negative control samples, devoid of the antibody of interest, were used to set the basal fluorescence. The graphics show an increase in fluorescence and in the percentage of positively stained elements, corresponding to differentiated cells, as compared with untreated cells [−(HA + BU + RA)]. The data shown are representative of six individual experiments.
![Figure 6 Flow cytometry analysis of cell lineage marker expression in stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs). The hAFSCs were cultured in the absence [−(HA + BU + RA)] or presence [+(HA + BU + RA)] of HA 2 mg/mL + BU 5 mM + RA 1 μM for 10 days. Cells were stained with primary antibodies specific for the marker of interest, in particular, α-sarcomeric actinin, α-MHC, and vWF, and with fluorescein isothiocyanate-conjugated secondary antibody. In order to validate the results, negative control samples, devoid of the antibody of interest, were used to set the basal fluorescence. The graphics show an increase in fluorescence and in the percentage of positively stained elements, corresponding to differentiated cells, as compared with untreated cells [−(HA + BU + RA)]. The data shown are representative of six individual experiments.](/cms/asset/9929c00f-48d6-443d-909f-03336d77601e/dddt_a_44706_f0006_c.jpg)
Assessment of morphologic modifications induced by HA + BU + RA during HAFCC differentiation towards the cardiovascular phenotype
Cardiac lineage commitment was further inferred from the observation that HA + BU + RA exposure was associated with a typical myofbrillar organization, as highlighted by electron microscopy analysis (). In untreated cells, the nucleus, located in the cell periphery, showed an indented aspect (, thin arrow), with small areas of heterochromatin and a nucleolus (thick arrow) very evident. The cytoplasm appeared homogeneous with few organelles. TEM observation at higher magnification of the untreated cell cytoplasm showed poor presence of organelles; in particular, there were only some cisterns of the rough endoplasmic reticulum (, arrow). In HA + BU + RA-treated cells, the cytoplasm appeared to be full of organelles, in particular mitochondria. The nucleus had an irregular shape with numerous indents (, thin arrow), and heterochromatin near the membrane and nucleolus (thick arrow) was very visible. Treated cells observed with higher magnification TEM revealed the rich presence of organelles, ie, numerous mitochondria with lamellar crests (, thin arrow), and vacuoles (, asterisk).
Figure 7 Morphologic analysis of stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) during HA + BU + RA-induced differentiation toward the cardiovascular phenotype. hAFSCs were cultured in the absence (A, C, E and G) or presence (B, D, F and H) of HA 2 mg/mL + BU 5 mM + RA 1 μM for 10 days. TEM analysis (A–D) or SEM analysis (E–H). (A) TEM imaging of untreated cells, 3,000x, scale bar 5 μm; (B) TEM imaging of HA + BU + RA-treated cells, 4,400x, scale bar 5 μm; (C) TEM imaging of untreated cells, 7,000x, scale bar 5 μm; (D) TEM imaging of treated cells, 12,000x, scale bar 1 μm; (E) SEM imaging of untreated cells, 1,600x, scale bar 10 μm; (F) SEM imaging of treated cells, 4,500x, scale bar 10 μm; (G) SEM imaging of untreated cells, 2,400x, scale bar 5 μm; (H) SEM imaging of treated cells, 8,000x, scale bar 5 μm. See text for more details.
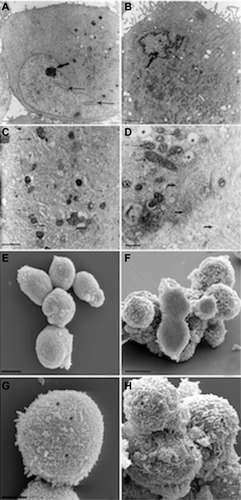
Exclusively, in cells treated with HA + BU + RA, numerous intracytoplasmic fibers were evident (, thick arrow), with an initial organization in bundles, extending parallel, although typical sarcomeric organization was not yet evident. In HA + BU + RA-treated cells, many mitochondria with large lamellar ridges were also present, unlike control cells, in which these organelles were less abundant and showed thinner ridges. Even the rough endoplasmic reticulum appeared more abundant in treated cells, as well as the number of intracytoplasmic vesicles.
Both cells had a nucleus with irregular morphology, with numerous indentations in dispersed chromatin and a nucleolus always evident. On SEM, a different morphology of the plasma membrane could be seen: it appeared substantially smooth or with little and short microvilli in control cells (, asterisk) on the other hand, the surface area of the HA + BU + RA-treated cells, taken at a higher magnification SEM, showed a large presence of microvilli (, asterisk), with different thicknesses and lengths, which almost completely covered the cell membrane. The presence of abundant rough endoplasmic reticulum vesicles and microvilli could be related to the synthetic activity and paracrine secretion of cells committed toward the cardiovascular phenotype.
Discussion
The present study highlights the fact that hAFSCs are committed toward cardiac and angiogenic phenotypes after exposure to a mixture of HA, BU, and RA. We observed that the percentage of cells positively stained for the cardiac phenotype marker, alpha-sarcomeric actinin, was higher when hAFSCs were treated with HA + BU + RA. In these cells, the appearance of this cardiac lineage-specific protein was preceded by a significant increase in expression of the cardiogenic genes GATA-4 and Nkx-2.5 and their corresponding proteins, and expression of the prodynorphin gene, a well known orchestrator of cardiogenesis.Citation16,Citation17 The appearance of a cardiac phenotype was further inferred by electron microscopy analysis, which clearly revealed an initial stage of sarcomeric organization in HA + BU + RA-treated cells.
In this study, we have highlighted consistent expression of the two vasculogenic genes, vascular endothelial growth factor and hepatocyte growth factor, in HA + BU + RA-treated cells; in particular, von Willebrand factor was upregulated in these hAFSCs, when compared with untreated control cells, as assessed by real-time PCR and Western blotting. Further, confocal microscopy and flow cytometry analysis confirmed the vascular commitment of hAFSCs by revealing a significant increase in von Willebrand factor-positive elements when hAFSCs were exposed to HA + BU + RA. As expected, cells committed toward the cardiac and vascular phenotypes showed downregulation of expression of the pluripotency regulators Nanog, Sox2, and Oct, both at the transcriptional and protein levels. These findings further confirmed a commitment of hAFSCs toward specific cellular phenotypes. Vascular differentiation of human mesenchymal stem cells is very important for tissue transplantation in regenerative medicine, because it represents a source which is working rapidly for tissue regeneration. Preclinical and clinical studies reported that the improvement in cardiac function and tissue regeneration observed after mesenchymal stem cell transplantation seems to depend on the release of paracrine factors.
Human mesenchymal stem cells have been found to express vascular endothelial growth factor, hepatocyte growth factor, and other arteriogenic cytokines promoting vasculogenesis through paracrine mechanisms in vitro and in vivo.Citation22–Citation25 In addition, undifferentiated hAFSCs have been used in the recovery of neurogenic and cardiac function in ischemic mouse and rat stroke models.Citation26,Citation27
However, despite these features, the cardiovascular commitment of human mesenchymal stem cells is an extremely low-yield process and the differentiated elements have a poor chance of survival. Other authors have described differentiation of hAFSCs toward vascular phenotypes with a higher yield, using media containing a mixture of specific growth factors and shear force stimuli.Citation28 Indeed, genetically modified mesenchymal stem cells have been shown to have a higher regenerative potential than nonengineered cells. In particular, some authors have demonstrated viability and engraftment from cells overexpressing secretable factors with cardioprotective properties.Citation29–Citation33
An alternative approach to improve paracrine release of angiogenic factors such as vascular endothelial growth factor and hepatocyte growth factor has been to precondition human mesenchymal stem cells of the fetal membrane (FMhMSCs), bone marrow, and dental pulp with HBR, which elicits a remarkable increase in cell survival and differentiation.Citation10 To this end, transplantation of FMhMSCs preconditioned ex vivo into infarcted rat myocardium with HBR primed a normalization of myocardial performance and a significant decrease of scar tissue through paracrine mechanisms. Of note, even mesenchymal stem cells obtained from other sources, such as human adipose-derived stem cells pretreated with HA + BU + RA favored survival of islet transplantation in streptozotocin-diabetic rats.Citation11
In the present work, we observed that HA + BU + RA-treated hAFSCs have a higher yield of cardiovascular differentiation, as compared with HA + BU + RA-treated FMhMSCs differentiated toward the same lineages (unpublished data). The possibility that hAFSCs may represent a suitable source of potentially transplantable cells in large-scale allogenic settings is supported by a number of interrelated observations, including their easy isolation and growth, affordability of expansion even as clonal growth, ability to differentiate into all germ layers,Citation12,Citation34–Citation36 lack of immunogenicity due to low expression of the major histocompatibility complex class II antigen,Citation37 and the absence of signs of tumorigenicity in vitro and in vivo.Citation38,Citation39 On the other hand, a limitation to the therapeutic application of these cells lies in the fact that they can only be obtained from pregnant women.
We have previously shown that the effect of a mixed ester of HBR on cardiac differentiation in murine embryonic and human mesenchymal stem cells obtained from fetal membranes was related to increased expression of the cardiogenic transcription factor Nkx-2.5 through Smad4 binding to its own consensus Smad site (Maioli M et al Plos One 2010).Citation40 On the basis of these crucial data, we hypothesize that a similar mechanism is involved even when hAFSCs are exposed to HA + BU, HA + RA, BU + RA, or HA + BU + RA. Future experiments are needed to confirm this hypothesis.
Considering the positive outcome obtained in this study, we hypothesize that exposing hAFSCs to a mixture of HA + BU + RA could pave the way for a large-scale cell therapy approach, even if in vivo studies are needed in order to define further the fate of committed stem cells.
Disclosure
The authors declare no conflicts of interest in this work.
References
- Baksh D Song L Tuan RS Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy J Cell Mol Med 2004 8 3 301 316 15491506
- Pittenger MF Martin BJ Mesenchymal stem cells and their potential as cardiac therapeutics Circ Res 2004 95 1 9 20 15242981
- Zimmet JM Hare JM Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy Basic Res Cardiol 2005 100 6 471 481 16237508
- De Coppi P Bartsch GJr Siddiqui MM Isolation of amniotic stem cell lines with potential for therapy Nat Biotechnol 2007 25 1 100 106 17206138
- Sartore S Lenzi M Angelini A Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes Eur J Cardiothorac Surg 2005 28 5 677 684 16188450
- Iop L Chiavegato A Callegari A Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury Cell Transplant 2008 17 6 679 694 18819256
- Guan X Delo DM Atala A Soker S In vitro cardiomyogenic potential of human amniotic fluid stem cells J Tissue Eng Regen Med 2011 5 3 220 228 20687122
- Bollini S Pozzobon M Nobles M In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells Stem Cell Rev 2011 7 2 364 380 21120638
- Ventura C Maioli M Asara Y Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells J Biol Chem 2004 279 22 23574 23579 15044487
- Ventura C Cantoni S Bianchi F Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts J Biol Chem 2007 282 19 14243 14252 17363374
- Cavallari G Olivi E Bianchi F Mesenchymal stem cells and islet cotransplantation in diabetic rats: improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules Cell Transplant 2012 21 12 2771 2781 22472472
- Tsai MS Hwang SM Tsai YL Cheng FC Lee JL Chang YJ Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells Biol Reprod 2006 74 3 545 551 16306422
- Grepin C Robitaille L Antakly T Nemer M Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation Mol Cell Biol 1995 15 8 4095 4102 7623805
- Biben C Palmer S Elliott DA Harvey RP Homeobox genes and heart development Cold Spring Harb Symp Quant Biol 1997 62 395 403 9598374
- Ventura C Zinellu E Maninchedda E Maioli M Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells Circ Res 2003 92 6 623 629 12623878
- Ventura C Zinellu E Maninchedda E Fadda M Maioli M Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells Circ Res 2003 92 6 617 622 12623877
- Ventura C Maioli M Opioid peptide gene expression primes cardiogenesis in embryonal pluripotent stem cells Circ Res 2000 87 3 189 194 10926868
- Duda DG Fukumura D Jain RK Role of eNOS in neovascularization: NO for endothelial progenitor cells Trends Mol Med 2004 10 4 143 145 15162796
- He H Venema VJ Gu X Venema RC Marrero MB Caldwell RB Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src J Biol Chem 1999 274 35 25130 25135 10455194
- Kashyap V Rezende NC Scotland KB Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs Stem Cells Dev 2009 18 7 1093 1108 19480567
- Bianchi F Maioli M Leonardi E A new non-enzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates Cell Transplant 10 8 2012 [Epub ahead of print.]
- Kim DH Yoo KH Choi KS Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell Cytokine 2005 31 2 119 126 15919213
- Mayer H Bertram H Lindenmaier W Korff T Weber H Weich H Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation J Cell Biochem 2005 95 4 827 839 15838884
- Kinnaird T Stabile E Burnett MS Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms Circ Res 2004 94 5 678 685 14739163
- Caplan AI Dennis JE Mesenchymal stem cells as trophic mediators J Cell Biochem 2006 98 5 1076 1084 16619257
- Mirabella T Cilli M Carlone S Cancedda R Gentili C Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model Biomaterials 2011 32 15 3689 3699 21371750
- Tajiri N Acosta S Glover LE Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats PLoS One 2012 7 8 e43779 22912905
- Zhang P Baxter J Vinod K Tulenko TN Di Muzio PJ Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli Stem Cells Dev 2009 18 9 1299 1308 19508152
- Matsumoto R Omura T Yoshiyama M Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction Arterioscler Thromb Vasc Biol 2005 25 6 1168 1173 15831811
- Duan HF Wu CT Wu DL Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor Mol Ther 2003 8 3 467 474 12946320
- Huang J Zhang Z Guo J Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium Circ Res 2010 106 11 1753 1762 20378860
- Mangi AA Noiseux N Kong D Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts Nat Med 2003 9 9 1195 1201 12910262
- Li W Ma N Ong LL Bcl-2 engineered MSCs inhibited apoptosis and improved heart function Stem Cells 2007 25 8 2118 2127 17478584
- Cipriani S Bonini D Marchina E Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain Cell Biol Int 2007 31 8 845 850 17379545
- Kunisaki SM Fuchs JR Steigman SA Fauza DO A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells Tissue Eng 2007 13 11 2633 2644 17655491
- in ‘t Anker PS Scherjon SA Kleijburg-van der Keur C Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation Blood 2003 102 4 1548 1549 12900350
- Magatti M De Munari S Vertua E Gibelli L Wengler GS Parolini O Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities Stem Cells 2008 26 1 182 192 17901399
- Joo S Ko IK Atala A Yoo JJ Lee SJ Amniotic fluid-derived stem cells in regenerative medicine research Arch Pharm Res 2012 35 2 271 280 22370781
- Chen J Lu Z Cheng D Peng S Wang H Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells PLoS One 2011 6 5 e19964 21625520
- Maioli M Santaniello S Montella A Hyaluronan esters drive Smad gene expression and signaling enhancing cardiogenesis in mouse embryonic and human mesenchymal stem cells PLoS One 2010 5 11 e15151 21152044