Abstract
Inflammatory bowel disease (IBD) is a chronic, non-specific inflammatory condition characterized by recurring inflammation of the intestinal mucosa. However, the existing IBD treatments are ineffective and have serious side effects. The etiology of IBD is multifactorial and encompasses immune, genetic, environmental, dietary, and microbial factors. The nanoparticles (NPs) developed based on specific targeting methodologies exhibit great potential as nanotechnology advances. Nanoparticles are defined as particles between 1 and 100 nm in size. Depending on their size and surface functionality, NPs exhibit different properties. A variety of nanoparticle types have been employed as drug carriers for the treatment of inflammatory bowel disease (IBD), with encouraging outcomes observed in experimental models. They increase the bioavailability of drugs and enable targeted drug delivery, promoting localized treatment and thus enhancing efficacy. Nevertheless, numerous challenges persist in the translation from nanomedicine to clinical application, including enhanced formulations and preparation techniques, enhanced drug safety profiles, and so forth. In the future, it will be necessary for scientists and clinicians to collaborate in order to study disease mechanisms, develop new drug delivery strategies, and screen new nanomedicines. Nevertheless, numerous challenges persist in the translation from nanomedicine to clinical application, including enhanced formulations and preparation techniques, enhanced drug safety profiles, and so forth. In the future, it will be necessary for scientists and clinicians to collaborate in order to study disease mechanisms, develop new drug delivery strategies, and screen new nanomedicines.
Introduction
Inflammatory bowel disease (IBD) is a chronic, non-specific inflammatory disease that affects the intestinal tract.Citation1 The prevalence of IBD is highest in North America and Europe, where it is estimated that 6.9 million people globally have it.Citation2,Citation3 The prevalence of inflammatory bowel disease (IBD) has been on the rise in newly industrialised countries over the past two decades, reaching a point of accelerated growth.Citation2,Citation3 In contrast, the Western world is currently experiencing a phase of stabilisation in terms of incidence, with prevalence expected to remain at approximately one percent by 2030.Citation4 This presents a significant challenge to global public health.
The two main types of IBD are Crohn’s disease,Citation1 which typically affects the gastrointestinal tract in a segmented structure, and ulcerative colitis, where lesions primarily affect the colonic mucosa and submucosa in a continuous pattern.Citation5 Abdominal pain, diarrhea, bloody stools, and weight loss are only a few of the primary clinical symptoms. Additionally, the most typical extra-intestinal problems primarily affect the joints, skin, eyes, and bile ducts, significantly impacting the quality of life.Citation6,Citation7
Although the exact cause of IBD is still unknown,Citation8 several factors, including immunological, gastrointestinal, environmental, nutritional, and microbial infections, may be linked to IBD.Citation9,Citation10 The pathophysiology of IBD is characterised by a loss of function of the mucosal epithelial cell system, local immune cell responses, dysbiosis of the gut microbiota, and changes in the local environment of the tissues. Despite the lack of curative treatment for the eradication of IBD,Citation11 these physiological and pathological changes provide new targets for the development of targeted drug delivery systems for IBD. Inducing an initial remission and preventing relapse during remission are the general principles of pharmacological treatment of IBD.Citation12 Aminosalicylates, antibiotics, glucocorticoids, immunomodulators, and biologics are commonly used in conventional drug therapy for IBD. These drugs aim to improve the mucosal lining of the colon and repair, induce and maintain inflammatory remission.
A significant obstacle to effective treatment is drug delivery to the diseased site. Parenteral, transoral, and rectal enema are traditional delivery methods for IBD.Citation13 Because of its low cost of production, convenience of handling, and good compliance, the oral dose form is regarded as the most desirable and acceptable form of daily administration for treating IBD.Citation14,Citation15 However, the active ingredient is absorbed by the mucosal membrane of the alimentary tract and distributed throughout the body following oral formulation. The development of systemic adverse drug reactions can impact treatment outcomes, as they are influenced by notable variations in the gastrointestinal environment and between healthy and inflamed intestinal regions. Intrarectal administration can potentially deliver tissue concentrations even higher than oral administration and provide local treatment for IBD in the distal colon.Citation16 However, conventional enemas are ineffective in patient compliance because of their short retention time in the colorectal lumen and the need for frequent administration.Citation17,Citation18 Therefore, it is imperative to design effective drug delivery systems (DDSs) to deliver more drugs to the site of inflammation precisely.
Medical nanomaterials have come a long way in the last few years. Its goal is to design and manufacture materials with novel properties and functions on the scale of 1 to 1000 nm, namely nanoparticles (NPs).Citation19 NPs are small, have a large surface area, and have a unique shape. As a novel bioactive carrier, NPs increase the local drug concentration at the disease site to maximize drug efficacy. They have been significant in gastrointestinal diseases.Citation20 Significant advancements have been made in nanoparticle-based strategies for the treatment of inflammation and tumours. Several chronic diseases, such as osteoarthritis,Citation21 rheumatoid arthritisCitation22 and skin conditions,Citation23 have been treated with NSAIDs (non-steroidal anti-inflammatory drugs) or Glucocorticoids as either a primary or adjunctive treatment option. These diseases often require prolonged anti-inflammatory therapy. The developments in nanotechnology have markedly enhanced the accumulation of anti-inflammatory agents. Targeting is the key to treating IBD. Targeted delivery of IBD reduces systemic drug exposure and related side effects by releasing the drug directly into the inflamed tissue, lowering the frequency of administration to obtain the required dosage, and minimizing the non-specific distribution of the drug throughout the body. Currently, various NPs, including polymeric NPs, lipid-based NPs, liposomes, silica NPs, nanogels, shell-core NPs, and particle NPs, are used as drug carriers for treating IBD.Citation24
Several targeting strategies have been investigated so far, and they are often predicted by different physiological factors between the colonic and proximal sections of the gastrointestinal tract.Citation25 Its three primary divisions are passive, active, and hybrid targeting. This paper has reviewed and discussed the targeting and functional roles of nanopharmaceutical agents in treating IBD (). We have summarised the effectiveness and limitations of different types of delivery systems for the treatment of IBD (). Additionally, we have summarized the challenges and possible avenues for further study in this area.
Table 1 The Passive Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 2 The Active Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 3 The Hybrid Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 4 The Effectiveness and Limitations of Different Types of Drug Delivery Systems for the Treatment of IBD
Passive Targeting
The primary determinants of passive targeting are the physicochemical characteristics of the particle carrier itself (size, charge, etc). and the local microenvironment.Citation101 Thus, passive targeting methods for IBD may be achieved by altering the nanosize of the NPs and exploring the local microenvironmental characteristics of the intestine (pH, reactive oxygen species [ROS] levels, and overexpression of the digestive enzymes).
Targeting Based on Enhanced Permeability and Retention (EPR) Effect
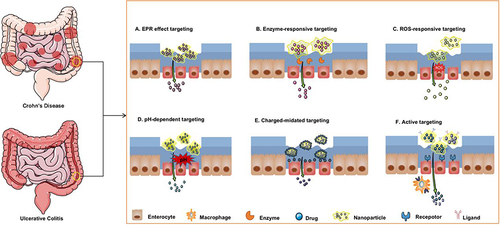
In normal tissues, microvascular gaps seem to be densified and structurally intact. In contrast, to enable particle adherence, the inflammatory intestine produces more mucus than normal tissue, and the small size of NPs increases their capacity to penetrate the mucus.Citation102 Furthermore, increased endothelial barrier permeability has been linked to epithelial injury and loss of intercellular tight junction chains produced by various factors (inflammatory mediators, cytokines, etc).Citation103,Citation104 NPs are taken up and retained in the inflammatory site by the infiltrating immune and inflammatory cells, such as macrophages, dendritic cells, and neutrophils.Citation105 This is known as the epithelial increased permeability and retention effect (EPR) ().Citation106 As a result, NPs can prolong their stay at the inflammation site by passively targeting it.
Figure 1 Strategies for inflammatory bowel disease treatment using nanoparticle-based drug delivery systems. Nanoparticles specifically target inflammatory colonic epithelial cells based on enhanced permeability and retention effects (A), specific enzyme levels (B), reactive oxygen species (ROS) levels (C), specific pH levels (D), electrostatic interactions (E), and ligand-receptor interactions (F).
Lamprecht et alCitation107 showed the advantageous impacts of nanoscale particle size for UC treatment by comparing the bioadhesive capabilities of fluorescent polystyrene particles measuring 10 µm, 1 µm, and 100 nm. When compared to a healthy colon, it was discovered that 100 nm particles adhered to an inflammatory colon in rats under oral administration compared to a healthy colon. Furthermore, the faster the drug was absorbed and the greater the therapeutic effect, the smaller the particle size.
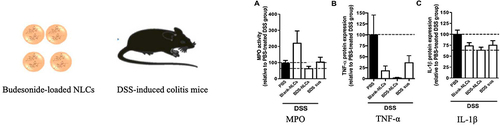
Solid lipid NPs (SLNs) have become an appealing drug delivery mechanism among the available nanocarriers.Citation108 Compared to other lipid NPs (liposomes, etc)., SLNs have superior stability, biocompatibility, and degradability in the gastrointestinal tract, along with the capacity to influence immune responses and anti-inflammatory properties.Citation109,Citation110 Beloqui et al developed nanostructured lipid carriers (BDS-NLC) containing budesonide (BDS) with an average diameter of approximately 200 nm.Citation26 Research conducted in vitro showed that BDS-NLC could encapsulate up to 95% of the drug and prolong its half-life in the colon. Similarly, it produced therapeutic benefits comparable to those of healthy control colon tissue by lowering the levels of myeloperoxidase (MPO), interleukin (IL) IL-1β, and tumor necrosis factor (TNF) TNF-α ().
Figure 2 The expression of myeloperoxidase activity (A), TNF-α (B), and IL-1β (C) was significantly decreased in the colon of mice treated with budesonide-nanostructured lipid carriers compared to the control group. Reprinted from Int J Pharm, volume 454(2), Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. 775–783, Copyright 2013, with permission from Elsevier.Citation26
Furthermore, protamine has been used to develop stable nanocapsules.Citation111 Jakubiak et al encapsulated cyclosporine A in protamine-coated nanocapsules.Citation27 The average particle size of these NPs was 160–180 nm. Although this nanocapsule showed good stability against trypsin in simulated trials, predicting its stability and drug release in vivo is challenging. In vitro experiments demonstrated that these NPs were superior to commercial agents in their ability to decrease IL-2 levels.
The treatment of experimental colitis with celecoxib (CXB) has demonstrated significant efficacy.Citation112,Citation113 A formulation of CXB nanomixed micelles (NMMs) was developed to investigate the adverse effects of colon-targeted agents to reduce CXB. The NMMs were then integrated into a novel pulsatile capsule with an average particle size <290 nm.Citation28 The capsule could be released in vitro in 88.35% of cases if the capsule is designed to target the colonic site. Furthermore, it demonstrates superior defense against acetic acid-induced experimental colitis models compared to regular capsules.
Ali et al designed NPs that target inflammatory colonic mucosa by inserting budesonide into poly(l-propylene-glycoside lactone) (PLGA) NPs with an average particle size of 200 nm.Citation29 Fluorescence analysis showed that while the NPs could be dispersed throughout the digestive tract in the colonic tissues of healthy mice, the particles appeared more at the inflamed site in inflamed mice. It is also important to note that the drug displayed a biphasic release pattern in vitro, releasing rapid at first, then slowly and continuously after that. They suggest that the initial rapid release could be because the drug molecules are just attached to the surface of the NPs rather than fully encapsulated.
Protein NPs have gathered attention recently because of their excellent biocompatibility and advantages in biodegradability.Citation114,Citation115 Covalently binding 5-aminosalicylic acid to hemoglobin produced the NPs with a diameter of about 220 nm.Citation30 Only 8% of the drug was absorbed and released within 4 h following the vitro simulation test. Data from their vivo trials also showed that 85% of the NPs could reach the colon and release the drug. This suggests that the system has outstanding stability and is able to slow drug release in the stomach.
The lack of polysaccharide-degrading enzymes in the human body may prevent the natural polysaccharide found in sawdust gum from being degraded in the upper gastrointestinal tract.Citation116 However, the microflora enzymes present in the colorectum can degrade it to produce fatty acids.Citation117,Citation118 A regimen with a mean particle size of 228 nm was shown to be optimized by Amaldoss et al after developing tamarind gum NPs loaded with rifaximin.Citation31 Compared to the control group, the NPs effectively lowered colonic inflammation. Furthermore, studies conducted on patients with IBD have demonstrated a significant increase in platelet counts in the literature.Citation119 However, they performed testing studies and discovered that neither the treatment group nor the blood fractions had significantly higher platelet levels.
Additionally, passive targeting based on the EPR effect has been observed by others. This could lead to some passive accumulation of NPs at the inflammation site and decrease the loaded drug’s adverse effects.Citation32–34 However, this single effect-based approach to drug delivery is not ideal. Furthermore, during the synthesis of NPs, the drug encapsulation rate may be satisfactory. However, variations in the preparation processes or formulations may cause NPs to have unstable properties, making it challenging to achieve acceptable outcomes. Furthermore, it is unlikely that the interactions of NPs with the tissues or cells in the inflamed colon will be the primary means of targeting the colon.Citation34
Targeting Based on Enzymes
The gastrointestinal tract contains various enzymes, including lysozyme, azo reductase, esterases, sphingomyelinase, etc.Citation11,Citation120 Furthermore, the enzyme secretion of patients with IBD significantly differed from that of healthy individuals. These digestive enzymes quickly degrade drugs, which reduces their therapeutic efficacy. The enzyme reaction pattern depends on certain enzymes to catalyze chemical reactions. The drug is released at the lesion site by surface-modified DDSs, which use the enzymes as stimuli to cause their degradation or morphological transformation ().Citation35–41
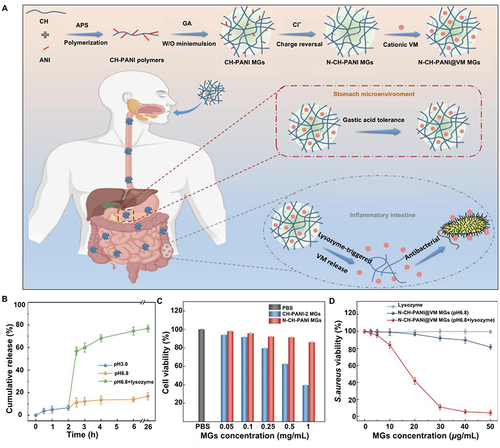
Intestinal pathogens cause aberrant lysozyme secretion in the colon by interfering with cellular function.Citation121,Citation122 Li et al developed a lysozyme-triggered chitosan polyaniline microgels loaded with vancomycin (VM).Citation35 The biodegradation of the microgel was triggered by lysozyme, which also cleaved the glycosidic bond and released VM (). According to an in vitro test, the drug was released in the inflammatory colon within 30 min, up to 76.9%. The microgel system inhibited S. aureus at the same concentration as the control without lysozyme. The Caco-2 cell line had an excellent biosafety profile with a cell survival rate of >86.1% in experiments.
Figure 3 Lysozyme-triggered release of vancomycin from chitosan microgels for treating inflammatory bowel disease. (A) Schematic representation and mechanism of action of lysozyme-triggered nanoparticles. (B) Determination of Caco-2 cell activity in various treatment groups. (C) Inhibitory effect of N-CH-PANI@VM MGs on Staphylococcus aureus in various environments. (D) Cumulative release of lysozyme-induced VM in various simulated environments. Adapted from J Adv Res, volume 43, Li X, Hetjens L, Wolter N, et al. Charge-reversible and biodegradable chitosan-based microgels for lysozyme-triggered release of vancomycin. 87–96, Copyright 2023, with permission from Elsevier.Citation35
Moreover, azo reductase is the most widely used enzyme for azo polymer adhesion, hydrogels, coatings, etc.Citation123 Additionally, the researchers developed multilayer-coated mesoporous silica (MSs), which activates azo reductase generated by intestinal microorganisms to release loaded drugs.Citation36 According to the test findings, mice in the oral-free drug group had a drug concentration 35 times lower at the colonic site than the mice. Notably, aryl hydrocarbon receptor activation by tryptophan-functionalized chitosan can protect the integrity of the intestinal barrier and enhance intestinal homeostasis when it is transformed into metabolites by intestinal flora.
Silica as a drug carrier NP in biomedical applications has advanced significantly. Researchers have developed a pre-drug system to treat colitis by loading 5-aminosalicylic acid onto silica NPs (SiNP).Citation37 Studies revealed that mice in the SiNP group accumulated six times as much drug in the inflamed tissue as in the control group, significantly decreasing the drug dosage needed for treatment. Experiments conducted on mice have demonstrated that the nanodrug selectively accumulates in inflamed tissues and prolongs the presentation duration to achieve a therapeutic impact with a delayed release. Although esterases can gradually initiate the catabolic conversion of precursor drugs, as demonstrated by drug release experiments, further in vivo research is required to understand this phenomenon fully.
Furthermore, natural polyphenols have drawn much attention as safe compounds with free radical scavenging and antioxidant properties.Citation124,Citation125 Consequently, researchers designed a DDSs encapsulated with dexamethasone (DEX) by self-assembling polyphenols (tannins) and polymers.Citation38 When esterase is present at a concentration of 30 U/mL, up to 62% of DEX is released. According to pharmacofluorescence imaging, the fluorescence intensity of the inflamed mouse colon was shown to be four times higher than that of the healthy colon. Furthermore, PPNP-DEX had a better therapeutic impact on colitis-affected mice than PPNP and free DEX. Research has shown that non-degradable polyethylene glycol (PEG) compounds can produce anti-polyethylene glycol antibodies in vivo, making PEG drugs biologically inactive.Citation126 However, they discovered little impact of anti-PEG antibodies on oral PEG drugs by fluorescence imaging, contrary to the report.
Sphingolipid liposomes were also used to develop the NPs,Citation39 and ICG was fluorescently labeled because sphingomyelinase is present outside of cells during cellular stress.Citation127 The results of the experiment showed that liposomes could be taken up by both epithelial cells and macrophages, thus accomplishing drug delivery. In the inflammatory colon, macrophages produced higher sphingomyelinase activity and greater drug phagocytosis than epithelial cells.
Furthermore, materials made of naturally occurring chemicals offer good biosafety and biocompatibility. Xu et al produced NP formulations with anti-inflammatory and antioxidant properties by packing DEX within curcumin and hydroxyethyl starch micelles.Citation42 In vitro release assays demonstrated that the negatively charged outer surface of NPs aided in their binding to inflammatory colon cells, and the α-amylase increased the drug release rate. NPs decreased the severity of inflammatory lesions and improved the effectiveness of free DEX compared to the untreated group.
Targeting Based on ROS
Free radicals, such as superoxide (O2) and −OH, and non-free radicals, like singlet oxygen (1O2) and hydrogen peroxide (H2O2), make up the majority of ROS.Citation128,Citation129 Bowel inflammation is caused by pro-inflammatory mediators, including TNF-α and IL-1, produced when ROS triggers the NF-κB signaling cascade.Citation130 Oxidative damage in the colon is caused by an excess of ROS and an imbalance of antioxidants in the intestinal mucosa of patients with IBD.Citation129,Citation131,Citation132 Activated phagocytes and leukocytes at the site of colitis are the primary source of the increased production of ROS.Citation133 According to a study, patients with IBD had 10- to 100-fold higher ROS concentrations in intestinal inflammation than healthy individuals.Citation134,Citation135 Redox-responsive DDSs have gained attention from researchers to improve targeted drug delivery in inflamed intestinal areas ().Citation43–47
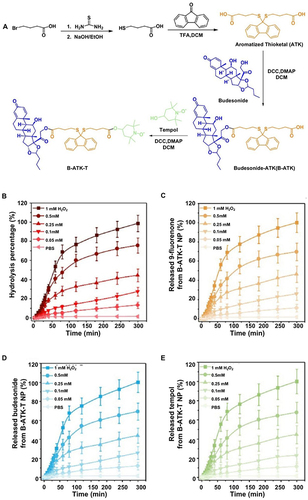
Drug-drug coupling systems, with their high drug loading and minimal side effects, have been suggested as a novel approach. Li et al developed spherical nanostructures by self-assembling ROS-sensitive aromatized thione linkers with the anti-inflammatory drug budesonide and the antioxidant tempol ().Citation43 Interestingly, the drug loading of the NPs was more significant (41% and 16%) than the loading of the two drugs in the PLGA NPs (6% and 3%). In the simulated environment experiment, the ROS-dependent release pattern led to nearly full release (99% and 98%) of these NPs for both drugs. Conversely, only 44% and 18% of the drug was released from PLGA NPs. The concurrent release of the two drugs allowed for the synergistic anti-inflammatory and antioxidant effects.
Figure 4 Sensitive reactive oxygen species-responsive B-ATK-T nanoparticles (NP) for treating irritable bowel disease. (A) The synthetic process of B-ATK-T. (B) Hydrolysis rate of B-ATK-T NP at various concentrations of hydrogen peroxide (H2O2). The release profiles of 9-fluorenone (C), budesonide (D), and tempol (E) from B-ATK-T NP at varying concentrations of H2O2 concentrations. Reprinted from J Control Release, volume 316, Li S, Xie A, Li H, et al. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. 66–78, copyright 2019, with permission from Elsevier.Citation43
Additionally, superoxide dismutase (SOD) breaks down superoxide to form hydrogen peroxide, which catalase then breaks down into water. Zhang et al produced NPs (Tpl/OxbCD NP) by encapsulating the free radical scavenger Tempol (Tpl) in oxidation-responsive b-cyclodextrin, which releases cargo molecules by scavenging ROS components. According to drug imaging, OxbCD NPs had a higher targeting effectiveness than control PLGA NPs and accumulated 2.5 times more fluorescence intensity in mouse colon tissue than in normal mice. The oral Tpl/OxbCD NPs group showed a significant reduction in symptoms in three mice colitis models, with more efficacy than the free radical scavenger Tpl and -based control nanomedicine.Citation44
In addition, IBD makes it easier for pathogens, such as intestinal bacteria, to enter the bloodstream and invade other organs. The goal of the design was a drug delivery system that scavenges ROS from the inflamed colon while also delivering antioxidant drugs to the bloodstream to reduce systemic inflammation. Researchers have developed silica-containing redox NPs that can scavenge ROS when loaded with silymarin.Citation45 According to the findings of in vivo experiments conducted on mice, the blood uptake of silymarin was significantly increased by the antioxidant carrier (siRNP). Additionally, the damage to the inflamed colonic mucosa was decreased considerably by the synergistic antioxidant effect of the drug and carrier.
Targeting Based on pH Levels
In contrast to the colon and rectum, which have pH values between 7.1 and 7.5, the stomach has an acidic pH.Citation136 Drug protection from gastrointestinal conditions and delayed drug release in acidic pH conditions can be achieved by NPs with a pH-sensitive design (). Scientists have developed drugs that are unique to the colon due to variations in the pH of the various gastrointestinal tract organs. Several pH-sensitive nanostructures, such as nanospheres, nanocapsules, and nano-polymers encapsulating other materials, have been developed. Additionally, colon-targeted drug delivery systems were designed using pH-dependent polymers, including methacrylic acid and methyl methacrylate (Eudragit® S 100, Eudragit® L, Eudragit® FS and Eudragit® P4135 F),Citation137 hydroxypropyl methyl phthalate cellulose, and few other polymers.Citation14,Citation48–62 Eudragit® polymer is one of the most widely used synthetic copolymers for colonic drug delivery.Citation138
The ionization of carboxyl functional groups makes the Eudragit® s100 resistant to invasion of the upper gastrointestinal tract, and it becomes soluble at pH >7.Citation139,Citation140 Qelliny et alCitation48 synthesized NPs were loaded with budesonide, and their surface was coated with pH-sensitive Eudragit® s100. Studies conducted in vitro show that up to 72% of its maximum short-term cumulative release occurs at pH 7.4. Additionally, studies conducted on animals suggested that it had a more significant therapeutic effect on UC than the drug suspension in its free form.
Furthermore, Zhang et al developed hybrid drug delivery systems by encapsulating PLGA NPs loaded with berberine within an Eudragit® FS 30D matrix that has already been pre-encapsulated with berberine ().Citation49 This pH-sensitive system immediately releases the drug-loaded NPs and berberine upon reaching the colon for lysis. The PLGA NPs are then absorbed by the colonic mucosa and gradually breakdown to maintain the sustained release of the drug. This pH-sensitive nanosystem accomplishes drug release into the tissue instantly and continuously. It promotes better therapeutic efficacy and patient compliance by lowering the amount of drug required and the frequency of administration.
Figure 5 The novel nano-delivery system MNEHDS for treating irritable bowel disease. (A) The manufacturing process of MNEHDS. (B) Berberine (BBR) drug release rates in various simulated environments. The changes in BBR concentrations were investigated at various intervals in the plasma (C), colon (D), and feces (E). Reprinted from Zhang L, Li M, Zhang G, et al. Micro- and nanoencapsulated hybrid delivery system (MNEHDS): a novel approach for colon-targeted oral delivery of berberine. Mol Pharmaceut. 2021;18(4):1573–1581. Copyright © 2021 American Chemical Society.Citation49
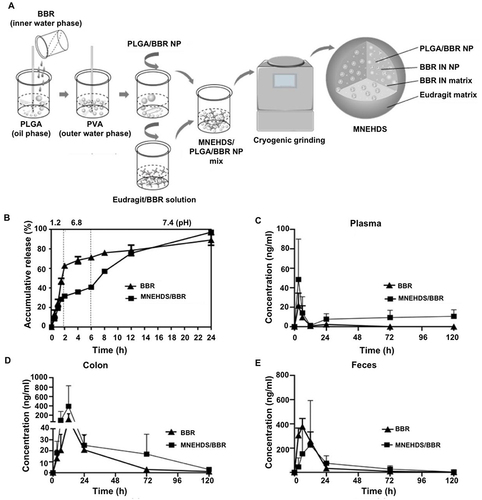
The process of creating polymer NPs involves grafting polyacrylamide (PAAm) onto the backbone of xanthan gum (XG).Citation50 Upon additional NP hydrolysis, the PAAm amide functional group is transformed into a carboxylic acid (-COOH) group, creating a pH-sensitive copolymer.Citation141 Moreover, coliform bacteria can activate XG. Therefore, the NPs are very selective for colonic targeting. At a pH of 6.8, 3 h were needed to release approximately 65% of the drug. In vitro tests on rats have revealed that the drug release rate is <15% with an acidic pH. However, within 8 h, the drug release rate may reach 100% if the pH of the solution is increased to 6.8 and intestinal contents are added.
Additionally, IL-1 receptor antagonist (IL-1Ra)-containing alginate/chitosan microcapsules were prepared.Citation51 Chitosan is a cationic polysaccharide.Citation142 The electrostatic interaction between the two is diminished in weak alkaline solutions because chitosan has a lower positive charge than alginate. This lets alginate absorb water and swell in an inflammatory colon environment, releasing the drug.Citation143 Furthermore, the microcapsules acquired an ultimate cumulative release rate of 86.2% in vitro. The microcapsules decreased the dose-induced colitis in mice, partially allowing the drug to accumulate in the colon.
Meissner et al developed a pH-sensitive Eudragit P-4135F polymer for colonic delivery of drug-loaded NPs to increase drug delivery efficiency and tolerability.Citation52 The polymer can break down and release the drug at pH >7.2. In vitro tests demonstrated that after 30 min, 100% of the loaded drug release at pH 7.4 could be achieved. Moreover, the oral NP formulation outperformed the free oral drug, although it was less effective in alleviating experimental colitis than subcutaneous administration.
The pH-dependent delivery mechanism may keep the drugs from dispersing before they reach the colon site. However, patients with IBD have a more acidic pH range in their colons,Citation136 which leads to partial drug release from the target site.Citation144 Researchers have created pH-dependent systems with alternative drug delivery systems, such as ROS-dependent or enzyme-triggered systems, to overcome the limitations of single pH-dependent DDSs.Citation63–67
Naeem et al developed a pH- and azo reductase-sensitive azo polyurethane and Eudragit® S100 NPs.Citation63 Compared to single-trigger ES NPs, the NPs provide superior therapeutic efficacy by preventing a sudden release of the drug in the ileum and delivering an adequate amount to the inflamed colon. Budesonide is then sustained and released by an enzymatic reaction compared to single-trigger ES NPs. Pilot tests have demonstrated that the NPs are more stable than the pH-dependent type alone, preventing early drug release and enabling targeted colonic drug delivery.
The researchers developed mesoporous silica NPs, coated them with hydrolyzed starch, and placed them inside capsules containing Eudragit® FS 30D.Citation64 The nanosystem made it possible to alleviate the adverse effects of the drug and increase drug concentration at the colonic inflammatory site. Mesoporous silica NPs are released from the nanocapsules at colonic pH and are endocytically transported into colon cells after amylase stimulation.
The researchers developed an antioxidant-responsive dextrose (OxiDEX) NPs loaded with rifaximin.Citation65 The pH-responsive polymer hydroxypropyl methylcellulose acetate succinate was then used to encapsulate the NPs in chitosan surfaces. A pH- and ROS-responsive nanodelivery system was formed. Upon entering the colon and passing the acidic environment of the stomach, the NPs will release RIF in response to a trigger that increases ROS levels. In vitro experiments have demonstrated that the system can initiate the release mechanism at intestinal pH (6.8) and that, in the presence of H2O2, NPs can release >60% of the drug.
In addition, Wang et al developed infliximab-loaded polyphenol-containing PEG polymer self-assembled NPs.Citation66 In the stomach, the NPs aggregated into large-sized NPs. Then, at neutral pH in the colon, they reversibly transformed into small-sized negatively charged NPs (~100 nm). The antibody drug is then released from the NPs when they bind to the inflammatory colonic site through charge interactions and are impacted by high ROS concentrations in the mucosa. The favorable colonic targeting specificity and excellent therapeutic efficacy of the NPs were demonstrated in vivo in mice with colitis.
Targeting Based on Positive Charge
Surface-negatively charged DDSs has a high molecular target in the form of the positively charged protein (transferrin), which is overexpressed on the inflamed epithelial surface of IBD.Citation145 Negatively charged particles exhibit preferential adherence to injured sections of the colon through electrostatic interactions with these proteins ().Citation68–73
Surfactants can impact the targeting efficiency of NPs. The NPs with negatively charged surfaces were created using polysorbate 20 as a surfactant.Citation68 In 30 min, the colonic site may release 80% of the loaded drug. The same NPs interacted two to three times more with macrophages (RAW 264.7 cells) than with enterocytes (C2BBe1 cells), which is an interesting aspect that implies a simple cell line is not a sufficient model of inflamed tissue in vivo.
Furthermore, heparin has a significant negative charge on the outer surface. Zhang et al developed NPs targeting inflammatory colon (HEP-HSA NPs) that use the electrostatic interactions at the region of intestinal inflammation to load both biological agents and small molecule drugs.Citation69 In vitro assays revealed that the NPs had a more potent anti-inflammatory impact than NPs loaded with a single drug. Notably, it was discovered that there was a negative correlation between the diameter of HEP-HSA NPs and mucosal binding, with larger NPs preferentially binding to inflamed mucosa. And the investigation indicates that the smaller particle-size NPs enter the submucosa deeper.
Active Targeting
Drug distribution was improved in the inflamed areas of the colon by the EPR effect, which was facilitated by the particle size and surface physicochemical properties of NPs. However, this only promoted the accumulation of DDSs in colitis tissues. Insufficient target cell absorption efficiency and low intracellular drug release restrict anti-inflammatory drug therapeutic efficacy. Therefore, developing nanocarriers capable of actively targeting inflammatory cells may enable more precise targeting of colonic disease and minimize adverse side effects more effectively.
Specific antigens or receptors, such as the mannose, scavenger, folate, CD44, and chemokine receptors, are significantly overexpressed by epithelial cells and activated macrophages during the development of IBD. The interaction between particular receptors expressed at the diseased site and targeting ligands on the surface of the vector has increased targeting specificity. It also increases the degree of endocytosis and the bioadhesion of drug agents to particular cells ().Citation75–94
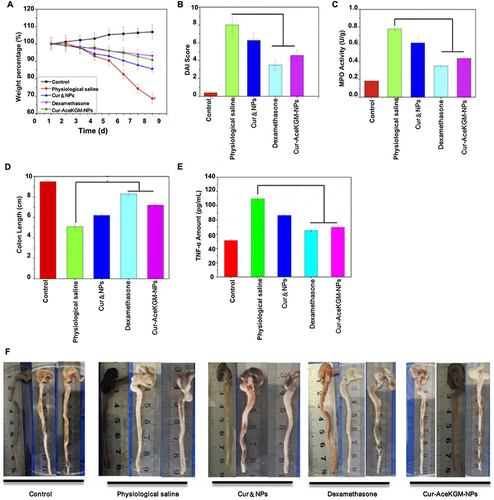
The mannose receptor is overexpressed explicitly on the surface of macrophages at the site of inflammation.Citation146 Upon contact with this receptor system, NPs are rapidly internalized through receptor-mediated endocytosis, resulting in targeted drug delivery. Wang et al developed a naturally occurring polysaccharide-based NP that targets binding to macrophage mannose receptors.Citation75 According to the experimental results, NPs exhibited 81% drug release within 48 h, and the MPO levels of the mice were decreased. The NPs offer sustained release of curcumin and effective therapeutic outcomes compared to oral free curcumin administration ().
Figure 6 AceKGM nanoparticles for the treatment of irritable bowel disease. The percentage change in mice from various treatment groups body weight (A), Disease Activity Index score (B), myeloperoxidase activity (C), colon length (D), and TNF-α content (E). (F) Mice colonic tissues from various treatment groups. Reprinted from Wang C, Guo Z, Liang J, et al. An oral delivery vehicle based on konjac glucomannan acetate targeting the colon for inflammatory bowel disease therapy. Front Bioeng Biotechnol. 2022;10:1025155. Creative Commons.Citation75
Phosphatidylglycerophosphate methyl ester (PGP-Me) is a ligand for the scavenger receptor, which is highly expressed in macrophages and dendritic cells.Citation147 SOD was delivered via nanovesicles containing PGP-Me, which also could promote endocytosis of the drug carried by macrophages.Citation76 The study showed that mouse macrophages took up the nanosystem 6.4 times more than liposomal NPs. The activity of the enzymes it contains remained unchanged when exposed to conditions similar to oral administration, compared to the control group.
Furthermore, Le et al developed NPs functionalized on folic acid surfaces that were loaded with antioxidant enzymes.Citation77 They next evaluated a mechanism dependent on cellular endocytosis mediated by the folic acid receptor. Its PEG coating keeps antioxidant enzymes from breaking down. In vitro, cellular uptake tests demonstrated that the NPs could be absorbed by macrophages and epithelial cells and displayed a powerful solid fluorescent signal compared to controls. Moreover, the results of in vivo tests showed that intrarectal administration significantly decreased colitis symptoms in mice models by downregulating the production of pro-inflammatory cytokines.
Moreover, hyaluronic acid is a primary gastrointestinal mucosal epithelial extracellular matrix component, enabling interaction with overexpressed CD44 receptors.Citation148,Citation149 Budesonide-loaded hyaluronic acid nanosystems (HANPs) were designed.Citation78 Compared to uncoated nanocomplexes, the HANPs enhanced cell adhesion and uptake in vitro experiments. Moreover, when HANPs and the exact dosage of free drugs were used in inflammatory cell models, HANPs showed higher anti-inflammatory effects on the secretion of inflammatory factors.
Chemokine receptor (CCR5) can be expressed on the macrophage surface.Citation150 Gong et al combined the chemokine ligand CCL4 with PLGA NPs to allow it to bind to the macrophage surface receptor CCR5, significantly improving the targeting ability of the drug.Citation79 Fluorescence staining showed that colonic macrophages could take up the NPs. The NPs not only improved the dysbiosis of the intestinal flora, but also promoted the repair of the intestinal barrier function by loading spleen tyrosine kinase inhibitors and decreasing the production of cytokines and chemokines.
Overall, the active targeting of ligands attached to the surface of nanodelivery systems is a promising strategy for treating IBD. Targeted ligands and targeted receptors expressed at inflammatory areas may improve the bioadhesion of drug formulations to particular cells and increase the degree of drug endocytosis. However, further in vivo research is required to evaluate the effectiveness of different strategies.
Hybrid Targeting
Apart from the above mentioned passive or active targeting strategies that rely on single factors (pH, enzymes, ROS, receptors, etc)., researchers have attempted to design targeting strategies that rely on multiple factors to overcome the multiple biological challenges encountered with orally delivered nanoparticle systems.Citation95,Citation97–100 These targeting strategies, which combine different NP triggers, take advantage of the benefits of a single form while potentially mitigating its disadvantages to attain maximum effectiveness.
Mannose was abundant in cationic konjac glucomannan (cKGM) and selectively identified mannose receptors on the membranes of macrophages.Citation151,Citation152 The researchers used methacrylate-based gelatin (GelMA) loaded with cKGM and ASO nanocomposite and embedded in pH-sensitive Eudragit FS30D to create pH and mannose receptor-responsive nanocomposites.Citation95 The alkaline environment of the colon promotes the release of nanocomplexes, and colonic macrophages can absorb ASO through mannose receptor-mediated endocytosis. According to the experimental data, colitis mice may benefit from the targeted release of nanonucleic acids into their colons, which may help reduce inflammation and mitigate damage. This could have an impact on how IBD is treated.
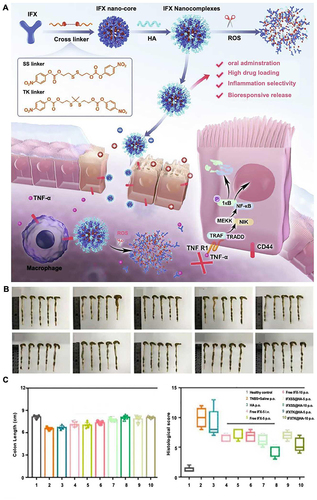
Additionally, infliximab was loaded into the oral NP delivery system by combining it with ROS-reactive cross-linkers and altering it with hyaluronic acid.Citation97 Two synthetic ROS-responsive cross-linkers, SS and TK linkers, are highly sensitive to ROS to protect the integrity of NPs and allow the release of antibodies in the inflamed intestinal mucosa. Hyaluronic acid-modified NPs target CD44 receptors and improve NP uptake by macrophages and colonic epithelial cells. In vivo experiments revealed that the NPs were more effective in terms of therapeutic efficacy than in the intravenous administration of infliximab ().
Figure 7 (A) IFXSS@HA/IFXTK@HA drug synthesis process and irritable bowel disease treatment mechanism. Colonic tissues (B), colonic length, and histopathologic histologic scores (C) of mice post-treatment in each group. Adapted from Chem Eng J, volume 445, Li X, Fang S, Yu Y, et al. Oral administration of inflammatory microenvironment-responsive carrier-free infliximab nanocomplex for the targeted treatment of inflammatory bowel disease. 136438, Copyright 2022, with permission from Elsevier.Citation97
Furthermore, carbon dots (CDs) have become essential nanomaterials due to their excellent stability and biocompatibility.Citation153 Researchers have prepared mannosylated nanocomposites by covalent polymerization of mannosylated NPs (Man-NPs) with CDs, and the glycosylation process is negatively charged due to the carboxyl group possessed on the main chain of inulin.Citation98 Man-NPs can bind selectively to the mannose receptor on the macrophage surface, leading to preferential cellular absorption.
Other Functional Effects
Other methods based on nano-delivery systems can be used to treat and diagnose IBD, in addition to using passive and active targeting strategies to target inflammatory colon tissue for drug action.
Immune regulation has a role in the pathogenesis of IBD. The spleen is the largest lymphoid organ and can regulate the immune system. A splenic-targeted PEG liposome (ST-H2S lipo) loaded with H2S donors was developed to treat UC by immunomodulation.Citation32 According to a fluorescence assay against drug release, the fluorescence intensity of liposomes loaded with H2S donors was higher than that of controls. ST-H2S lipo exhibited significant absorption in the spleen following the intravenous drug administration. On the other hand, both conventional long-circulating liposomes (LC-H2S lipo) and ST-H2S lipo accumulated in the colon, with LC-H2S lipo demonstrating a higher absorption rate. Compared to LC-H2S liposomes, ST-H2S liposomes had a more substantial immunomodulatory effect and a better therapeutic effect.
Restoring colon homeostasis using a microbiota-based strategy may be an effective IBD treatment. Nanomedicines containing components of cell membranes show promise as a therapeutic approach for managing a range of inflammatory diseases.Citation46 Scientists have recently developed a nanosystem with both antioxidant and anti-inflammatory functions (SeM@EM) by coating the surface of mesoporous silica NPs with a natural E. coli membrane that acted as a ROS scavenger. It was demonstrated that the NPs reduced inflammation and improved the adhesion of the drug. It is also remarkable how the NPs regulated the intestinal homeostatic balance and the growth of good intestinal microbiota.
Imaging IBD can be complex because the routinely used contrast agents (iodine-based and barium-based) are usually non-specific for the site of inflammation in IBD. Nahaet al developed a cerium oxide NP (Dex-CeNP) coated with dextrose anhydride as a contrast agent for IBD diagnostic imaging.Citation154 The presence of dextran provides good NP stability, biocompatibility, and specificity. Cerium oxide is also an antioxidant, neutralizing free radicals and reducing inflammation. Dex-CeNPs provide significant computed tomography contrast in the colon and accumulate in colitis-affected tissues. Notably, oral doses can nearly completely leave the body in 24 h.
Cerium dioxide NPs exhibit diverse enzymatic properties, such as superoxide dismutase and catalase activities, in addition to their capacity to scavenge hydroxyl radicals. Zhao et al combined cerium dioxide NPs and negatively charged montmorillonite to create the nanoenzyme complex.Citation70 When administered orally, the nanosystem targets the positively charged, inflamed colon and, in addition to its antioxidant properties, acts as montmorillonite to reduce bleeding.
Challenges and Future Perspectives
Despite substantial advancements in treating IBD based on nanodelivery techniques, there are still some issues and inefficiencies in the development process.
Formulation improvement of drug preparation. Increased drug release from the colonic site and improved therapeutic efficacy can arise from formulation optimization of the product. For instance, multiple NPs were developed using various formulation ratios, and it was ultimately discovered that capryol 90 may be used as the carrier to enable complete drug release.Citation48
Improvement of the preparation process. Some preparation methods require a time-consuming, multi-step process with production scale restrictions, making the prepared NPs less stable The drugs made partially by self-assembly have inadequate drug loading and low encapsulation rates. Problems regarding drug stability, loading discrepancies, and dimensional variations in the nano-delivery platform may also occur in large-scale production, whereas they do not occur in small batch production. Therefore, additional research on nano/micro-targeted drug delivery and developing new preparation processes is required to obtain a straightforward and dependable medication production.
Different results may stem from different experimental models. In vitro simulation experiments using NPs can produce positive findings regarding anti-inflammatory and antioxidant results. However, there are differences between experimental models (rodents) and human patient species. In vivo modeling of drug release and predicting stability of the gastrointestinal tract is challenging due to the complexity and individual heterogeneity of the gastrointestinal tract. For instance, the relevant targeting and therapeutic effect will be lessened if inflammation exists in other sites. Further research should be done to find an animal model that can accurately represent human IBD disease and imitate the pathophysiological environment of human IBD.
Optimal drug properties. Despite the excellent performance of the prepared NPs in trials, new designs must still be found to increase the precise release rate of the drug. The intended clinical outcome cannot be achieved by focusing on just one factor; instead, multiple combinations of strategies must be used. IBD treatment should include improving intestinal flora and re-establishing intestinal balance. Drug biocompatibility and biosafety should also be considered because nanocarriers may be toxic to the liver, kidneys, or other organs during their breakdown, metabolism, and excretion. Further experimental design and validation are required for some experimental results that did not investigate and understand the origins of the occurrence.
There are a number of potential issues to be addressed from the translation of nanomedicines to the clinic, including insufficient understanding of the mechanisms and chemical structure materials of NPs, safety profiles, regulatory and legal challenges. Therefore, the absorption and binding mechanisms during gastrointestinal transit still need to be studied in depth for the development of more advanced DDSs with more rational use of the pathological and physiological microenvironment. It is also essential to assess the long-term toxicity of DDSs and to develop relevant regulatory programmes. For drugs, more extensive multi-centre clinical studies are also needed to validate their efficacy. Concerted efforts by scientists and clinicians are needed in the development of nanomaterial drug delivery systems for the treatment of inflammatory diseases. The regulatory situation and ethical considerations pertaining to the development and application of nanoparticle-based therapeutics are also important factors to be taken into account in the context of translational research and clinical applications.
We are glad to note the innovative theories and strategies that nanotechnology has contributed to treating IBD and the diagnostic advancements it has brought about. The following qualities should be included in a perfect nanomedicine: A) it should be simple to make and can be mass-produced; B) it should be stable, with high drug loading and excellent drug release rates; C) it should have high target specificity, acting directly on the inflammatory colon site and releasing the drug continuously; and D) it should be easy to breakdown and absorb by the human body and should have good biosafety.
Conclusions
There is no complete treatment plan for IBD because it is a chronic idiopathic inflammatory disease with an unknown etiology. Every traditional therapeutic drug and treatment has disadvantages and causes more adverse effects and causes more adverse effects. Nano-agents, which can target and have various functional effects through both passive and active targeting, have been produced with the advancement of nanotechnology. Nanomedicines have demonstrated superior experimental therapeutic results. Currently, research on the clinical application of drug preparation, experimental design, and clinical application of drugs is still unsatisfactory. But these will be the areas of focus for future research. Future research will tend to the following aspects. Investigate the pathophysiological mechanism of the disease in more detail; develop novel drug delivery strategies by combining the research features of active and passive targeting; examine novel experimental animal models to provide adequate pathological information for experiments; and screen optimal targeting drugs for early release into clinical practice.
Author Contributions
JG: Writing-reviewing and editing. JL: Conceptualization and methodology. ZL: Software. HW: Visualization and supervision. ZM: Funding acquisition. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi:10.1038/nature10208
- Alatab S, Sepanlou SG, Ikuta K. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. doi:10.1016/S2468-1253(19)30333-4
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi:10.1038/s41575-020-00360-x
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–429. doi:10.1056/NEJMra020831
- Ott C, Schoelmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10(10):585–595. doi:10.1038/nrgastro.2013.117
- Ardizzone S, Puttini PS, Cassinotti A, et al. Extraintestinal manifestations of inflammatory bowel disease. Digestive Liver Dis. 2008;40:S253–S259. doi:10.1016/S1590-8658(08)60534-4
- Ganji-Arjenaki M, Rafieian-Kopaei M, Malekzadeh M. Phytotherapies in inflammatory bowel disease. J Res Med Sci. 2019;24:24. doi:10.4103/jrms.JRMS_363_18
- Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49. doi:10.1038/nrgastro.2017.136
- Bruner LP, White AM, Proksell S. Inflammatory bowel disease. Prim Care. 2023;50(3):411–427. doi:10.1016/j.pop.2023.03.009
- Zhang S, Langer R, Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2017;16:82–96. doi:10.1016/j.nantod.2017.08.006
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi:10.1053/j.gastro.2020.12.031
- Lautenschläger C, Schmidt C, Fischer D, et al. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Delivery Rev. 2014;71:58–76. doi:10.1016/j.addr.2013.10.001
- Coco R, Plapied L, Pourcelle V, et al. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm. 2013;440(1):3–12. doi:10.1016/j.ijpharm.2012.07.017
- Ghosh S, Ghosh S, Sil PC. Role of nanostructures in improvising oral medicine. Toxicol Rep. 2019;6:358–368. doi:10.1016/j.toxrep.2019.04.004
- Kietzmann D, Moulari B, Béduneau A, et al. Colonic delivery of carboxyfluorescein by pH-sensitive microspheres in experimental colitis. Eur J Pharm Biopharm. 2010;76(2):290–295. doi:10.1016/j.ejpb.2010.06.013
- Kruis W, Kiudelis G, Racz I, et al. Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, non-inferiority trial. Gut. 2009;58(2):233–240. doi:10.1136/gut.2008.154302
- Date AA, Halpert G, Babu T, et al. Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease. Biomaterials. 2018;185:97–105. doi:10.1016/j.biomaterials.2018.09.005
- Jeevanandam J, Barhoum A, Chan YS, et al. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050–1074. doi:10.3762/bjnano.9.98
- Brakmane G, Winslet M, Seifalian AM. Systematic review: the applications of nanotechnology in gastroenterology. Aliment. Pharmacol Ther. 2012;36(3):213–221. doi:10.1111/j.1365-2036.2012.05179.x
- Pontes AP, Welting TJM, Rip J, et al. Polymeric nanoparticles for drug delivery in osteoarthritis. Pharmaceutics. 2022;14(12):2639. doi:10.3390/pharmaceutics14122639
- Marasini N, Er G, Fu C, et al. Development of a hyperbranched polymer-based methotrexate nanomedicine for rheumatoid arthritis. Acta Biomater. 2022;142:298–307. doi:10.1016/j.actbio.2022.01.054
- Mai Y, Ouyang Y, Yu M, et al. Topical formulation based on disease-specific nanoparticles for single-dose cure of psoriasis. J Control Release. 2022;349:354–366. doi:10.1016/j.jconrel.2022.07.006
- Brusini R, Varna M, Couvreur P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv Drug Delivery Rev. 2020;157:161–178. doi:10.1016/j.addr.2020.07.010
- Gazzaniga A, Maroni A, Sangalli ME, et al. Time-controlled oral delivery systems for colon targeting. Expert Opin Drug Delivery. 2006;3(5):583–597. doi:10.1517/17425247.3.5.583
- Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm. 2013;454(2):775–783. doi:10.1016/j.ijpharm.2013.05.017
- Jakubiak P, Thwala LN, Cadete A, et al. Solvent-free protamine nanocapsules as carriers for mucosal delivery of therapeutics. Eur Polym J. 2017;93:695–705. doi:10.1016/j.eurpolymj.2017.03.049
- El-Hady SM, AbouGhaly MH, El-Ashmoony MM, et al. Colon targeting of celecoxib nanomixed micelles using pulsatile drug delivery systems for the prevention of inflammatory bowel disease. Int J Pharm. 2020;576:118982.
- Ali H, Weigmann B, Collnot E-M, et al. Budesonide loaded PLGA nanoparticles for targeting the inflamed intestinal mucosa-pharmaceutical characterization and fluorescence imaging. Pharm Res. 2016;33(5):1085–1092. doi:10.1007/s11095-015-1852-6
- Vaezi Z, Aghdaei HA, Sedghi M, et al. Hemoglobin bio-adhesive nanoparticles as a colon-specific delivery system for sustained release of 5-aminosalicylic acid in the effective treatment of inflammatory bowel disease. Int J Pharm. 2022;616:121531.
- Amaldoss MJN, Ahmed I, Kumar J, et al. Therapeutic efficacy of rifaximin loaded tamarind gum polysaccharide nanoparticles in TNBS induced IBD model Wistar rats. Rep Pract Oncol Radiother. 2021;26(5):712–729. doi:10.5603/RPOR.a2021.0100
- Oh C, Lee W, Park J, et al. Development of spleen targeting H 2 s donor loaded liposome for the effective systemic immunomodulation and treatment of inflammatory bowel disease. ACS nano. 2023;17:4327–4345. doi:10.1021/acsnano.2c08898
- Huguet-Casquero A, Xu Y, Gainza E, et al. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int J Pharm. 2020;586:119515.
- Kim JM, Kim DH, Park HJ, et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J Nanobiotechnol. 2020;18(1). doi:10.1186/s12951-020-00693-4
- Li X, Hetjens L, Wolter N, et al. Charge-reversible and biodegradable chitosan-based microgels for lysozyme-triggered release of vancomycin. J Adv Res. 2023;43:87–96. doi:10.1016/j.jare.2022.02.014
- Cheng S, Shen H, Zhao S, et al. Orally administered mesoporous silica capped with the cucurbit 8 uril complex to combat colitis and improve intestinal homeostasis by targeting the gut microbiota. Nanoscale. 2020;12(28):15348–15363. doi:10.1039/D0NR03037F
- Moulari B, Pertuit D, Pellequer Y, et al. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008;29(34):4554–4560. doi:10.1016/j.biomaterials.2008.08.009
- Wang X, Yan -J-J, Wang L, et al. Rational design of polyphenol-poloxamer nanovesicles for targeting inflammatory bowel disease therapy. Chem Mater. 2018;30(12):4073–4080. doi:10.1021/acs.chemmater.8b01173
- Penate Medina T, Pan J, Damoah C, et al. Utilizing sphingomyelinase sensitizing liposomes in imaging intestinal inflammation in dextran sulfate sodium-induced murine colitis. Biomedicines. 2022;10(2):413. doi:10.3390/biomedicines10020413
- Priyam A, Shivhare K, Yadav S, et al. Enhanced solubility and self-assembly of amphiphilic sulfasalazine-PEG-OMe (S-PEG) conjugate into core-shell nanostructures useful for colonic drug delivery. Colloids Surfaces, A. 2018;547:157–167.
- Ferri D, Gaviña P, Parra M, et al. Mesoporous silica microparticles gated with a bulky azo derivative for the controlled release of dyes/drugs in colon. Royal Soc Open Sci. 2018;5(8):180873. doi:10.1098/rsos.180873
- Xu C, Chen S, Chen C, et al. Colon-targeted oral nanoparticles based on ROS-scavenging hydroxyethyl starch-curcumin conjugates for efficient inflammatory bowel disease therapy. Int J Pharm. 2022;623:121884.
- Li S, Xie A, Li H, et al. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J Control Release. 2019;316:66–78. doi:10.1016/j.jconrel.2019.10.054
- Zhang Q, Tao H, Lin Y, et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials. 2016;105:206–221. doi:10.1016/j.biomaterials.2016.08.010
- Thu-Ha Thi N, Trinh N-T, Tran HN, et al. Improving silymarin oral bioavailability using silica-installed redox nanoparticle to suppress inflammatory bowel disease. J Control Release. 2021;331:515–524. doi:10.1016/j.jconrel.2020.10.042
- Xu J, Shi T, Zhang Y, et al. Probiotic-inspired nanomedicine restores intestinal homeostasis in colitis by regulating redox balance, immune responses, and the gut microbiome. Adv Mater. 2022;35(3):2207890.
- Zhang D, Wei Y, Chen K, et al. Biocompatible Reactive Oxygen Species (ROS)-responsive nanoparticles as superior drug delivery vehicles. Adv Healthcare Mater. 2015;4(1):69–76. doi:10.1002/adhm.201400299
- Qelliny MR, Aly UF, Elgarhy OH, et al. Budesonide-loaded eudragit s 100 nanocapsules for the treatment of acetic acid-induced colitis in animal model. AAPS Pharm Sci Tech. 2019;20(6). doi:10.1208/s12249-019-1453-5
- Zhang L, Li M, Zhang G, et al. Micro- and nanoencapsulated hybrid delivery system (MNEHDS): a novel approach for colon-targeted oral delivery of berberine. Mol Pharmaceut. 2021;18(4):1573–1581. doi:10.1021/acs.molpharmaceut.0c00970
- Mutalik S, Suthar NA, Managuli RS, et al. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int J Biol Macromol. 2016;86:709–720. doi:10.1016/j.ijbiomac.2015.11.092
- Cao J, Cheng J, Xi S, et al. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur J Pharm Biopharm. 2019;137:112–121. doi:10.1016/j.ejpb.2019.02.011
- Meissner Y, Pellequer Y, Lamprecht A. Nanoparticles in inflammatory bowel disease: particle targeting versus pH-sensitive delivery. Int J Pharm. 2006;316(1–2):138–143. doi:10.1016/j.ijpharm.2006.01.032
- Jiang J, Xiao J, Zhao Z, et al. One-step prepared nano-in-micro microcapsule delivery vehicle with sequential burst-sustained drug release for the targeted treatment of inflammatory bowel disease. Mater Chem Front. 2021;5(16):6027–6040. doi:10.1039/D1QM00589H
- Ali H, Weigmann B, Neurath MF, et al. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167–177. doi:10.1016/j.jconrel.2014.03.039
- Markam R, Bajpai J, Bajpai AK. Synthesis of ginger derived nanocarriers (GDNC) and study of in vitro release of 5-amino salicylic acid (5-ASA) as an anti inflammatory drug. J Drug Delivery Sci Technol. 2019;50:355–364. doi:10.1016/j.jddst.2019.01.039
- Dong Y, Zhou Z, Ding H, et al. Preparation and properties of a pH sensitive carrier based on three kinds of polymer blend to control the release of 5-amino salicylic acid. Pharmaceut Develop Technol. 2014;19(8):960–967. doi:10.3109/10837450.2013.846372
- Jacob EM, Borah A, Pillai SC, et al. Garcinol encapsulated ph-sensitive biodegradable nanoparticles: a novel therapeutic strategy for the treatment of inflammatory bowel disease. Polymers. 2021;13(6):862. doi:10.3390/polym13060862
- Duan H, Lü S, Gao C, et al. Mucoadhesive microparticulates based on polysaccharide for target dual drug delivery of 5-aminosalicylic acid and curcumin to inflamed colon. Colloids Surf B Biointerfaces. 2016;145:510–519. doi:10.1016/j.colsurfb.2016.05.038
- Qu Z, Wong KY, Moniruzzaman M, et al. One-pot synthesis of pH-responsive eudragit-mesoporous silica nanocomposites enable colonic delivery of glucocorticoids for the treatment of inflammatory bowel disease. Adv Ther. 2021;4(2). doi:10.1002/adtp.202000165
- Kesharwani SS, Ahmad R, Bakkari MA, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–179.
- Kshirsagar SJ, Bhalekar MR, Patel JN, et al. Preparation and characterization of nanocapsules for colon-targeted drug delivery system. Pharmaceut Develop Technol. 2012;17(5):607–613. doi:10.3109/10837450.2011.557732
- Nguyen CTH, Webb RI, Lambert LK, et al. Bifunctional succinylated epsilon-polylysine-coated mesoporous silica nanoparticles for pH-responsive and intracellular drug delivery targeting the colon. ACS Appl Mater Interfaces. 2017;9(11):9470–9483. doi:10.1021/acsami.7b00411
- Naeem M, Kim W, Cao J, et al. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf B Biointerfaces. 2014;123:271–278. doi:10.1016/j.colsurfb.2014.09.026
- Gonzalez-Alvarez M, Coll C, Gonzalez-Alvarez I, et al. Gated mesoporous silica nanocarriers for a “two-step” targeted system to colonic tissue. Mol Pharmaceut. 2017;14(12):4442–4453. doi:10.1021/acs.molpharmaceut.7b00565
- Bertoni S, Liu Z, Correia A, et al. pH and reactive oxygen species-sequential responsive nano-in-micro composite for targeted therapy of inflammatory bowel disease. Adv Funct Mater. 2018;28(50):1806175.
- Wang X, Yan J, Wang L, et al. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics. 2020;10(23):10808–10822. doi:10.7150/thno.47601
- Teruel AH, Pérez-Esteve É, González-álvarez I, et al. Smart gated magnetic silica mesoporous particles for targeted colon drug delivery: new approaches for inflammatory bowel diseases treatment. J Control Release. 2018;281:58–69. doi:10.1016/j.jconrel.2018.05.007
- Wachsmann P, Moulari B, Béduneau A, et al. Surfactant-dependence of nanoparticle treatment in murine experimental colitis. J Control Release. 2013;172(1):62–68. doi:10.1016/j.jconrel.2013.07.031
- Zhang S, Cho WJ, Jin AT, et al. Heparin-coated albumin nanoparticles for drug combination in targeting inflamed intestine. Adv Healthcare Mater. 2020;9(16):2000536.
- Zhao S, Li Y, Liu Q, et al. An Orally Administered CeO 2 @montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv Funct Mater. 2020;30(45). doi:10.1002/adfm.202004692
- Shrestha N, Xu Y, Prévost JRC, et al. Impact of PEGylation on an antibody-loaded nanoparticle-based drug delivery system for the treatment of inflammatory bowel disease. Acta Biomater. 2022;140:561–572. doi:10.1016/j.actbio.2021.12.015
- Li X, Yu M, Zhu Z, et al. Oral delivery of infliximab using nano-in-microparticles for the treatment of inflammatory bowel disease. Carbohydr Polym. 2021;273:118556. doi:10.1016/j.carbpol.2021.118556
- Sunnap O, Subramanian S, Vemula PK, et al. Zingerone-encapsulated solid lipid nanoparticles as oral drug-delivery systems to potentially target inflammatory diseases. Chemnanomat. 2022;8(12). doi:10.1002/cnma.202200388
- Zhang S, Ermann J, Succi MD, et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci, trans med. 2015;7(300). doi:10.1126/scitranslmed.aaa5657
- Wang C, Guo Z, Liang J, et al. An oral delivery vehicle based on konjac glucomannan acetate targeting the colon for inflammatory bowel disease therapy. Front Bioeng Biotechnol. 2022;10:1025155.
- Schilrreff P, Simioni YR, Jerez HE, et al. Superoxide dismutase in nanoarchaeosomes for targeted delivery to inflammatory macrophages. Colloids Surf B Biointerfaces. 2019;179:479–487. doi:10.1016/j.colsurfb.2019.03.061
- Le Z, He Z, Liu H, et al. Antioxidant enzymes sequestered within lipid-polymer hybrid nanoparticles for the local treatment of inflammatory bowel disease. ACS Appl Mater Interfaces. 2021;13(47):55966–55977. doi:10.1021/acsami.1c19457
- Vafaei SY, Esmaeili M, Amini M, et al. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr Polym. 2016;144:371–381. doi:10.1016/j.carbpol.2016.01.026
- Gong W, Yu J, Zheng T, et al. CCL4-mediated targeting of spleen tyrosine kinase (Syk) inhibitor using nanoparticles alleviates inflammatory bowel disease. Clin Translat Med. 2021;11(2). doi:10.1002/ctm2.339
- Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
- Shabana S, Hamouda HI, Abdalla M, et al. Multifunctional nanoparticles based on marine polysaccharides for apremilast delivery to inflammatory macrophages: preparation, targeting ability, and uptake mechanism*. Int J Biol Macromol. 2022;222:1709–1722. doi:10.1016/j.ijbiomac.2022.09.225
- Huang Z, Gan J, Jia L, et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi:10.1016/j.biomaterials.2015.01.013
- Sinhmar GK, Shah NN, Rawal SU, et al. Surface engineered lipid nanoparticle-mediated site-specific drug delivery system for the treatment of inflammatory bowel disease. Artif Cells Nanomed Biotechnol. 2018;46:565–578. doi:10.1080/21691401.2018.1463232
- Mao Y, Han M, Chen C, et al. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale. 2021;13(47):20157–20169. doi:10.1039/D1NR06015E
- Herminia Higa L, Schilrreff P, Briski AM, et al. Bacterioruberin from Haloarchaea plus dexamethasone in ultra-small macrophage-targeted nanoparticles as potential intestinal repairing agent. Colloids Surf B Biointerfaces. 2020;191:110961.
- Higa LH, Jerez HE, de Farias MA, et al. Ultra-small solid archaeolipid nanoparticles for active targeting to macrophages of the inflamed mucosa. Nanomedicine. 2017;12(10):1165–1175. doi:10.2217/nnm-2016-0437
- Qiu H, Gong H, Bao Y, et al. Reactive oxygen species-scavenging hollow MnO 2 nanozymes as carriers to deliver budesonide for synergistic inflammatory bowel disease therapy. Biomater. Sci. 2022;10(2):457–466. doi:10.1039/D1BM01525G
- Bao M, Wang K, Li J, et al. ROS scavenging and inflammation-directed polydopamine nanoparticles regulate gut immunity and flora therapy in inflammatory bowel disease. Acta Biomater. 2023;161:250–264. doi:10.1016/j.actbio.2023.02.026
- Naserifar M, Hosseinzadeh H, Abnous K, et al. Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 2020;262:118555. doi:10.1016/j.lfs.2020.118555
- Huang Y, Guo J, Gui S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-alpha siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur J Pharm Sci. 2018;125:232–243. doi:10.1016/j.ejps.2018.10.009
- Wei F, Lang Y, Shen Q, et al. Osteopontin-loaded PLGA nanoparticles enhance the intestinal mucosal barrier and alleviate inflammation via the NF-kappa B signaling pathway. Colloids Surf B Biointerfaces. 2020;190:110952.
- Lee Y, Sugihara K, Gillilland MG, et al. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nature Mater. 2020;19(1):118. doi:10.1038/s41563-019-0462-9
- Kotla NG, Burke O, Pandit A, et al. An orally administrated hyaluronan functionalized polymeric hybrid nanoparticle system for colon-specific drug delivery. Nanomaterials. 2019;9(9):1246. doi:10.3390/nano9091246
- Du Y, Liu Y, Sun L, et al. cRGD peptide incorporated with patchouli alcohol loaded silk fibroin nanoparticles for enhanced targeting of inflammatory sites in colitis. Biomat Advan. 2022;140:101231.
- Gan J, Liu Y, Sun L, et al. Orally administrated nucleotide-delivery particles from microfluidics for inflammatory bowel disease treatment. Appl Mater Today. 2021;25:101231.
- Chen Y, Feng J, Chen Y, et al. ROS-responsive nano-medicine for navigating autophagy to enhance targeted therapy of inflammatory bowel disease. Int J Pharm. 2024;659:124117. doi:10.1016/j.ijpharm.2024.124117
- Li X, Fang S, Yu Y, et al. Oral administration of inflammatory microenvironment-responsive carrier-free infliximab nanocomplex for the targeted treatment of inflammatory bowel disease. Chem Eng J. 2022;445:136438.
- Sun Q, Arif M, Chi Z, et al. Macrophages-targeting mannosylated nanoparticles based on inulin for the treatment of inflammatory bowel disease (IBD). Int J Biol Macromol. 2021;169:206–215. doi:10.1016/j.ijbiomac.2020.12.094
- Feng J, Wang Y, Lv Y, et al. XA pH-responsive and colitis-targeted nanoparticle loaded with shikonin for the oral treatment of inflammatory bowel disease in mice. Mol Pharmaceut. 2022;19(11):4157–4170. doi:10.1021/acs.molpharmaceut.2c00550
- Lv Y, Ren M, Yao M, et al. Colon-specific delivery of methotrexate using hyaluronic acid modified pH-responsive nanocarrier for the therapy of colitis in mice. Int J Pharm. 2023;635:122741. doi:10.1016/j.ijpharm.2023.122741
- Attia MF, Anton N, Wallyn J, et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi:10.1111/jphp.13098
- Lai SK, Wang -Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Delivery Rev. 2009;61(2):158–171. doi:10.1016/j.addr.2008.11.002
- Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74(7):787–800. doi:10.1139/y96-081
- Schulzke JD, Ploeger S, Amasheh M, et al. Epithelial tight junctions in intestinal inflammation. Molecu Struct Funct Tight Junct. 2009;1165:294–300.
- Lamprecht A, Yamamoto H, Takeuchi H, et al. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther. 2005;315(1):196–202. doi:10.1124/jpet.105.088146
- Lamprecht A. IBD Selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7(6):311–312. doi:10.1038/nrgastro.2010.66
- Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18(6):788–793. doi:10.1023/A:1011032328064
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349–358. doi:10.4103/0250-474X.57282
- Bondì ML, Montana G, Craparo EF, et al. Lipid nanoparticles as delivery vehicles for the Parietaria judaica major allergen Par j 2. Int J Nanomed. 2011;6:2953–2962. doi:10.2147/IJN.S24264
- Harel-Adar T, Mordechai TB, Amsalem Y, et al. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108(5):1827–1832. doi:10.1073/pnas.1015623108
- Song W, Shen L, Wang Y, et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat Commun. 2018;9. doi:10.1038/s41467-018-04605-x
- Zwolinska-Wcislo M, Krzysiek-Maczka G, Ptak-Belowska A, et al. antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis. Importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J Physiol Pharmacol. 2011;62(3):357–368.
- Ribeiro Paiotti AP, Miszputen SJ, Oshima CTF, et al. Effect of COX-2 inhibitor after TNBS-induced colitis in wistar rats. J Molec Histol. 2009;40(4):317–324. doi:10.1007/s10735-009-9243-0
- Hong S, Choi DW, Kim HN, et al. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):604. doi:10.3390/pharmaceutics12070604
- Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11:11. doi:10.1186/1477-3155-11-11
- Nayak AK, Pal D. Tamarind seed polysaccharide: an emerging excipient for pharmaceutical use. Indian J Pharm Educ Res. 2017;51(2):S136–S146. doi:10.5530/ijper.51.2s.60
- Nakajima N, Ishihara K, Matsuura Y. Dietary-fiber-degrading enzymes from a human intestinal Clostridium and their application to oligosaccharide production from nonstarchy polysaccharides using immobilized cells. Appl Microbiol Biotechnol. 2002;59(2–3):182–189. doi:10.1007/s00253-002-1015-7
- Ahn MY, Shin KH, Kim D-H, et al. Characterization of a Bacteroides species from human intestine that degrades glycosaminoglycans. Can J Microbiol. 1998;44(5):423–429. doi:10.1139/w98-027
- Collins CE, Rampton DS. Review article: platelets in inflammatory bowel disease--pathogenetic role and therapeutic implications. Aliment Pharmacol Ther. 1997;11(2):237–247. doi:10.1046/j.1365-2036.1997.153328000.x
- Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl. 1997;222:41–44. doi:10.1080/00365521.1997.11720716
- Fahlgren A, Hammarström S, Danielsson Å, et al. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131(1):90–101. doi:10.1046/j.1365-2249.2003.02035.x
- Rubio CA. The natural antimicrobial enzyme lysozyme is up-regulated in gastrointestinal inflammatory conditions. Pathogens. 2014;3(1):73–92. doi:10.3390/pathogens3010073
- Roldo M, Barbu E, Brown JF, et al. Azo compounds in colon-specific drug delivery. Expert Opin Drug Delivery. 2007;4(5):547–560. doi:10.1517/17425247.4.5.547
- Cao YH, Cao RH, Brakenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13(7):380–390. doi:10.1016/S0955-2863(02)00204-8
- Ayissi VBO, Ebrahimi A, Schluesenner H. Epigenetic effects of natural polyphenols: a focus on SIRT1-mediated mechanisms. Mol Nutr Food Res. 2014;58(1):22–32. doi:10.1002/mnfr.201300195
- Ishida T, Wang X, Shimizu T, et al. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122(3):349–355. doi:10.1016/j.jconrel.2007.05.015
- Sakata A, Ochiai T, Shimeno H, et al. Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology. 2007;122(1):54–64. doi:10.1111/j.1365-2567.2007.02612.x
- Campbell EL, Colgan SP. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2019;16(2):106–120. doi:10.1038/s41575-018-0079-5
- Simmonds NJ, Rampton DS. Inflammatory bowel disease--a radical view. Gut. 1993;34(7):865–868. doi:10.1136/gut.34.7.865
- Schulze-Osthoff K, Bauer MK, Vogt M, et al. Oxidative stress and signal transduction. Internat J Vitam Nutr Res. 1997;67(5):336–342.
- Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344(8926):859–861. doi:10.1016/S0140-6736(94)92831-2
- Pavlick KP, Laroux FS, Fuseler J, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33(3):311–322. doi:10.1016/S0891-5849(02)00853-5
- Chen L, You Q, Hu L, et al. The antioxidant procyanidin reduces reactive oxygen species signaling in macrophages and ameliorates experimental colitis in mice. Front Immunol. 2017;8:1910. doi:10.3389/fimmu.2017.01910
- Simmonds NJ, Allen RE, Stevens TRJ, et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103(1):186–196. doi:10.1016/0016-5085(92)91112-H
- Sedghi S, Fields JZ, Klamut M, et al. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34(9):1191–1197. doi:10.1136/gut.34.9.1191
- Nugent SG, Kumar D, Rampton DS, et al. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48(4):571–577. doi:10.1136/gut.48.4.571
- Palugan L, Cerea M, Zema L, et al. Coated pellets for oral colon delivery. J Drug Delivery Sci Technol. 2015;25:1–15. doi:10.1016/j.jddst.2014.12.003
- Naik JB, Waghulde MR. Development of vildagliptin loaded Eudragit microspheres by screening design: in vitro evaluation. J Pharm Invest. 2018;48(6):627–637. doi:10.1007/s40005-017-0355-3
- Madhavi M, Madhavi K, Jithan AV. Preparation and in vitro/in vivo characterization of curcumin microspheres intended to treat colon cancer. J Pharm Bioallied Sci. 2012;4(2):164–171. doi:10.4103/0975-7406.94825
- Asfour MH, Mohsen AM. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J Adv Res. 2018;9:17–26. doi:10.1016/j.jare.2017.10.003
- Kulkarni RV, Sa B. Evaluation of pH-Sensitivity and Drug Release Characteristics of (Polyacrylamide-Grafted-Xanthan)-Carboxymethyl Cellulose-Based pH-Sensitive interpenetrating network hydrogel beads. Drug Dev Ind Pharm. 2008;34(12):1406–1414. doi:10.1080/03639040802130079
- Das S, Chaudhury A, Ng K-Y. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int J Pharm. 2011;406(1–2):11–20. doi:10.1016/j.ijpharm.2010.12.015
- Ways TMM, Lau WM, Khutoryanskiy VV. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers. 2018;10(3):267. doi:10.3390/polym10030267
- Maroni A, Moutaharrik S, Zema L, et al. Enteric coatings for colonic drug delivery: state of the art. Expert Opin Drug Delivery. 2017;14(9):1027–1029. doi:10.1080/17425247.2017.1360864
- Tirosh B, Khatib N, Barenholz Y, et al. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol Pharmaceut. 2009;6(4):1083–1091. doi:10.1021/mp9000926
- Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci USA. 1986;83(8):2501–2505. doi:10.1073/pnas.83.8.2501
- Leonel Parra F, Stoll IK, Zwick M, et al. Make It Simple: (SR-A1+TLR7) Macrophage Targeted NANOarchaeosomes. Front Bioeng Biotechnol. 2018;6:6. doi:10.3389/fbioe.2018.00006
- Tripodo G, Trapani A, Torre ML, et al. Hyaluronic acid and its derivatives in drug delivery and imaging: recent advances and challenges. Eur J Pharm Biopharm. 2015;97:400–416. doi:10.1016/j.ejpb.2015.03.032
- de la Motte CA, Kessler SP. The role of hyaluronan in innate defense responses of the intestine. Int J Cell Biol. 2015;2015:481301. doi:10.1155/2015/481301
- Mukaida N, Sasaki S-I, Baba T. CCL4 Signaling in the Tumor Microenvironment. In: Birbrair A, editor. Tumor Microenvironment: The Role of Chemokines, Pt A. Springer; 2020:23–32.
- Schreiber S, Perkins SL, Teitelbaum SL, et al. Regulation of mouse bone marrow macrophage mannose receptor expression and activation by prostaglandin E and IFN-gamma. J Iimmunol. 1993;151(9):4973–4981. doi:10.4049/jimmunol.151.9.4973
- Lane KB, Egan B, Vick S, et al. Characterization of a rat alveolar macrophage cell line that expresses a functional mannose receptor. J Leukoc Biol. 1998;64(3):345–350. doi:10.1002/jlb.64.3.345
- Janus L, Piatkowski M, Radwan-Praglowska J. Microwave-assisted synthesis and characterization of Poly(L-lysine)-Based Polymer/carbon quantum dot nanomaterials for biomedical purposes. Materials. 2019;12(23):3825. doi:10.3390/ma12233825
- Naha PC, Hsu JC, Kim J, et al. Dextran-coated cerium oxide nanoparticles: a computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. Acs Nano. 2020;14(8):10187–10197. doi:10.1021/acsnano.0c03457
