Abstract
Purpose
Oxycodone is a potent μ- and κ-opioid receptor agonist that can relieve both somatic and visceral pain. We assessed oxycodone- vs sufentanil-based multimodal analgesia on postoperative pain following major laparoscopic gastrointestinal surgery.
Methods
In this randomised double-blind controlled trial, 40 adult patients were randomised (1:1, stratified by type of surgery) to receive oxycodone- or sufentanil-based multimodal analgesia, comprising bilateral transverse abdominis plane blocks, intraoperative dexmedetomidine infusion, flurbiprofen axetil, and oxycodone- or sufentanil-based patient-controlled analgesia. The co-primary outcomes were time-weighted average (TWA) of visceral pain (defined as intra-abdominal deep and dull pain) at rest and on coughing during 0–24 h postoperatively, assessed using the numerical rating scale (0–10) with a minimal clinically important difference of 1.
Results
All patients completed the study (median age, 64 years; 65% male) and had adequate postoperative pain control. The mean (SD) 24-h TWA of visceral pain at rest was 1.40 (0.77) in the oxycodone group vs 2.00 (0.98) in the sufentanil group (mean difference=−0.60, 95% CI, −1.16 to −0.03; P=0.039). Patients in the oxycodone group had a significantly lower 24-h TWA of visceral pain on coughing (2.00 [0.83] vs 2.98 [1.26]; mean difference=−0.98, 95% CI, −1.66 to −0.30; P=0.006). In the subgroup analyses, the treatment effect of oxycodone vs sufentanil on the co-primary outcomes did not differ in terms of age (18–65 years or >65 years), sex (female or male), or type of surgery (colorectal or gastric). Secondary outcomes (24-h TWA of incisional and shoulder pain, postoperative analgesic usage, rescue analgesia, adverse events, and patient satisfaction) were comparable between groups.
Conclusion
For patients undergoing major laparoscopic gastrointestinal surgery, oxycodone-based multimodal analgesia reduced postoperative visceral pain in a statistically significant but not clinically important manner.
Trial Registration
Chinese Clinical Trial Registry (ChiCTR2100052085).
Introduction
Laparoscopic gastrointestinal surgery is a widely performed procedure, with comparable safety and long-term outcomes to conventional open laparotomy.Citation1–3 Patients undergoing major laparoscopic gastrointestinal surgery often experience moderate to severe postoperative pain, especially visceral pain. Visceral pain is deep and dull pain inside the abdomen, which cannot be accurately localized. The pathological mechanisms of visceral pain are very complex, involving surgical manipulations, peritoneal inflammation, visceral ischemia, and visceral hypersensitivity due to peripheral and central pathway sensitization.Citation4 Visceral pain can trigger autonomic nerve reflexes, such as nausea, vomiting, sweating, and cardiovascular and respiratory changes,Citation5,Citation6 and may provoke negative emotional responses.Citation7 Early postoperative visceral pain is also related to chronic pain following surgery.Citation8
Opioids are the cornerstones of postoperative analgesia but are associated with adverse effects. Multimodal analgesic strategy with opioids, non-opioid analgesics, and local anaesthetics has been used to enhance analgesia and reduce opioid-related adverse events.Citation9–11 Oxycodone, a semisynthetic μ- and κ-opioid receptor agonist may provide a better treatment for both somatic and visceral pain after laparoscopic surgery than other opioids (morphine, fentanyl, sufentanil, or alfentanil).Citation12–14 Oxycodone has been shown to be effective for postoperative pain control and well-tolerated across a variety of surgical procedures and patient populations. Compared with morphine, oxycodone is associated with a reduced incidence of side effects including hallucinations, nightmares, and histamine-related symptoms (such as itching and hypotension).Citation15 In the context of multimodal analgesia provision, the effects of oxycodone administration on postoperative pain outcomes after laparoscopic surgery are not fully understood.
Therefore, we designed this randomised controlled trial comparing oxycodone-based with sufentanil-based multimodal analgesia among patients undergoing major laparoscopic gastrointestinal surgery. We hypothesized that the use of oxycodone-based analgesic regimen would be superior to sufentanil-based analgesia in terms of early visceral pain relief in these patients.
Methods
Study Design
This researcher-initiated, prospective, randomised, double-blind, controlled study was approved by the Institutional Review Board of First Affiliated Hospital of Soochow University (no. 2021–920) on September 23, 2021. The trial was registered prior to patient enrollment at the Chinese Clinical Trial Registry (Registration no: ChiCTR2100052085; Principal investigator: Ke Peng; Date of registration: October 16, 2021; Accessible via https://www.chictr.org.cn/showproj.html?proj=135273). This study complied with the Declaration of Helsinki. Written informed consent was obtained from all patients. The implementation and reporting of this trial conformed to the Consolidated Standards of Reporting Trials guidelines (Supplementary material).
Patients
Adult patients with ASA physical status I–III undergoing major laparoscopic gastrointestinal surgery (defined as surgical time ≥90 min, involving at least partial resection of stomach or intestine) were recruited. Exclusion criteria included emergency surgery; body mass index ≥35 kg m−2; allergy to medications in this study; severe cardiopulmonary disease (myocardial infarction, heart failure, or respiratory failure); severe cerebrovascular disease (hemorrhagic or ischemic stroke); severe liver (Child-Pugh class C) or renal failure (requiring renal replacement therapy); cognitive impairment, use of antipsychotics, alcoholism, long-term use of opioids or other analgesics; inability to understand the rating scales or refusal for participation.
Randomisation
A researcher who was not involved in the other parts of the study performed the randomisation in a ratio of 1:1 and stratified by type of surgery (gastric or colorectal). Randomisation results were concealed in sealed opaque envelopes. A research nurse assigned eligible patients to an oxycodone group or a sufentanil group. Both oxycodone and sufentanil were colorless solutions constituted with normal saline in identical syringes and patient-controlled analgesia (PCA) containers. All patients, anaesthesiologists, perioperative care team, and researchers who collected postoperative outcome data were blinded throughout the study.
Anaesthesia and Interventions
The baseline mean blood pressure (MBP) and heart rate (HR) values were obtained in surgical wards preoperatively. Patients received the ASA standard monitoring during surgery. For patients in both groups, anaesthesia was induced with i.v. propofol 1.5–2 mg kg−1, sufentanil 0.2 μg kg−1, and cisatracurium 0.2 mg kg−1. Anaesthesia was maintained with sevoflurane, titrated to a minimal alveolar concentration at 0.7–1.2. Patients received remifentanil infusion of 0.05–0.2 μg kg−1 min−1 and cisatracurium 0.1 mg kg−1 as required. The ventilation was adjusted to maintain the end-tidal carbon dioxide at 35–45 mmHg. Dexamethasone 8 mg and ondansetron 4 mg were administered for prophylaxis of nausea and vomiting. Hypotension (a reduction in MBP >30% of baseline), hypertension (an increase in MBP >30% of baseline), bradycardia (HR <45 beats min−1), and tachycardia (HR >100 beats min−1) were treated.
Patients in both groups received a multimodal analgesia consisting of the following aspects: (1) transverse abdominis plane (TAP) blocks were conducted before skin incision using a subcostal approach in laparoscopic gastric surgery or a lateral approach at the level of the umbilicus in colorectal surgery. With patients in the supine position, the rectus abdominis, external oblique, internal oblique, and transversus abdominis muscles were identified by ultrasonography. A 22G 8-cm block needle was advanced using the in-plane technique until the needle tip reached the fascia above the transversus abdominis muscle. After negative aspiration, 0.375% ropivacaine 20 mL was injected at both sides; (2) intraoperative dexmedetomidine infusion at 0.4 μg kg−1 h−1 until 30 min before skin closure; (3) i.v. flurbiprofen axetil 50 mg at the end of surgery; and (4) a loading dose of oxycodone 0.2 mg kg−1 or sufentanil 0.2 μg kg−1, followed by oxycodone- or sufentanil-based PCA until 48 h after surgery. The PCA system contained oxycodone 100 mg or sufentanil 100 μg diluted with normal saline to a volume of 100 mL (no background infusion, bolus of 1 mL, lockout time of 5 min). Rescue analgesia with additional oxycodone 5 mg (the oxycodone group) or sufentanil 5 μg (the sufentanil group) could be given to treat moderate to severe pain, defined as a score ≥4 on a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (the worst pain imaginable). To standardize the doses of sufentanil and oxycodone, equivalent milligrams of morphine was calculated according to the literature: i.v. sufentanil 1 μg = i.v. oxycodone 1 mg = i.v. morphine 1 mg.Citation16,Citation17 The conversion ratio between parenteral morphine and oxycodone ranged from 0.65 to 1.5, and we selected a ratio of 1.0 in our study based on the previous studies.Citation18,Citation19
A researcher who was blinded to the allocation assessed postoperative pain (visceral, incisional, and shoulder pain) on the NRS at 1, 3, 6, 18, 24, and 48 h postoperatively. The three types of pain were defined as previously reported: visceral pain was intra-abdominal deep and dull pain; incisional pain was pain located at the abdominal wall; and shoulder pain was pain referred to the shoulder.Citation20,Citation21
Study Outcomes
The co-primary outcomes were visceral pain at rest and on coughing during the first 24 h after surgery. The 24-h overall pain was expressed as time-weighted average (TWA) which was calculated by plotting the area under the curve of NRS pain scores during 24 h and dividing that number by 24.Citation22
The secondary outcomes included incisional and shoulder pain at rest and on coughing during 24 h postoperatively; PCA usage during 24 and 48 h; need for rescue analgesia; sedation in PACU and ward (defined as ≥2 on a 5-point level of sedation scale);Citation23 nausea and vomiting; use of antiemetics; respiratory depression, constipation, and dizziness; patient satisfaction (very satisfied, satisfied, neutral, or dissatisfied). The exploratory outcomes were quality of recovery (QoR)-15 scores on postoperative days 1, 2, 3, and 30; 30-d post-surgical pain (defined as NRS scores ≥1);Citation24 and 30-d death.
Statistical Analysis
According to our institutional data, the mean (standard deviation [SD]) 24-h NRS visceral pain on coughing was 3.5 (1.0) in patients undergoing major laparoscopic gastrointestinal surgery with sufentanil-based PCA. For patients with postoperative acute pain, the established minimum clinically important difference is 1 (on a 0–10 scale).Citation25 We hypothesized that the oxycodone-based analgesia would reduce the visceral pain score to 2.5 (1.0). To detect this difference at a significance level of 0.05 and a power of 80%, 17 patients were required in each group. After accounting for possible dropouts, we enrolled a total of 40 patients (n = 20 in each group). The sample size calculation was conducted using the PASS 15.0 (NCSS, LCC, Kaysville, UT, USA).
Data normality was assessed using the Shapiro–Wilk test. Continuous data were presented as mean (SD) if they were normally distributed and as median (interquartile range [IQR]) if not. Categorical data were presented as n (%). Data were analysed using the unpaired t test, Mann–Whitney U-test, χ2 test, or Fisher exact test, as appropriate. For pain scores at several postoperative timepoints, multiple unpaired t test was used with corrections for multiple comparisons using the false discovery rate and a two-stage step-up method of Benjamini, Krieger, and Yekutieli. The interventional effects of oxycodone vs sufentanil were evaluated using the mean/median difference or odds ratios (OR) with the 95% confidence interval (CI). Additionally, subgroup analyses of the co-primary outcomes were conducted in terms of age (18–65 years or >65 years), sex (female or male), and type of surgery (colorectal or gastric).
The outcome data were analysed in the intention-to-treat population (including all randomised patients who had surgery). For the two co-primary outcomes, a two-sided P <0.025 indicates a statistically significant difference (Bonferroni correction, 0.05/2). For the perioperative data and secondary and exploratory outcomes, no multiple comparison correction was applied. All analyses were done using the SPSS 25.0 (IBM SPSS, Chicago, IL, USA).
Results
Patient Characteristics
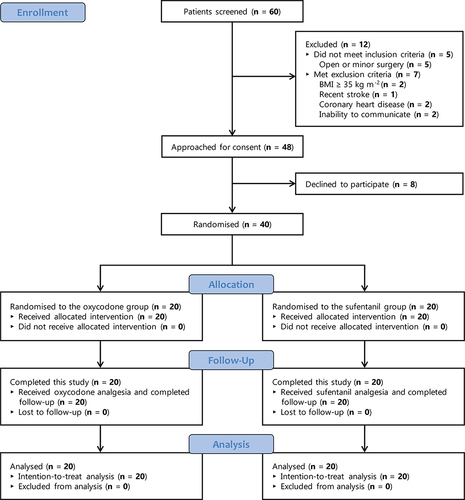
A total of 60 patients were screened from December 1, 2021 to May 31, 2022 (). Of them, 12 were excluded, 8 declined to participate, and 40 were randomised and included in the intention-to-treat analysis. All patients had available data on the primary outcomes.
shows the characteristics of patients. The median (IQR) age was 67.5 (61.3–71.8) years in the oxycodone group and 62 (57.3–70) years in the sufentanil group. Overall, 26 of 40 (65%) patients were male. In both groups, 95% of patients were at ASA classification I or II, 35% underwent laparoscopic gastric surgery, and 65% had laparoscopic colorectal surgery. shows that the two groups are comparable in terms of perioperative values of MBP and HR, haemodynamic events, use of anaesthetics and analgesics, use of other medications, duration of surgery, duration of anaesthesia, and length of postoperative hospital stay. The mean (SD) length of PACU stay was 51.7 (21.2) min in the oxycodone group and 69.6 (29.6) min in the sufentanil group.
Table 1 Patient Characteristics
Table 2 Perioperative Data
Primary Outcomes
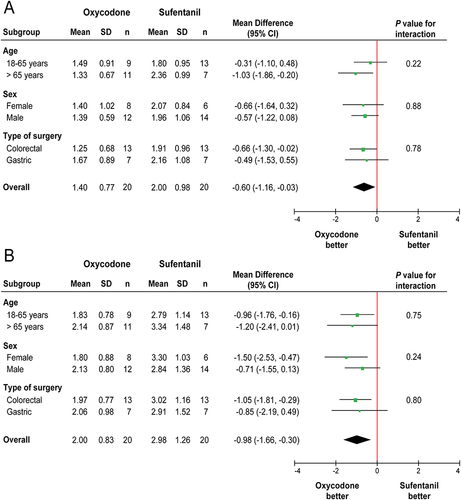
The mean (SD) TWA of visceral pain score at rest during the first 24 h postoperatively was 1.40 (0.77) in the oxycodone group vs 2.00 (0.98) in the sufentanil group (difference=−0.60, 95% CI, −1.16 to −0.03; P=0.039), which was not statistically significant ().
Table 3 Study Outcomes
The 24-h TWA of visceral pain score on coughing was significantly lower in the oxycodone group compared with the sufentanil group (mean [SD], 2.00 [0.83] vs 2.98 [1.26]; difference=−0.98, 95% CI, −1.66 to −0.30; P=0.006) (); however, this difference did not reach the minimum clinically important difference of 1.
Additionally, the treatment effect of oxycodone vs sufentanil did not differ in terms of age (18–65 years or >65 years), sex (female or male), or type of surgery (colorectal or gastric) in the subgroup analyses of 24-h visceral pain at rest () and on coughing ().
Secondary and Exploratory Outcomes
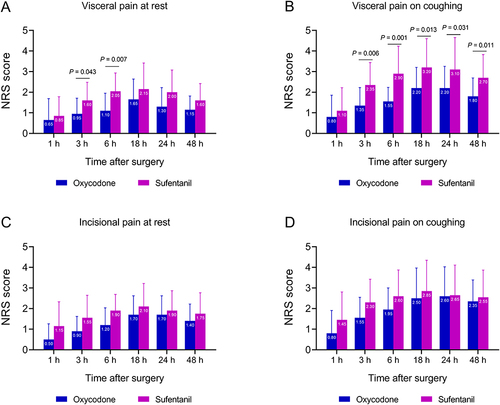
Patients in the oxycodone group had lower visceral pain scores at rest at 3 h (mean [SD], 0.95 [0.76] vs 1.60 [0.88]; P=0.043) and 6 h postoperatively (mean [SD], 1.10 [0.85] vs 2.05 [0.89]; P=0.007) (), and lower visceral pain scores on coughing from 3 h (mean [SD], 1.35 [0.88] vs 2.35 [1.09]; P=0.006) to 48 h after surgery (mean [SD], 1.80 [0.89] vs 2.70 [1.13]; P=0.011) (). The two groups had comparable incisional pain scores at rest and on coughing ( and ). The median shoulder pain scores were 0 in both groups ().
Figure 3 Visceral pain and incisional pain during the first 48 h postoperatively. (A) Visceral pain at rest. (B) Visceral pain on coughing. (C) Incisional pain at rest. (D) Incisional pain on coughing. NRS, numerical rating scale.
There were no significant between-group differences in PCA usage, need for rescue analgesia, postoperative sedation, nausea and vomiting, use of antiemetics, dizziness, patient satisfaction, i.v. morphine equivalent doses, QoR-15 scores, and 30-d post-surgical pain (). No patients developed respiratory depression or constipation. No death event occurred within 30 days after surgery.
Discussion
This study suggested that in the context of multimodal analgesia, oxycodone- vs sufentanil-based postoperative analgesia reduced 24-h visceral pain on coughing in patients undergoing major laparoscopic gastrointestinal surgery; however, this reduction in pain scores did not achieve the minimum clinically important difference. Meanwhile, both analgesic regimens provided adequate postoperative pain control, with a good safety profile and high patient satisfaction.
Visceral pain originates within internal organs, distinguishing it from somatic pain. Visceral pain tends to elicit significant discomfort and negative emotional responses,Citation26 which is attributed to the dual innervation and central projection to emotion-controlling areas.Citation7 Currently, postoperative pain after laparoscopic surgical procedures is often treated with the combined use of opioid medications and nonsteroidal anti-inflammatory drugs, but sometimes the pain can be difficult to treat, especially the visceral components.
Oxycodone produces effective postoperative analgesia through acting on both opioid μ and κ receptors.Citation14,Citation20,Citation27–30 The κ-opioid receptors locate within the gastrointestinal tract are significant factors for anti-nociception in the visceral pain system.Citation31 The analgesic efficacy of oxycodone is associated with its plasma concentration, suggesting a peripheral effect mediated through κ-receptors.Citation32 With the activation of κ-opioid receptors, oxycodone presents a distinct pharmacological profile compared to other opioids such as morphine and fentanyl in which analgesia is mediated by μ receptors located mainly in the central nervous system.
A recent meta-analysis showed that oxycodone led to better analgesia than other opioids (morphine, fentanyl, sufentanil, and alfentanil) in laparoscopic surgery.Citation12 In that meta-analysis, the majority of included studies were conducted in patients undergoing laparoscopic cholecystectomy and gynecological surgery. However, it is still unclear to what extent oxycodone-based postoperative analgesia compared with sufentanil-based analgesia would affect postoperative pain, especially visceral pain, in patients who had major laparoscopic gastrointestinal surgery.
Many multimodal analgesia strategies are currently being investigated to reduce opioid-related adverse effects. In our study, the multimodal analgesia strategy comprised bilateral TAP blocks after anaesthesia induction, intraoperative dexmedetomidine infusion, flurbiprofen axetil administration at the end of surgery, and postoperative analgesia with oxycodone or sufentanil. Owing to the use of these analgesic techniques, postoperative pain was successfully treated in all patients, with a mean NRS score ranging from 1.4 to 3.1.
Adverse effects associated with postoperative oxycodone or sufentanil analgesia include nausea, vomiting, constipation, dizziness, and respiratory depression. In our study, all patients received the prophylactic use of dexamethasone and ondansetron. Postoperative nausea and vomiting occurred in 5 patients (25%) in the oxycodone group and 3 (15%) in the sufentanil group; of them, only 1 patient in each group needed rescue antiemetics. Four patients (20%) in the oxycodone group and 2 (10%) in the sufentanil group experienced dizziness. No patients developed respiratory depression or constipation. The two multimodal analgesia strategies showed similar clinical efficacy and safety, without significant adverse events.
The strengths of this trial include (1) the prospective, randomised, double-blind, controlled study design; (2) the primary focus on oxycodone-based analgesia and visceral pain in patients undergoing major laparoscopic gastrointestinal surgery; (3) the rigorous consideration of stratified randomisation by the type of surgery; and (4) the implementation of multimodal pain management (TAP blocks, dexmedetomidine, flurbiprofen axetil, and PCA) for patients in both groups.
Nonetheless, this study is limited by the small sample size, relatively healthy patients (95% at ASA status I or II), and lack of postoperative gastrointestinal function assessment (such as time to first flatus and time to first oral feeding). Additionally, the reduced visceral pain in the oxycodone group did not translate into higher QoR-15 scores in this pilot study. Last, we showed that only 1 patient in each group had 30-d post-surgical pain, but we did not evaluate chronic pain (eg pain at 3 months or later after surgery). A larger multicentre trial is required to ascertain the optimal multimodal analgesic strategy on postoperative pain and quality of recovery in this patient population.
In conclusion, our results suggest that for patients undergoing major laparoscopic gastrointestinal surgery, the oxycodone-based multimodal analgesia decreased postoperative visceral pain in a statistically but not clinically meaningful manner. For these surgical patients, postoperative pain can be satisfactorily treated using the multimodal analgesic strategy with either oxycodone or sufentanil.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Peng received funding from the Suzhou Medical Health Science and Technology Innovation Project and Beijing Red Lilac Public Welfare Development Center Clinical Research Project. Dr. Yang received funding from the National Natural Science Foundation of China. Dr. Cheng received funding from the Beijing Red Lilac Public Welfare Development Center Clinical Research Project. No other disclosures were reported.
Data Sharing Statement
All data and materials generated or used in this study (including individual deidentified participant data, Case Report Form, and Informed Consent Form) are available upon reasonable request to the corresponding author. Data can be accessible after the publication of this article without time limit.
Additional information
Funding
References
- Huang C, Liu H, Hu Y, et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surgery. 2022;157(1):9–17. doi:10.1001/jamasurg.2021.5104
- Liu F, Huang C, Xu Z, et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: the CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6(10):1590–1597. doi:10.1001/jamaoncol.2020.3152
- Niitsu H, Hinoi T, Kawaguchi Y, et al. Laparoscopic surgery for colorectal cancer is safe and has survival outcomes similar to those of open surgery in elderly patients with a poor performance status: subanalysis of a large multicenter case-control study in Japan. J Gastroenterol. 2016;51(1):43–54. doi:10.1007/s00535-015-1083-y
- Johnson AC, Greenwood-Van Meerveld B. The Pharmacology of Visceral Pain. Adv Pharmacol. 2016;75:273–301. doi:10.1016/bs.apha.2015.11.002
- Chou YJ, Ou YC, Lan KC, Jawan B, Chang SY, Kung FT. Preemptive analgesia installation during gynecologic laparoscopy: a randomized trial. J Minim Invasive Gynecol. 2005;12(4):330–335. doi:10.1016/j.jmig.2005.05.005
- Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106(1):11–18. doi:10.1097/00000542-200701000-00007
- Gebhart GF, Bielefeldt K. Physiology of Visceral Pain. Compr Physiol. 2016;6(4):1609–1633. doi:10.1002/cphy.c150049
- Blichfeldt-Eckhardt M, Ording H, Andersen C, Licht P, Toft P. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155(11):2400–2407. doi:10.1016/j.pain.2014.09.019
- Pirie K, Traer E, Finniss D, Myles P, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129(3):378–393. doi:10.1016/j.bja.2022.05.029
- Wick E, Grant M, Wu C. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: a Review. JAMA Surgery. 2017;152(7):691–697. doi:10.1001/jamasurg.2017.0898
- Sjövall S, Kokki M, Kokki H. Laparoscopic surgery: a narrative review of pharmacotherapy in pain management. Drugs. 2015;75(16):1867–1889. doi:10.1007/s40265-015-0482-y
- Li Y, Dou Z, Yang L, Wang Q, Ni J, Ma J. Oxycodone versus other opioid analgesics after laparoscopic surgery: a meta-analysis. Eur. J. Med. Res. 2021;26(1):4. doi:10.1186/s40001-020-00463-w
- Lenz H, Sandvik L, Qvigstad E, Bjerkelund C, Raeder J. A comparison of intravenous oxycodone and intravenous morphine in patient-controlled postoperative analgesia after laparoscopic hysterectomy. Anesthesia Analg. 2009;109(4):1279–1283. doi:10.1213/ane.0b013e3181b0f0bb
- An Y, Zhao L, Wang T, et al. Preemptive oxycodone is superior to equal dose of sufentanil to reduce visceral pain and inflammatory markers after surgery: a randomized controlled trail. BMC Anesthesiology. 2019;19(1):96. doi:10.1186/s12871-019-0775-x
- Kokki H, Kokki M, Sjovall S. Oxycodone for the treatment of postoperative pain. Expert Opin Pharmacother. 2012;13(7):1045–1058. doi:10.1517/14656566.2012.677823
- Treillet E, Laurent S, Hadjiat Y. Practical management of opioid rotation and equianalgesia. J Pain Res. 2018;11:2587–2601. doi:10.2147/JPR.S170269
- Turan A, Atim A, Dalton JE, et al. Preoperative angiotensin-converting enzyme inhibitor use is not associated with increased postoperative pain and opioid use. Clin J Pain. 2013;29(12):1050–1056. doi:10.1097/AJP.0b013e318287a258
- Han L, Su Y, Xiong H, et al. Oxycodone versus sufentanil in adult patient-controlled intravenous analgesia after abdominal surgery: a prospective, randomized, double-blinded, multiple-center clinical trial. Medicine. 2018;97(31):e11552. doi:10.1097/MD.0000000000011552
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007–1012. doi:10.1111/j.1365-2044.2010.06498.x
- Jiang Z, Zhou G, Song Q, Bao C, Wang H, Chen Z. Effect of Intravenous Oxycodone in Combination With Different Doses of Dexmedetomidine on Sleep Quality and Visceral Pain in Patients After Abdominal Surgery: a Randomized Study. Clin J Pain. 2018;34(12):1126–1132. doi:10.1097/ajp.0000000000000645
- Koch S, Ahlburg P, Spangsberg N, Brock B, Tønnesen E, Nikolajsen L. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta anaesthesiologica Scandinavica. 2008;52(6):845–850. doi:10.1111/j.1399-6576.2008.01643.x
- Brinck E, Maisniemi K, Kankare J, Tielinen L, Tarkkila P, Kontinen V. Analgesic Effect of Intraoperative Intravenous S-Ketamine in Opioid-Naïve Patients After Major Lumbar Fusion Surgery Is Temporary and Not Dose-Dependent: a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Anesthesia Analg. 2021;132(1):69–79. doi:10.1213/ane.0000000000004729
- Lin T, Yeh Y, Lin F, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102(1):117–122. doi:10.1093/bja/aen320
- Wu XD, Zeng FF, Yu XX, et al. Development and Validation of a Prediction Model for Chronic Post-Surgical Pain After Thoracic Surgery in Elderly Patients: a Retrospective Cohort Study. J Pain Res. 2022;15:3079–3091. doi:10.2147/JPR.S368295
- Myles P, Myles D, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. doi:10.1093/bja/aew466
- Golubović S, Golubović V, Cindrić-Stancin M, Tokmadzić VJ. Intraperitoneal analgesia for laparoscopic cholecystectomy: bupivacaine versus bupivacaine with tramadol. Pain. 2009;33(1):299–302.
- Feng X, Yang P, Liao Z, Zhou R, Chen L, Ye L. Comparison of oxycodone and sufentanil in patient-controlled intravenous analgesia for postoperative patients: a meta-analysis of randomized controlled trials. Chin Med J. 2023;136(1):45–52. doi:10.1097/CM9.0000000000002259
- Quan Z, Luo C, Chi P, Wang L, He H. Analgesic Effects of Oxycodone Relative to Those of Sufentanil, in the Presence of Midazolam, During Endoscopic Injection Sclerotherapy for Patients With Cirrhosis and Esophageal Varices. Anesthesia Analg. 2018;127(2):382–386. doi:10.1213/ane.0000000000003305
- Kwon YS, Jang JS, Lee NR, et al. A Comparison of Oxycodone and Alfentanil in Intravenous Patient-Controlled Analgesia with a Time-Scheduled Decremental Infusion after Laparoscopic Cholecystectomy. Pain Res Manag. 2016;2016:7868152. doi:10.1155/2016/7868152
- Dang SJ, Li RL, Wang J, et al. Oxycodone vs Sufentanil in Patient-Controlled Intravenous Analgesia After Gynecological Tumor Operation: a Randomized Double-Blind Clinical Trial. J Pain Res. 2020;13:937–946. doi:10.2147/JPR.S236933
- Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123(1):28–36. doi:10.1016/j.pain.2006.02.006
- Staahl C, Upton R, Foster DJ, et al. Pharmacokinetic-pharmacodynamic modeling of morphine and oxycodone concentrations and analgesic effect in a multimodal experimental pain model. J Clin Pharmacol. 2008;48(5):619–631. doi:10.1177/0091270008314465