Abstract
Background
Recent studies have shown that expression of G protein-coupled receptor kinase 6 (GRK6) and β-arrestin1 in the striatum is closely associated with hyperactive dopamine receptors in rats with levodopa-induced dyskinesia (LID). Our research group has shown that Tianqi antitremor granules have a significant effect on the motor complications of Parkinson’s disease (PD). However, whether Tianqi antitremor granules have an effect on the behavioral manifestations and expression of GRK6 and β-arrestin1 in rats with LID is unknown.
Methods
Rats with PD received twice daily intraperitoneal injections of levodopa for 4 weeks to induce dyskinesia. Rats with LID were randomly divided into five groups: an LID-control group, an LID group, a levodopa plus Tianqi antitremor granules as traditional Chinese medicine (TCM)-low group, a levodopa plus TCM-medium group, and levodopa plus TCM-high group. Peak intensity of rotations was measured. GRK6 and β-arrestin1 expression in the striatum of the dyskinetic rats was observed by immunohistochemistry and Western blotting.
Results
Pulsatile treatment with levodopa induced abnormal involuntary movements in rats with PD similar to LID in patients with PD. We found that repeated levodopa administration increased peak rotations in dyskinetic rats. However, peak rotations were decreased in rats given levodopa plus the different doses of Tianqi antitremor granules. In accordance with changed behavior, GRK6 and β-arrestin1 expression was decreased in rats with PD and was persistently low in rats with LID, but this decrease was prevented by coadministration of levodopa and Tianqi antitremor granules.
Conclusion
Tianqi antitremor granules ameliorated levodopa-induced dyskinetic behavior, reversed the decrease in GRK6 and β-arrestin1 expression, and acted as a useful adjunctive medicine for the treatment of LID.
Introduction
Parkinson’s disease (PD) is one of the common neurodegenerative disorders, and is characterized by resting tremor, rigidity, bradykinesia, and postural abnormality.Citation1 The disease is caused by degeneration of dopaminergic neurons in the substantia nigra and consequent depletion of dopamine content in the striatum.Citation2 Due to the depletion of dopamine, dopamine receptors (mainly D1 and D2) in the striatal neurons become hyperactive.Citation3 Recent evidence suggests that G protein-coupled receptor kinase 6 (GRK6) and β-arrestin1 expression in the striatum is closely associated with the hyperactive dopamine receptors seen in rats with levodopa-induced dyskinesia (LID).Citation4 We have recently reported that GRK6 and β-arrestin1 expression is altered in rats with PD and LID, and that these may be effective targets for treatment of dyskinesia.Citation5
For many years, levodopa has been considered to be the most efficacious drug for the treatment of PD in clinical practice.Citation6 However, long-term administration of levodopa induces motor complications, particularly LID and motor fluctuations.Citation7 Therefore, prevention and treatment of PD in combination with other drugs, such as traditional Chinese medicine (TCM) is urgent. Over centuries, our ancestors have accumulated a large body of experience in the treatment of PD using TCM.Citation8 It was assumed that PD was caused by a deficiency of Qi, Yin, Yang, liver, and kidney, and an excess of wind, fire, phlegm, and ecchymosis.Citation9 Thus, the treatment principles for PD were summarized as including replenishment of Qi and blood, nourishing Yin, suppressing excessive Yang, calming the liver to prevent wind, and cleaning and activating the channels and collaterals.Citation10 Since 2009, our research group has treated the motor complications of PD using Tianqi antitremor granules, mentioned in “Mi Fang Ding Zhen Wan” and recorded in the diagnosis and treatment principles of Kentang Wang, with significant effect.Citation11
The present study investigated the effect of Tianqi antitremor granules on the behavior of dyskinetic rats. The effect of these granules on GRK6 and β-arrestin1 expression was also investigated to determine their efficacy and mechanisms of action.
Materials and methods
Animals
Ninety adult female Sprague-Dawley rats (weighing 180–220 g) were used in this study. The rats were housed in the Institute of Experimental Animal Science, Shanghai Jiaotong University School of Medicine, at a constant temperature of 23°C, and had free access to food and water. All animal procedures were approved by the institutional review board of Xinhua Hospital and were performed according to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Materials
Apomorphine, 6-hydroxydopamine (6-OHDA), levodopa methyl ester, benserazide, and ascorbic acid were obtained from Sigma Chemical Co (St Louis, MO, USA). Rabbit polyclonal antibodies against GRK6 and β-arrestin1 were purchased from Abcam Inc (Cambridge, UK). Mouse anti-β-actin antibody, horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulin (Ig)G secondary antibodies were obtained from Beyotime Institute of Biotechnology (Shanghai, People’s Republic of China). Methanol was supplied by Jiangsu Yonghua Fine Chemical Products Co, Ltd (Jiangsu, People’s Republic of China). Water was obtained from a Milli-Q purification system (Millipore, Molsheim, France).
TCM composition
The herbal granules used in this study were sourced from Jiangyin Tianjiang Pharmaceutical Co, Ltd (Jiangsu, People’s Republic of China). The formula is from “Mi Fang Ding Zhen Wan”, recorded in the diagnosis and treatment principles written by Kentang Wang, a TCM doctor from the Ming Dynasty.Citation11 Three groups of TCM are needed, ie, a TCM-medium group (including Tian ma 15 g, Huang qi 15 g, Shou di huang 15 g, Bai shao yao 20 g), a TCM-low group (each dose being 20% lower than in the medium-dose group), and a TCM-high group (each dose being 20% more than in the medium-dose group).
6-OHDA lesions and testing
Each rat head was mounted onto a stereotaxic apparatus (Narishige, Tokyo, Japan). Next, 6-OHDA (8 μg/4 μL) was injected into the right medial forebrain bundle. The coordinates were calculated according to the rat brain atlas, ie, bregma 4.5 mm, lateral 0.9 mm, and dura 7.5 mm.Citation12 Three weeks after surgery, the 6-OHDA-lesioned rats were injected intraperitoneally with apomorphine 0.25 mg/kg. The number of rotations was counted visually for each rat. Rats showing at least seven full turns per minute away from the lesioned side were deemed to be successfully lesioned and were selected for further study.
Treatment protocol
All rats with confirmed PD were treated with levodopa to induce LID. All dyskinetic rats with abnormal involuntary movement (AIM) scores >8 were divided into five groups: an LID-control group (n = 6), an LID group (n = 6), a levodopa plus TCM-low group (n = 6), a levodopa plus TCM-medium group (n = 6), and a levodopa plus TCM-high group (n = 6). Dyskinetic rats in the LID-control group were cotreated with vehicle (twice daily, by intraperitoneal injection) for 29 days. The LID group was treated with levodopa methylester (25 mg/kg with 6.25 mg/kg benserazide by intraperitoneal injection twice daily for 29 days), while the rest of the rats were cotreated with levodopa/benserazide and different doses of Tianqi antitremor granules, serving as the levodopa plus TCM-low, levodopa plus TCM-medium, and levodopa plus TCM-high groups. In addition, rats in the PD group (n = 6) and sham group (n = 6, given sham 6-OHDA injections without expressing PD symptoms) were treated with subcutaneous vehicle daily for 4 weeks.
Measurement of behavior
Rats were monitored for AIMs using a procedure similar to a method described previously.Citation13 Briefly, on test days, each rat was placed in a plastic tray 5 minutes before drug treatment. After injection, the rat was assessed for orolingual, limb, axial, and locomotor movements. At 20-minute intervals, AIMs were rated for 60 seconds over a period of 3 hours, during which a severity score between 0 and 4 was assigned to each AIM category. Finally, the four types of AIM scores were summed at each time point. The peak intensity of rotation was measured as the peak number of contralateral turns in any 5-minute interval.
Immunohistochemistry
Rats were sacrificed by deep anesthesia using 3% pentobarbital, and their ascending aortas were perfused with cold phosphate-buffered saline followed by 4% paraformaldehyde. The brains were rapidly extracted, immersed in 4% paraformaldehyde overnight, and dehydrated by graded alcohol. The brains were then embedded in paraffin, cut into coronal sections 5 μm thick, and subjected to immunohistochemistry assays using the avidin-biotin-peroxidase complex technique with diaminobenzidine as a substrate. The sections were incubated with an affinity-purified polyclonal antibody against GRK6 and β-arrestin1 overnight at 4°C. We computed the integrated optical density for quantitative analysis of the intensity of GRK6 and β-arrestin1 immunostaining. Images from six random nonoverlapping areas in each section were captured using a BH-2 microscope (Olympus, Tokyo, Japan) fitted with a MicroImage video camera at ×400 magnification to gain a mean value for each section. Digitally fixed images were analyzed using an Image Pro Plus analyzer (Media Cybernetics Inc, Rockville, MD, USA).
Western blotting
Striatal tissues were lysed in lysis buffer containing phenylmethanesulfonyl fluoride. The supernatant was collected after centrifugation at 12,000 g for 10 minutes at 4°C. After measurement of protein concentration using a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA), samples containing 40 μg protein were loaded onto 10% sodium dodecyl sulfate polyacrylamide gel. The proteins were transferred onto a 0.2 mm polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membranes were then blocked in 5% instant nonfat dried milk for 2 hours at room temperature and incubated with primary antibodies corresponding to GRK6 (1:500), β-arrestin1 (1:500), or β-actin (1:1000) at 4°C overnight. On the second day, the membranes were washed and incubated with a horseradish peroxidaseconjugated secondary antibody (1:1000) for 2 hours at room temperature. Immunoblot signals were visualized in the chemiluminescent solution with a gel imager (Millipore, Billerica, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Analysis of variance followed by least significant difference post hoc comparison tests were used to analyze the experimental data among the different groups. P < 0.05 was considered to be statistically significant.
Results
Motor response alterations
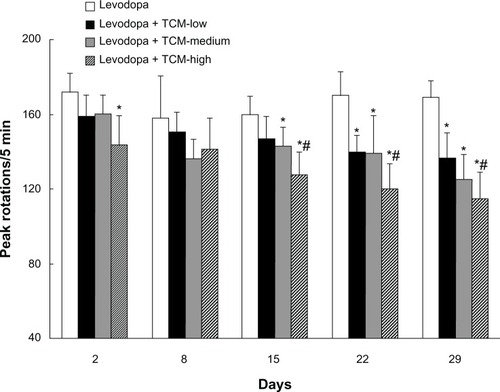
As shown in , dyskinetic rats treated with levodopa for 29 days expressed LID, as evidenced by increased peak rotations. In contrast, peak rotations were decreased in dyskinetic rats given levodopa plus the different doses of Tianqi antitremor granules. Peak rotations on days 22 and 29 were lower in the TCM-low group than in the LID group (treated with levodopa only). There was no difference in peak rotations between the TCM-low group and the TCM-medium group. Similarly, peak rotations were not different between the TCM-high and TCM-medium groups, while rats in the TCM-high group showed lower peak rotations than the TCM-low group on days 15, 22, and 29.
Figure 1 Effect of Tianqi antitremor granules on peak rotations in dyskinetic rats.
Abbreviation: TCM, traditional Chinese medicine.
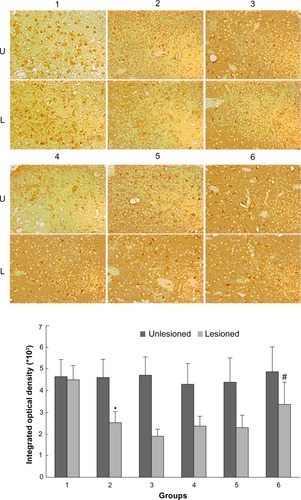
Effect of Tianqi antitremor granules on GRK6 levels in dyskinetic rats
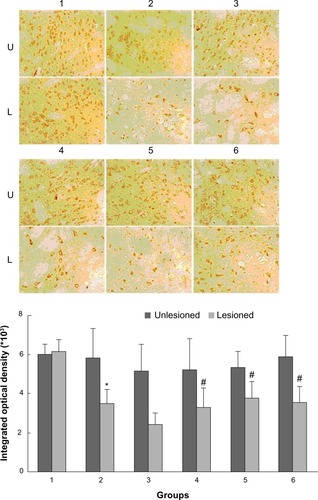
As shown in , rats with PD showed a significant decrease in GRK6 expression in the lesioned striatum relative to the nonlesioned striatum (81.42% ± 5.94% of the value on the nonlesioned side). Lower GRK6 expression persisted in the LID-control rats treated chronically with levodopa plus vehicle (73.66% ± 3.43% of the nonlesioned side), which is accordance with our previous work. Further, GRK6 expression in the LID rats continued to decrease following treatment with levodopa for 29 days (56.32% ± 4.45% of the nonlesioned side). Interestingly, coadministration of levodopa plus TCM had a different effect on GRK6 expression. It was found that GRK6 expression in the lesioned striatum for TCM-low (79.12% ± 3.15% of the nonlesioned side) was not different from that in the LID group. In contrast, TCM-medium (83.98% ± 4.50% of the nonlesioned side) and TCM-high (80.56% ± 4.74% of the nonlesioned side) prevented the decrease in GRK6 expression in dyskinetic rats. There was no difference in this regard between the TCM-medium group and the TCM-high group. Further, immunohistochemistry confirmed the results obtained on Western blotting ().
Figure 2 Western blotting results confirming the effect of administration of Tianqi antitremor granules on GRK6 expression in dyskinetic rats.
Abbreviations: GRK6, G protein-coupled receptor kinase 6; PD, Parkinson’s disease; LID, levodopa-induced dyskinesia; TCM, traditional Chinese medicine.
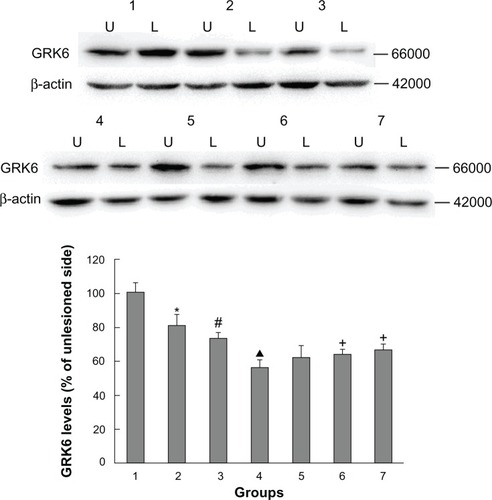
Figure 3 Effect of Tianqi antitremor granules on levels of GRK6 in dyskinetic rats.
Abbreviations: GRK6, G protein-coupled receptor kinase 6; LID, levodopa-induced dyskinesia; TCM, traditional Chinese medicine.
Effect of Tianqi antitremor granules on β-arrestin1 levels in dyskinetic rats
In addition to measuring GRK6 expression, we also examined striatal β-arrestin1 levels in all groups of animals. We have previously reported that β-arrestin1 levels are decreased in rats with PD and that this decrease persists in rats with LID. As shown in , β-arrestin1 levels in rats with PD were significantly reduced (76.66% ± 5.12% of the nonlesioned side), and decreased further in LID-control rats (65.44% ± 6.68% of the nonlesioned side). Further, LID rats receiving intermittent levodopa had lower expression of β-arrestin1 (48.7% ± 4.08% of the nonlesioned side) than LID rats injected with saline. In agreement with the behavioral analysis, β-arrestin1 expression increased progressively on treatment with levodopa plus Tianqi antitremor granules for 29 days, while lowering peak rotations. We found that the degree of TCM-low (51.62% ± 3.26% of the nonlesioned side), TCM-medium (53.74% ± 2.81% of the nonlesioned side), and TCM-high (56.54% ± 3.52% of the nonlesioned side) were slight, with the TCM-high group showing the greatest increase. These results are in accordance with findings on immunohistochemistry ().
Figure 4 Western blotting results confirmed the effect of administration of Tianqi antitremor granules on β-arrestin1 expression in dyskinetic rats.
Abbreviations: PD, Parkinson’s disease; LID, levodopa-induced dyskinesia; TCM, traditional Chinese medicine.
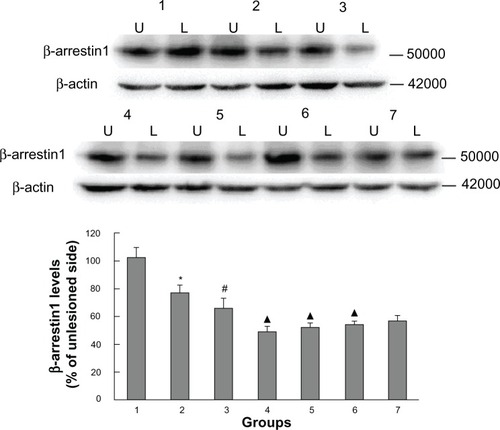
Figure 5 Effect of Tianqi antitremor granules on levels of β-arrestin1 in dyskinetic rats.
Abbreviations: LID, levodopa-induced dyskinesia; TCM, traditional Chinese medicine.
Discussion
PD is a common neurodegenerative disorder that affects the basal ganglia.Citation14 So far, treatment with levodopa (using the dopamine precursor levodopa) to enhance synaptic transmission remains the gold standard for PD.Citation15 However, long-term treatment with levodopa has side effects.Citation16 Hence, many researchers have started to combine western medicine with TCM to treat PD and LID.Citation17 In this study, we used levodopa plus Tianqi antitremor granules comprised of Tian Huang qi, Shou di huang, and Bai shao ya. Tian ma Huang qi act as the sovereign drug, while Shou di huang, Bai shao yao, and Dang gui act as the minister drug. The herbal formula is designed to replenish Qi and blood, wind, and expectorate stagnant phlegm.
Rats rendered hemiparkinsonian by 6-OHDA and treated twice daily with levodopa develop AIMs similar dyskinesia in PD patients.Citation18 In this study, we found that rats treated repeatedly with levodopa showed an increase in peak rotations, but that coadministration of Tianqi antitremor granules attenuated AIMs and reduced peak rotations. Rats in the TCM-low group showed a marked decrease in peak rotations on day 22. Medium-dose and high-dose TCM reduced peak rotations earlier than low-dose TCM on day 15 day 2, respectively. These data indicate that Tianqi antitremor granules could ameliorate levodopa-induced dyskinetic behavior, and the greater the dose, the better the effect.
G protein-coupled receptor kinases and arrestins two major protein families that primarily regulate receptor desensitization of G protein-coupled receptors.Citation19 G protein-coupled receptor kinases phosphorylate the agonist receptor, and arrestins then bind to the phosphorylated receptor, thereby desensitizing the receptor.Citation20 In this way, cell signaling is inhibited.Citation21 As we know, dopamine receptors, which belong to the G protein-coupled receptor family, become hypersensitive after progressive depletion of dopamine neurons in PD.Citation22 Studies have shown that GRK6 and β-arrestin1 play a crucial role in the sensitivity of dopamine related to the occurrence of PD and LID.Citation23 We found the quantity of GRK6 and β-arrestin1 in the striatum rats with PD decreased and continued to decrease in rats striatum.Citation5 These results are in accordance with what we have previously reported. After coadministration of Tianqi antitremor granules and levodopa, we observed an increasing abundance of GRK6 and β-arrestin1 in the striatal fractions. Immunohistochemistry showed that GRK6 expression levels were increased in the three groups treated with Tianqi antitremor granules. Meanwhile, we also detected a marked increase in GRK6 expression in the TCM-medium TCM-high groups by Western blotting. Although immunohistochemistry showed increased β-arrestin1 expression in the three groups, only the increase in the TCM-high group was statistically significant. Further, the results of Western blotting showed that β-arrestin1 expression was reduced in rats treated with levodopa or levodopa plus TCM, while rats treated with levodopa plus TCM showed less reduction in β-arrestin1 expression than rats treated with levodopa alone, and the TCM-high group showed the best effect. In summary, medium-dose and high-dose TCM can increase GRK6 expression in dyskinetic rats, and β-arrestin1 expression is only sensitive to high-dose TCM. These observations are consistent with the fact that Tianqi antitremor granules modulate the susceptibility of the dopamine receptor by adjusting expression of GRK6 and β-arrestin1 in the striatum.
In conclusion, to the authors’ knowledge, this study provides the first evidence that Tianqi antitremor granules ameliorate LID in rats with PD, reverse the decrease in GRK6 and β-arrestin1, and are a useful adjunctive treatment for LID.
Acknowledgments
The study was supported by grants from the Projects of National Science Foundation of China (81071025, 81171203, 81171204, 81200871) and Projects of the Shanghai Committee of Science and Technology (11nm0503300, 11410708900, 12XD1403800).
Disclosure
The authors report no conflicts of interest in this work.
References
- Luquin MR Garcia-Ruiz PJ Marti MJ Levodopa in the treatment of Parkinson’s disease: myths and realities Rev Neurol 2012 55 669 688 Spanish 23172094
- Chen JJ Swope DM Pharmacotherapy for Parkinson’s disease Pharmacotherapy 2007 27 161S 173S 18041936
- Calabresi P Picconi B Tozzi A Di Filippo M Dopamine-mediated regulation of corticostriatal synaptic plasticity Trends Neurosci 2007 30 211 219 17367873
- Ahmed MR Bychkov E Gurevich VV Benovic JL Gurevich EV Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment J Neurochem 2008 104 1622 1636 17996024
- Wu N Song L Yang X Yuan W Liu Z NMDA receptor regulation of levodopa-induced behavior and changes in striatal G protein-coupled receptor kinase 6 and β-arrestin-1 expression in parkinsonian rats Clin Interv Aging 2013 8 347 352 23569367
- Stocchi F Tagliati M Olanow CW Treatment of levodopa-induced motor complications Mov Disord 2008 23 Suppl 3 S599 S612 18781681
- Del Sorbo F Albanese A Levodopa-induced dyskinesias and their management J Neurol 2008 255 Suppl 4 32 41 18821084
- Kim TH Cho KH Jung WS Lee MS Herbal medicines for Parkinson’s disease: a systematic review of randomized controlled trials PloS One 2012 7 e35695 22615738
- Li XZ Zhang SN Liu SM Lu F Recent advances in herbal medicines treating Parkinson’s disease Fitoterapia 2012 84C 273 285 23266574
- Wang Y Xie CL Lu L Fu DL Zheng GQ Chinese herbal medicine paratherapy for Parkinson’s disease: a meta-analysis of 19 randomized controlled trials Evid Based Complement Alternat Med 2012 2012 534861 23008740
- Huang TK Zhang DB Neuropathy and Psychosis of Traditional Chinese Medicine Beijing, China Chinese Medical Science Publishers 2000 Extrapyramidal disease 426 427
- Paxinos G Watson C The Rat Brain in Stereotaxic Coordinates San Diego, CA Academic Press 2007
- Morgese MG Cassano T Cuomo V Giuffrida A Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors Exp Neurol 2007 208 110 119 17900568
- Guridi J Gonzalez-Redondo R Obeso JA Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson’s disease Parkinsons Dis 2012 2012 943159 23125942
- Cenci MA L-DOPA-induced dyskinesia: cellular mechanisms and approaches to treatment Parkinsonism Relat Disord 2007 13 Suppl 3 S263 S267 18267248
- Fabbrini G Brotchie JM Grandas F Nomoto M Goetz CG Levodopainduced dyskinesias Mov Disord 2007 22 1379 1389 17427940
- Yuan CX Ye Q Wang J Zhang Y Yuan CG Effects of traditional Chinese herbal medicine on the neurobehavioral manifestations and the activity of dopamine D2 receptor in corpora striatum of rats with levodopa-induced dyskinesias Zhong Xi Yi Jie He Xue Bao 2008 6 1024 1028 Chinese 18847536
- Deumens R Blokland A Prickaerts J Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway Exp Neurol 2002 175 303 317 12061862
- Gurevich EV Tesmer JJ Mushegian A Gurevich VV G protein-coupled receptor kinases: more than just kinases and not only for GPCRs Pharmacol Ther 2012 133 40 69 21903131
- DeWire SM Ahn S Lefkowitz RJ Shenoy SK Beta-arrestins and cell signaling Annu Rev Physiol 2007 69 483 510 17305471
- Gurevich VV Gurevich EV The structural basis of arrestin-mediated regulation of G-protein-coupled receptors Pharmacol Ther 2006 110 465 502 16460808
- Gainetdinov RR Bohn LM Sotnikova TD Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice Neuron 2003 38 291 303 12718862
- Bezard E Gross CE Qin L Gurevich VV Benovic JL Gurevich EV L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain Neurobiol Dis 2005 18 323 335 15686961