Abstract
Parkinson’s disease (PD) is the second-most common age-dependent neurodegenerative disorder and is caused by severe degeneration of dopaminergic neurons in the substantia nigra pars compacta. Unfortunately, current treatment only targets symptoms and involves dopamine replacement therapy, which does not counteract progressive degeneration. MicroRNAs (miRNAs) are a class of small RNA molecules implicated in post-transcriptional regulation of gene expression during development. Recent studies show that miRNAs are playing an important role in the pathophysiology of PD. miRNA-based therapy is a powerful tool with which to study gene function, investigate the mechanism of the disease, and validate drug targets. In this review, we focus on the recent advances of the use of miRNAs in the pathogenesis of PD.
Introduction
Parkinson’s disease (PD) is the second-most common age-dependent neurodegenerative disorder and it affects roughly 1% of the population over 50.Citation1 The disease is clinically characterized by bradykinesia, resting tremor, cogwheel rigidity, and eventually postural instability.Citation2 PD was first described in 1817 by James Parkinson,Citation3 and it was reported in 1893 that these symptoms were attributable to severe degeneration of dopaminergic neurons in the substantia nigra pars compacta.Citation4 However, as the disease advances, the pathology spreads to involve other brain regions, including cingulate gyrus, the amygdala, and higher cortical regions, resulting in dementia and psychiatric manifestations in affected patients.
Pathologically, abnormal accumulation of the presynaptic protein α-synuclein in the brain, forming Lewy bodies, is a hallmark of disease.Citation5 While PD is primarily a sporadic disease in nature, to date, at least five genes have been linked with this multigenic disease including α-synuclein, leucine-rich repeat kinase 2 (LRRK2), parkin, phosphatase and tensin homolog-induced kinase 1 (PINK1), and DJ-1.Citation6–Citation9 In addition, cumulative evidence indicates that dysfunction of these pathways plays a direct role in the etiology of Parkinson’s disease. Recent success in using microRNA (miRNA) as therapeutic targets holds substantial promise for treatment of the complex Parkinson’s disease.
miRNAs are a class of endogenous and small non-coding RNA molecules, approximately 21–24 nucleotides in length, which play an important function in post-transcriptional regulation of gene expression during development. The first miRNA, lin-4, was discovered in Caenorhabditis elegans in 1993.Citation10 This class of small non-coding RNAs is highly evolutionarily conserved and induces translational repression or degradation of a target messenger (mRNA), according to the biogenesis and mechanism outlined below.Citation11,Citation12
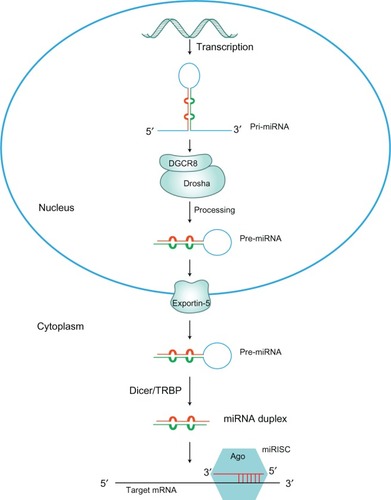
miRNA genes are mainly transcribed by RNA polymerase II into a primary transcript forming a self-folded hairpin structure,Citation13,Citation14 which is recognized and processed by the RNAse III enzyme Drosha in the nucleus, to yield a 70 base pair (bp) precursor miRNA (pre-miRNA).Citation15 Pre-miRNAs are subsequently transported from the nucleus to the cytoplasm by an exportin-5 dependent mechanism. Once in the cytosol, pre-miRNAs are further processed by a second RNAse III enzyme, Dicer, into a 23 bp double stranded miRNA/miRNA*.Citation16 One strand is loaded into the RNA-induced silencing complex (RISC), which requires the presence of proteins belonging to the Argonaute family. Complementarily, mature miRNAs guide RISC to their target mRNAs via base-pairing interactions between the 5′ end of the miRNA and the 3′ untranslated region (3′UTR) of the target mRNA,Citation11,Citation12 which results in mRNA translational repression or degradation.Citation17 shows the miRNA biogenesis pathway.Citation18–Citation20
Figure 1 The miRNA biogenesis pathway.
Abbreviations: Ago, argonaute; GTP, guanosine triphosphate; miRISC, miRNA-induced silencing complex; miRNA, microRNA; pri-miRNA, primary miRNA; pre-miRNA, precursor miRNA; TRBP, trans-activation response element RNA binding protein.
Two common mechanisms for miRNA mediated gene regulation have been described above: translational repression and mRNA degradation.Citation21 In fact, a recent study showed that decreasing miRNA levels by destabilization of mammalian target mRNAs is a major mechanism in miRNA gene repression.Citation22 An individual miRNA is able to target up to a few hundred different mRNAsCitation23 and therefore, is able to regulate the expression of multiple and diverse proteins involved in a biological process.Citation24 Recent evidence suggests that translation regulation is an important process in the pathophysiology of PD. It is hardly surprising that the number of roles assigned to miRNAs in the pathogenesis of PD is rapidly expanding.
In this review we focus on the recent advances in the use of miRNAs in pathological processes of PD.
miRNAs and SNCA
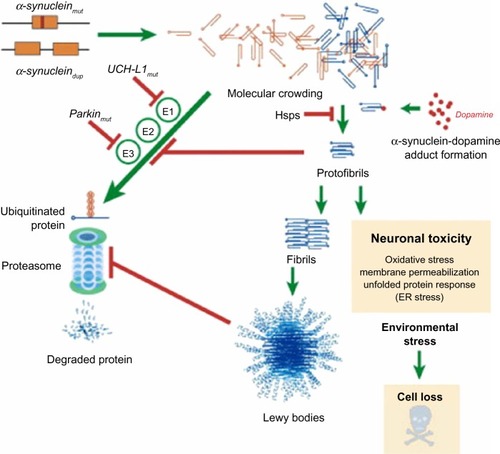
The α-synuclein gene (SNCA), encoding the protein SNCA, is one of the key genes playing a central role in the pathogenesis of PD.Citation25 In PD, α-synuclein is the key protein that aggregates and accumulates in fibrillar form in the pathological hallmark lesions known as Lewy bodies and Lewy neuritis in vulnerable neurons.Citation26,Citation27 It is almost certain that the presence of α-synuclein-rich Lewy bodies is caused by inefficient clearance of α-synuclein. In in vitro studies, α-synuclein protein was shown to undergo concentration-dependent self-aggregation by a nucleation-dependent mechanism.Citation28 Factors that reduce protein clearance efficiency will tip the balance in favor of protein aggregation and will promote the development of inclusions ().Citation29 Additionally, it was shown in a series of works that excess α-synuclein is deleterious to neurons and in particular dopamine neurons, the death of which is responsible for the characteristic motor features of PD.Citation30–Citation33
Figure 2 Putative mechanisms of α-synuclein neurodegeneration in familial PD.
Abbreviations: PD, Parkinson’s disease; UCH-L1, ubiquitin carboxy-terminal hydrolase L1; UPP, ubiquitin proteasome pathway.
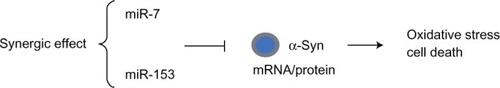
To date, two specific miRNAs were recently shown to regulate α-synuclein levels post-transcriptionally: miR-7Citation34 and miR-153,Citation35 which are abundantly expressed in the brain. The human α-synuclein mRNA has a 3′-UTR, which has potential functional significance in regulating the stability of the mRNA and the rate of protein translation. Further, it was shown that the brain-enriched miRNAs, miR-7 and miR-153, preferentially bind to the SNCA mRNA 3′-UTR and significantly downregulate α-synuclein expression in PD mouse models ().Citation34,Citation35 Recently, miR-7 has been reported to be specifically expressed during neuroblastoma differentiation, cortical development, embryonic stem cell differentiation, and control neurite outgrowth in vitro.Citation36 Therefore, it is conceivable that depletion of certain miRNA species resulting in upregulated expression of α-synuclein levels in a PD brain would subsequently indicate a functional role of these miRNAs in the disease process.
Figure 3 MicroRNA (miR-7 and miR-153) regulation of SNCA in PD.
Abbreviations: 3′-UTR, 3′ untranslated region; α-syn, α-synuclein; mRNA, messenger RNA; miRNA, microRNA; PD, Parkinson’s disease.
miR-7, which is expressed mainly in neurons, is predicted to bind to the α-synuclein 3′-UTR with its seed sequence complementary to nucleotides 119–127, which is conserved in humans, rats, mice, dogs, and chickens.Citation34 Transfection of α-synuclein-expressed cells with miR-7 showed that α-synuclein protein expressions and mRNA levels were both significantly reduced, indicating that miR-7 promotes the degradation of its target mRNA. Thus, the main mechanism of the downregulation of α-synuclein expression by miR-7 is decreased mRNA stability rather than alteration of translation rate. Furthermore, this effect would be lost with mutation of the miR-7 target sequence and in the absence of the 3′-UTR, which confirmed that repression of α-synuclein expression by miR-7 requires the 3′-UTR of the α-synuclein mRNA. It has been confirmed that miR-7 actively represses α-synuclein protein expression; on the contrary, endogenous levels of α-synuclein protein are significantly increased by using specific anti-miRNA inhibitors against miR-7.Citation31 Further, the expression of miR-7 is decreased in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxicated mouse model of PD, which may contribute to the increased α-synuclein expression in this model. The role of miR-7 in downregulating α-synuclein expression post-transcriptionally has been confirmed in several cellular systems.Citation35 Importantly, miR-7 mediated repression of α-synuclein expression protects against the impaired proteasomal functionCitation37,Citation38 and cytotoxicityCitation39,Citation40 associated with α-synuclein overexpression. Antisense inhibition of miR-7 in HeLa cells has also been found to downregulate cell growth and increase apoptosis,Citation41 suggesting that miR-7 has a protective role by preventing oxidative stress.Citation34,Citation42
miR-153 is another miRNA species shown to down-regulate α-synuclein expression by targeting the α-synuclein 3′-UTR where the seed match is located between bases 462 and 468, a sequence that is conserved across vertebrate species.Citation35 In particular, it was shown that miR-153 is also predominantly expressed in the brain,Citation43 specifically binding to the α-synuclein mRNA 3′-UTR and downregulating its mRNA and protein levels.Citation35 Overexpression of miR-153 significantly reduces endogenous α-synuclein levels, whereas miR-153 inhibition enhances translation of a luciferase construct bearing the α-synuclein 3′-UTR in primary neurons.
In a recent work, quantitative analysis of gene transcripts and SNCA protein showed that miR-7 and miR-153 have an additive effect in downregulating α-synuclein expression.Citation35 miR-7 and miR-153 alone produced a 33% and a 12% decrease, respectively, in reporter gene expression, while the combination of the two microRNAs, miR-153/7, resulted in 47% repression, indicating that there is significant synergy between miR-7 and miR-153 to repress α-synuclein mRNA levels and protein expression. These findings reveal that first, miR-7 and miR-153 are synergistic in their effect; second, the 3′-UTR of SNCA mRNA is necessary to regulate SNCA protein expression; third, miRNAs do not interact with the coding region of SNCA to regulate SNCA protein expression; and fourth, miRNAs act at the pre-translational level.Citation35 Regulation of this mRNA level is likely due to miR-7- and miR-153-induced elevation of mRNA deadenylation and uncapping, as they completely complement SNCA mRNA.Citation44,Citation45 Specifically, both miR-7 and miR-153, as well as α-synuclein mRNA and protein, are found in highest levels in nervous tissue, particularly in the midbrain, hippocampus, and cortex, and their expression is restricted to neurons rather than astrocytes.Citation34,Citation35 However, in other organs, such as the lungs and heart, the level of these RNA transcripts was considerably lower. Interestingly, miR-7, miR-153, and SNCA mRNA show highest expression in the midbrain, indicating that deregulation of their expression levels may be important in the pathogenesis of PD. This correspondence in expression pattern is maintained during prenatal and postnatal development, suggesting that miR-7 and miR-153, which are co-expressed with SNCA through a transcription feed-forward loop,Citation35 play a significant role in regulation of α-synuclein levels in patients with familial or sporadic PD.
miRNAs and LRRK2
LRRK2, the protein known as dardarin, was identified in 2004 as being responsible for the most common known genetic form of PD, with autosomal dominant inheritance and low penetrance.Citation46,Citation47 In addition to the growing body of knowledge showing that specific miRNAs target and regulate the expression of disease-associated genes, LRRK2 in a recent study that while specific miRNAs regulate the expression of disease-associated genes, a disease-linked protein can also conversely regulate the miRNA pathway.Citation48
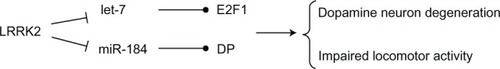
LRRK2 is supposed to exhibit GTPase activity, which is typical of ROCO family protein members. In the Drosophila model,Citation49 analysis of transgenic Drosophilia expressing the pathogenic mutant forms of human LRRK2 gene, I1915T or G2019S, demonstrated that defective LRRK2 does not possess GTPase activity. The LRRK2 gene therefore derepressed miRNA-mediated inhibition of the transcription factors E2F1 and DP,Citation48 which are involved in the control of cell cycle and survival,Citation49,Citation50 and thereby increased dopaminergic neuronal death.
miRNAs of the let-7 and miR-184* families were found to repress the translation of the two factors E2F1 and DP, respectively.Citation48 Pathogenic LRRK2 inhibited let-7 and miR-184* function and consequently E2F1 and DP were upregulated; such effects were dependent on LRRK2 kinase activity. Deletion of let-7, antagomir-mediated inhibition of let-7 and miR-184* actions, and blockage of binding sites for let-7 and miR-184* in 3′-UTRs of their target mRNAs, led to increased expression of E2F1/DP and development of toxic effects similar to that associated with pathogenic LRRK2, which resulted in reduced locomotor activity and dopamine neuron count (). On the other hand, increasing the levels of these two miRNAs partially attenuated the unfavorable effect of mutant LRRK2.Citation48 Recently, it has been shown that let-7b, a member of the let-7 miRNA family, regulates neural stem cell proliferation and differentiation by targeting the stem cell regulator TLX and the cell cycle regulator cyclin D1.Citation51 Overexpression of let-7b led to reduced neural stem cell proliferation and increased neural differentiation, whereas antisense knockdown of let-7b resulted in enhanced proliferation of neural stem cells.Citation51
Figure 4 LRRK2 regulates the microRNA (let-7 and miR-184*) network.
Abbreviation: LRRK2, leucine-rich repeat kinase 2.
The effect of action of pathogenic LRRK2 mutants on miRNA regulation might depend on their co-immunoprecipitation with components of the RISC. In addition, the RISC core protein argonaute-1 levels are negatively regulated by LRRK2 in aged, but not young, flies, and promoted the assocation of argonaute-1 with the increased phosphorylation of 4E-BP in relation to LRRK2.Citation48 These findings indicate that LRRK2 is playing an important role in the pathogenesis of PD, which is mediated through the miRNA pathway, thus paving the way for new therapeutic strategies for PD.
Although these studies demonstrate that translational regulation of E2F1 and DP by miRNAs let-7 and miR-184* is involved in LRRK2-mediated neurodegeneration, the complex mechanism by which mutant LRRK2 inhibits the miRNA pathway and causes neuronal death in the Drosophila still needs to be delineated in a mammalian system.
Parkin, DJ-1, and miRNAs
In accordance with numerous studies, the two genes PAPK2 and PAPK7 are also associated with the pathogenesis of recessive parkinsonism.Citation52–Citation56 Parkin protein is encoded by the PAPK2 gene, whose mutations on chromosome 6q25.2-27 will cause autosomal recessive juvenile parkinsonism (AR-JP), one of the most common familial forms of PD.Citation53 It has been reported that parkin participates in the proteasome-mediated degradation as a ubiquitin-protein ligase collaborating with the ubiquitin-conjugating enzyme UbcH7.Citation53 Furthermore, a study of more than 100 families with AR-JP described mutations in parkin, which showed loss of the ubiquitin-protein ligase activity, indicating that mutations in the PARK2 gene are the cause of the development of PD.Citation54
DJ-1, whose protein is encoded by the PARK7 gene, is a second protein involved in autosomal recessive primary parkinsonism with its homozygous mutations.Citation55,Citation56 DJ-1 plays a causal role in the cellular oxidative stress response, in that its deficiency displays proteasomal inhibition and an apparently normal initial burst of reactive oxygen species (ROS),Citation57 leading to oxidative stress and apoptosis.Citation58 Proteasomal inhibition and ROS have previously been correlated with PD pathology,Citation59 thus the hypothesis that DJ-1 mutations lead to PD because of an increased sensitivity to such stressors.Citation60 Some recent studies demonstrated that DJ-1 deficiency caused severe mitochondria fragmentation,Citation61–Citation63 which suggested that DJ-1 is also involved in the regulation of mitochondrial dynamics.Citation64 Additionally, some data indicates that DJ-1 can bind to parkin during oxidative stress and protect mitochondria from damage, and showing the common neuroprotective role of these two proteins.Citation65
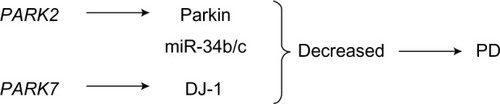
In PD patients, miR-34b and miR-34c are downregulated compared with healthy controls at early (premotor) stages in brain samples,Citation66 accompanied by a significant decrease in the concentrations of parkin and DJ-1 proteins.Citation67,Citation68 The levels of miR-34b and miR-34c are decreased by 40%–65% in affected brain areas such as the amygdala, substantia nigra, frontal cortex, and cerebellum.Citation66 In addition, depletion of miR-34b/miR-34c in differentiated SH-SY5Y neuroblastoma cells resulted in cell death associated with impaired mitochondrial function and oxidative stress, which are consistently recognized biochemical defects in PD brains ().Citation66 Nevertheless, it remains to be proven whether the decreased expression of these miRNAs results from the death of dopaminergic neurons or from their specific downregulation in surviving neurons.Citation66 Characterizing the physiological targets of miR-34b and miR-34c in neurons also needs to be better understood.
Figure 5 The role of PARK2 and PARK7 in the pathogenesis of PD.
Abbreviation: PD, Parkinson’s disease.
miRNA and other candidate genes
Study of the relative abundance of miRNAs in affected brain regions in patients with PD and their comparison to non-affected controls has been one strategy to understand the role of specific miRNAs in the disease. The work of Kim et al was the first study to focus on the expression profiles of 224 different miRNAs precursors obtained from brain regions of patients with PD and control subjects.Citation69–Citation70 This finding has been confirmed by quantitative reverse transcription polymerase chain reaction, ribonuclease protection assay, and Northern blotting. Within this miRNA panel, eight miRNAs appeared to be specifically enriched in the midbrain compared to the cerebral cortex and cerebellum. The expression of one of these miRNAs, miR-133b, has been found to be significantly deficient in the midbrain of PD patient samples compared to controls.Citation70 In addition, miR-133b appears to function as a negative regulator of dopaminergic neuron development, suggesting a direct link to dopamine homeostasis. The elevated level of miR-133b in primary embryonic rat midbrain cultures results in the decrease in dopamine release from neurons, while its lowered level has the opposite effect in number of dopamine neurons.Citation69
miR-133b is also downregulated in mouse dopamine neuron deficient models (Aphakia strain,Citation69 a naturally occurring mouse mutant, which can be used as a model of neuronal loss in human PD).Citation71 Aphakia mice have a mutation in pituitary homeobox 3 (Pitx3), which is considered to be a PD candidate gene and a marker of DA-neurons. Pitx3, a homeobox transcription factor that is required for dopaminergic neuron differentiation in the midbrain substantia nigra,Citation72–Citation74 was recently identified as an miR-133b target.Citation69 Additionally, two polymorphisms in Pitx3 have been associated with sporadic PD in humans.Citation75,Citation76
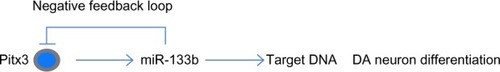
A murine model of PD suggested that miR-133b and Pitx3 regulate expression of each other via a negative feedback loop:Citation69 the Pitx3 gene induces miR-133b transcription, which in turn represses Pitx3 expression.Citation69 Thus, loss of Pitx3 also results in the loss of miR-133b in the PD model. Nevertheless, an important question remains unanswered concerning whether the loss of the miRNA contributes to the development of PD. However, in the in vitro dopamine neuron (DN) differentiation assay, overexpression of miR-133b targeted Pitx3 and resulted in the loss of DN differentiation.Citation77 Conversely, inhibition of miR-133b led to increased expression of DN markers, indicating enhanced DN differentiation. This negative feedback mechanism regulates midbrain dopaminergic neurons activity and terminal differentiation (). Although a miR-133b knockout mouse model has not yet been developed,Citation78 PD probably results from the loss of Pitx3-dependent gene expression rather than the lack of miR-133b. Therefore, whether miR-133b might have protective potential against dopaminergic neuronal neurodegeneration, remains to be verified.
Figure 6 miR-133b and Pitx3 form a negative feedback loop.
Abbreviations: DA, dopaminergic; Pitx3, pituitary homeobox 3.
Another important candidate gene associated with the development of PD is the fibroblast growth factor 20 gene (FGF20), a neurotrophic factor preferentially expressed in the substantia nigra, stimulating the maturation of dopaminergic neurons.Citation79,Citation80 Single nucleotide polymorphisms (SNPs) are small genetic variations in chromosomal DNA sequences where a single nucleotide is replaced by one of the other three nucleotides. SNPs in miRNA target sites will affect transcription and processing of miRNAs, as well as the interactions between miRNAs and their targets, potentially leading to various human diseases ranging from cancer to PD.Citation81 van der Walt et al demonstrated that the polymorphic variations in the FGF20 gene influence the risk of PD development, including two SNPs, rs1721100 and ss20399075, located within highly conserved sequences of the 3′-UTR.Citation82
More recently, another SNP, rs12720208, which is also located in the 3′-UTR of the FGF20 mRNA, was identified to have the strongest association with risk for PD.Citation83 This SNP lies within a predicted binding site for miR-433, which is highly expressed in the brain, leading to reduced miR-433 binding efficiency.Citation84 In an experimental model, the risk allele rs12720208 decreased the binding of the mRNA with miR-433 and resulted in the overexpression of FGF20. Furthermore, increased levels of FGF20 led to a concomitant increase in α-synuclein levels, which has previously been shown to cause PD through both overexpression and point mutations ().Citation85 Human brain samples with the miR-433 SNP demonstrated upregulation of both FGF20 and α-synuclein.Citation84 Thus, it is predicted that elevated FGF20 might predispose to PD through long-term upregulation of α-synuclein and further enhance neurogenesis early in life, but also create chronic elevation leading to degeneration during later life stages.Citation83 Conversely, subsequent reports found no association between FGF20 polymorphism and PD risk, or a relationship between the rs12720208 genotype, FGF20, and α-synuclein protein levels.Citation86,Citation87 Thus, FGF20 probably plays an important role in subpopulations of PD and should be a focus for future study.
Figure 7 MicroRNA (miR-433) regulation of FGF20 in PD.
Abbreviations: α-syn, α-synuclein; FGF20, fibroblast growth factor 20; PD, Parkinson’s disease; SNP, single-nucleotide polymorphism.

miRNA and PD therapy
The design of effective miRNA-based PD therapeutics faces an immediate challenge because of the complexity of neurodegenerative diseases. RNA interference has previously been exploited with great success in knocking down the expression of selected target genes and reducing protein levels involved in PD.Citation88,Citation89 As an example, targeting α-synuclein by infusion of chemically modified short interfering RNACitation90 or injection of small-hairpin-RNA-specific lentiviral vector systemsCitation91 notably decreased protein levels. The expectation has been proposed that RNA-interference-based therapies will be one of the major classes of drugs in the future,Citation92 which strongly supports the notion that miRNAs may be better candidates for regulating endogenous gene expression in RNA-based therapies.Citation93 As miRNA drugs do not appear to have the same issues with toxicity as short interfering RNA or small hairpin RNA based therapies,Citation94,Citation95 they are gaining more attention for their special potential in PD therapy.
In order to manipulate the expression levels of disease-related miRNAs in the hope of curing or prolonging disease onset, two miRNA-based therapeutic approaches are being investigated in vitro and in vivo. On the one hand, introduction of mature miRNA mimics, which are small RNA molecules that resemble miRNA precursors, can be used to effectively downregulate the expression of specific target proteins. The target protein can be any gene involved in disease pathogenesis, or the disease gene itself having a gain-of-function pathogenic mutation.Citation96 However, there are also two main challenges that must be conquered in the use of this miRNA-based approach before assessing their efficacy in humans. For one thing, delivery of any drug to the brain by peripheral administration faces the particular technical hurdle posed by delivery across the blood–brain barrier. For another, the off-target physiological effect is another downfall in manipulating the levels or function of a specific miRNA. Although synthetic miRNA mimics are frequently used in culture research, the efficacy of miRNA mimics in in vivo conditions have not been studied extensively.Citation97–Citation99 Presumably, adequate dose optimization of the miRNA mimic must be achieved to create persistent effects before clinical application.Citation96
On the other hand, creating a loss-of-function in the miRNA of interest can be achieved by exploiting synthetic anti-miRNA molecules.Citation100 These are single-stranded antisense oligonucleotides with complementary sequence that bind to and inactivate the miRNA target, thus blocking these overexpressed miRNAs. In a previous study, Stoffel’s group designed chemically modified, cholesterol-conjugated single-stranded RNA analogs complementary to miRNAs, which were termed as “antagomirs,” to help the RNA enter cells.Citation101 Another example is of modified locked nucleic acids anti-miRs that were designed specifically against miR-122, an abundant liver-specific miRNA.Citation102,Citation103 This approach has also been successfully used to reduce the amount of miR-122 without showing any toxicity in nonhuman primates and mice.Citation102,Citation103 Intravenous administration of chemically engineered oligonucleotides against several certain miR-NAs led to a marked reduction of corresponding miRNA levels in many organs, and the silencing of these miRNAs is specific, efficient, and long-lasting.Citation101 An elegant study further demonstrated the therapeutic feasibility and safety of miRNA inhibition of this approach in a primate disease model.Citation104 Therefore, the modified oligonucleotides, termed antagomirs, are efficient and specific silencers of endogenous miRNAs through intravenous injections or cerebrospinal fluid infusion, providing a potential role in miRNA-based therapy. The locked-nucleic-acids-modified oligonucleotides targeting miR-122 (SPC3649) method is currently being used in Phase I clinical trials for hepatitis C virus infection, probably becoming the first miRNA therapeutic target in humans.
Although miRNA-based therapies pose many unsolved challenges, including the specificity of miRNA function and the specific noninvasive delivery to the central nervous system, they still present exciting opportunities to combat humankind’s debilitating diseases.
Conclusion
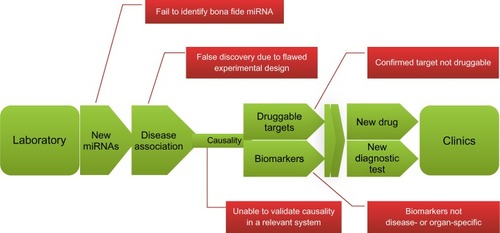
In the past few years, the field of miRNA study has revealed highly significant molecular mechanisms relevant to PD pathogenesis. Emerging evidence strongly indicates the important role of miRNAs in the pathogenesis of this disorder and the disturbance of processes associated with regulation of the expression of genes implicated in its development. However, complex challenges also remain and miRNA therapy or diagnosis may be many years away from entering the clinic ().Citation105 This review represents the tip of the iceberg in the identification of miRNAs and their targets involved in PD, which has been summarized in . We strongly believe that miRNA-based techniques will be helpful in addressing unresolved questions concerning PD via in vitro and in vivo approaches. Given the pace of new findings and the discovery of applications, miRNA is likely to remain a major new therapy for the foreseeable future.
Table 1 miRNAs potentially involved in PD pathophysiology
Figure 8 The road from laboratory to clinic: the promises and challenges of miRNA research.
Abbreviation: miRNA, microRNA.
Acknowledgments
The study was supported by the Projects of National Science Foundation of China (numbers 81071025, 81171203, 81171204, and 81200871), and Projects of the Shanghai Committee of Science and Technology, People’s Republic of China (numbers 11 nm0503300, 11410708900, and 12XD1403800).
Disclosure
The authors report no conflicts of interest in this work.
References
- Robinson PA Protein stability and aggregation in Parkinson’s disease Biochem J 2008 413 1 1 13 18537793
- Savitt JM Dawson VL Dawson TM Diagnosis and treatment of Parkinson disease: molecules to medicine J Clin Invest 2006 116 7 1744 1754 16823471
- Parkinson J An Essay on the Shaking Palsy London Sherwood, Neely and Jones 1817
- D’Amelio M Ragonese P Sconzo G Aridon P Savettieri G Parkinson’s disease and cancer: insights for pathogenesis from epidemiology Ann N Y Acad Sci 2009 1155 324 334 19250224
- Harraz MM Dawson TM Dawson VL MicroRNAs in Parkinson’s disease J Chem Neuroanat 2011 42 2 127 130 21295133
- Hamza TH Zabetian CP Tenesa A Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease Nat Genet 2010 42 9 781 785 20711177
- Dawson TM Dawson VL Molecular pathways of neurodegeneration in Parkinson’s disease Science 2003 302 5646 819 822 14593166
- Gasser T Molecular pathogenesis of Parkinson disease: insights from genetic studies Expert Rev Mol Med 2009 11 e22 19631006
- Simon-Sanchez J Schulte C Bras JM Genome-wide association study reveals genetic risk underlying Parkinson’s disease Nat Genet 2009 41 12 1308 1312 19915575
- Lee RC Feinbaum RL Ambros V The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14 Cell 1993 75 5 843 854 8252621
- Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function Cell 2004 116 2 281 297 14744438
- Bushati N Cohen SM microRNA functions Annu Rev Cell Dev Biol 2007 23 175 205 17506695
- Lee Y Kim M Han J MicroRNA genes are transcribed by RNA polymerase II EMBO J 2004 23 20 4051 4060 15372072
- Garzon R Marcucci G Croce CM Targeting microRNAs in cancer: rationale, strategies and challenges Nat Rev Drug Discov 2010 9 10 775 789 20885409
- Borchert GM Lanier W Davidson BL RNA polymerase III transcribes human microRNAs Nat Struct Mol Biol 2006 13 12 1097 1101 17099701
- Lee YS Nakahara K Pham JW Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways Cell 2004 117 1 69 81 15066283
- Bartel DP MicroRNAs: target recognition and regulatory functions Cell 2009 136 2 215 233 19167326
- Abe M Bonini NM MicroRNAs and neurodegeneration: role and impact Trends Cell Biol 2013 23 1 30 36 23026030
- Kuss AW Chen W MicroRNAs in brain function and disease Curr Neurol Neurosci Rep 2008 8 3 190 197 18541114
- Enciu AM Popescu BO Gheorghisan-Galateanu A MicroRNAs in brain development and degeneration Mol Biol Rep 2012 39 3 2243 2252 21643950
- Kosik KS The neuronal microRNA system Nat Rev Neurosci 2006 7 12 911 920 17115073
- Guo H Ingolia NT Weissman JS Bartel DP Mammalian microR-NAs predominantly act to decrease target mRNA levels Nature 2010 466 7308 835 840 20703300
- Lewis BP Burge CB Bartel DP Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets Cell 2005 120 1 15 20 15652477
- Liu NK Xu XM MicroRNA in central nervous system trauma and degenerative disorders Physiol Genomics 2011 43 10 571 580 21385946
- Fortin DL Troyer MD Nakamura K Kubo S Anthony MD Edwards RH Lipid rafts mediate the synaptic localization of alpha-synuclein J Neurosci 2004 24 30 6715 6723 15282274
- Spillantini MG Goedert M The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy Ann N Y Acad Sci 2000 920 16 27 11193145
- Spillantini MG Schmidt ML Lee VM Trojanowski JQ Jakes R Goedert M Alpha-synuclein in lewy bodies Nature 1997 388 6645 839 840 9278044
- Conway KA Lee SJ Rochet JC Ding TT Williamson RE Lansbury PT Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy Proc Natl Acad Sci U S A 2000 97 2 571 576 10639120
- Eriksen JL Dawson TM Dickson DW Petrucelli L Caught in the act: alpha-synuclein is the culprit in Parkinson’s Disease Neuron 2003 40 3 453 456 14642269
- Mutez E SNCA locus duplication carriers: from genetics to Parkinson disease phenotypes Hum Mutat 2011 32 4 E2079 E2090 21412942
- Sekine T Clinical course of the first Asian family with Parkinsonism related to SNCA triplication Mov Disord 2010 25 16 2871 2875 20818659
- Cullen V Acid beta-glucosidase mutants linked to gaucher disease, parkinson disease, and lewy body dementia alter alpha-synuclein processing Ann Neurol 2011 69 6 940 953 21472771
- Yavich L Tanila H Vepsalainen S Jäkälä P Role of alpha-synuclein in presynaptic dopamine recruitment J Neurosci 2004 24 49 11165 11170 15590933
- Junn E Lee KW Jeong BS Chan TW Im JY Mouradian MM Repression of alpha-synuclein expression and toxicity by microRNA-7 Proc Natl Acad Sci U S A 2009 106 31 13052 13057 19628698
- Doxakis E Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153 J Biol Chem 2010 285 17 12726 12734 20106983
- Chen H Shalom-Feuerstein R Riley J miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro Biochem Biophys Res Commun 2010 394 4 921 927 20230785
- Petrucelli L O’Farrell C Lockhart PJ Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons Neuron 2002 36 6 1007 1019 12495618
- Stefanis L Larsen KE Rideout HJ Sulzer D Greene LA Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death J Neurosci 2001 21 24 9549 9560 11739566
- Jiang H Wu YC Nakamura M Parkinson’s disease genetic mutations increase cell susceptibility to stress: mutant alpha-synuclein enhances H2O2- and Sin-1-induced cell death Neurobiol Aging 2007 28 11 1709 1717 16978743
- Junn E Mouradian MM Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine Neurosci Lett 2002 320 3 146 150 11852183
- Cheng AM Byrom MW Shelton J Ford LP Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis Nucleic Acids Res 2005 33 4 1290 1297 15741182
- Lau P de Strooper B Dysregulated microRNAs in neurodegenerative disorders Semin Cell Dev Biol 2010 21 7 768 773 20080199
- Farh KK Grimson A Jan C The widespread impact of mammalian MicroRNAs on mRNA repression and evolution Science 2005 310 5755 1817 1821 16308420
- Behm-Ansmant I Rehwinkel J Doerks T Stark A Bork P Izaurralde E mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes Genes Dev 2006 20 14 1885 1898 16815998
- Eulalio A Huntzinger E Nishihara T Rehwinkel J Fauser M Izaurralde E Deadenylation is a widespread effect of miRNA regulation RNA 2009 15 1 21 32 19029310
- Paisan-Ruiz C Jain S Evans EW Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease Neuron 2004 44 4 595 600 15541308
- Zimprich A Biskup S Leitner P Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology Neuron 2004 44 4 601 607 15541309
- Gehrke S Imai Y Sokol N Lu B Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression Nature 2010 466 7306 637 641 20671708
- Girling R Partridge JF Bandara LR A new component of the transcription factor DRTF1/E2F Nature 1993 362 6415 83 87 8446173
- Ingram L Munro S Coutts A La Thangue N E2F-1 regulation by an unusual DNA damage-responsive DP partner subunit Cell Death Differ 2011 18 1 122 132 20559320
- Zhao C Sun G Li S MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling Proc Natl Acad Sci U S A 2010 107 5 1876 1881 20133835
- Cookson MR Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways Cold Spring Harb Perspect Med 2012 2 9 a009415 22951446
- Shimura H Hattori N Kubo S Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase Nat Genet 2000 25 3 302 305 10888878
- Illarioshkin SN Periquet M Rawal N Mutation analysis of the parkin gene in Russian families with autosomal recessive juvenile parkinsonism Mov Disord 2003 18 8 914 919 12889082
- Bonifati V Rizzu P van Baren MJ Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism Science 2003 299 5604 256 259 12446870
- Ariga H Takahashi-Niki K Kato I Maita H Niki T Iguchi-Ariga SM Neuroprotective Function of DJ-1 in Parkinson’s Disease Oxid Med Cell Longev 2013 2013 683920 23766857
- Shendelman S Jonason A Martinat C Leete T Abeliovich A DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation PLoS Biol 2004 2 11 e362 15502874
- Demasi M Davies KJ Proteasome inhibitors induce intracellular protein aggregation and cell death by an oxygen-dependent mechanism FEBS Lett 2003 542 1–3 89 94 12729904
- Dauer W Przedborski S Parkinson’s disease: Mechanisms and models Neuron 2003 39 6 889 909 12971891
- Billia F Hauck L Grothe D Parkinson-susceptibility gene DJ-1/PARK7 protects the murine heart from oxidative damage in vivo Proc Natl Acad Sci U S A 2013 110 15 6085 6090 23530187
- Irrcher I Aleyasin H Seifert EL Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics Hum Mol Genet 2010 19 19 3734 3746 20639397
- Krebiehl G Ruckerbauer S Burbulla LF Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1 PLoS One 2010 5 2 e9367 20186336
- Thomas KJ McCoy MK Blackinton J DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy Hum Mol Genet 2011 20 1 40 50 20940149
- Wang X Petrie TG Liu Y Liu J Fujioka H Zhu X Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction J Neurochem 2012 121 5 830 839 22428580
- Mandemakers W Morais VA De Strooper B A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases J Cell Sci 2007 120 Pt 10 1707 1716 17502481
- Miñones-Moyano E Porta S Escaramís G MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function Hum Mol Genet 2011 20 15 3067 3078 21558425
- Shapshak P Molecule of the month: miRNA and Parkinson’s disease protein PARK2 Bioinformation 2013 9 8 381 382 23750085
- Saito Y Saito H MicroRNAs in cancers and neurodegenerative disorders Front Genet 2012 3 194 23056009
- Kim J Inoue K Ishii J A microRNA feedback circuit in midbrain dopamine neurons Science 2007 317 5842 1220 1224 17761882
- Hebert SS De Strooper B Alterations of the microRNA network cause neurodegenerative disease Trends Neurosci 2009 32 4 199 206 19268374
- Van den Munckhof P Luk KC Ste-Marie L Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons Development 2003 130 11 2535 2542 12702666
- Hwang DY Ardayfo P Kang UJ Semina EV Kim KS Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice Brain Res Mol Brain Res 2003 114 2 123 131 12829322
- Martinat C Bacci JJ Leete T Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype Proc Natl Acad Sci U S A 2006 103 8 2874 2879 16477036
- Nunes I Tovmasian LT Silva RM Burke RE Goff SP Pitx3 is required for development of substantia nigra dopaminergic neurons Proc Natl Acad Sci U S A 2003 100 7 4245 4250 12655058
- Fuchs J Mueller JC Lichtner P The transcription factor PITX3 is associated with sporadic Parkinson’s disease Neurobiol Aging 2009 30 5 731 738 17905480
- Bergman O Håkansson A Westberg L PITX3 polymorphism is associated with early onset Parkinson’s disease Neurobiol Aging 2010 31 1 114 117 18420308
- Li J Dani JA Le WD The Role of Transcription Factor Pitx3 in Dopamine Neuron Development and Parkinson’s Disease Curr Top Med Chem 2009 9 10 855 859 19754401
- Nelson PT Wang WX Rajeev BW MicroRNAs (miRNAs) in Neurodegenerative Diseases Brain Pathol 2008 18 1 130 138 18226108
- Ohmachi S Watanabe Y Mikami T FGF-20, a novel neurotrophic factor, preferentially expressed in the substantia nigra pars compacta of rat brain Biochem Biophys Res Commun 2000 277 2 355 360 11032730
- Ohmachi S Mikami T Konishi M Miyake A Itoh N Preferential neurotrophic activity of fibroblast growth factor-20 for dopaminergic neurons through fibroblast growth factor receptor-1c J Neurosci Res 2003 72 4 436 443 12704805
- Sethupathy P Collins FS MicroRNA target site polymorphisms and human disease Trends Genet 2008 24 10 489 497 18778868
- van der Walt JM Noureddine MA Kittappa R Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease Am J Hum Genet 2004 74 6 1121 1127 15122513
- Wang WX Rajeev BW Stromberg AJ The expression of microRNA miR-107 decreases early in Alzheimer‘s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1 J Neurosci 2008 28 5 1213 1223 18234899
- Davis TH Cuellar TL Koch SM Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus J Neurosci 2008 28 17 4322 4330 18434510
- Wang G van der Walt JM Mayhew G Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein Am J Hum Genet 2008 82 2 283 289 18252210
- Wider C Dachsel JC Soto AI FGF20 and Parkinson‘s disease: no evidence of association or pathogenicity via alpha-synuclein expression Mov Disord 2009 24 3 455 459 19133659
- de Mena L Cardo LF Coto E FGF20 rs12720208 SNP and microRNA-433 variation: no association with Parkinson’s disease in Spanish patients Neurosci Lett 2010 479 1 22 25 20471450
- Bumcrot D Manoharan M Koteliansky V Sah DW RNAi therapeutics: a potential new class of pharmaceutical drugs Nat Chem Biol 2006 2 12 711 719 17108989
- de Fougerolles A Vornlocher HP Maraganore J Lieberman J Interfering with disease: a progress report on siRNA-based therapeutics Nat Rev Drug Discov 2007 6 6 443 453 17541417
- Lewis J Melrose H Bumcrot D In vivo silencing of alpha-synuclein using naked siRNA Mol Neurodegener 2008 3 19 18976489
- Sapru MK Yates JW Hogan S Jiang L Halter J Bohn MC Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi Exp Neurol 2006 198 2 382 390 16455076
- Blow N Small RNAs: delivering the future Nature 2007 450 7172 1117 1120 18075597
- Mack G MicroRNA gets down to business Nat Biotechnol 2007 25 6 631 638 17557095
- McBride JL Boudreau RL Harper SQ Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi Proc Natl Acad Sci U S A 2008 105 15 5868 5873 18398004
- Boudreau RL McBride JL Martins I Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice Mol Ther 2009 17 6 1053 1063 19240687
- Junn E Mouradian MM MicroRNAs in neurodegenerative diseases and their therapeutic potential Pharmacol Ther 2012 133 2 142 150 22008259
- Boissonneault V Plante I Rivest S Provost P MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1 J Biol Chem 2009 284 4 1971 1981 18986979
- Hébert SS Horré K Nicolai L Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression Proc Natl Acad Sci U S A 2008 105 17 6415 6420 18434550
- Zhao X He X Han X MicroRNA-mediated control of oligodendrocyte differentiation Neuron 2010 65 5 612 626 20223198
- Meister G Landthaler M Dorsett Y Tuschl T Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing RNA 2004 10 3 544 550 14970398
- Krützfeldt J Rajewsky N Braich R Silencing of microRNAs in vivo with ‘antagomirs’ Nature 2005 438 7068 685 689 16258535
- Elmén J Lindow M Schütz S LNA-mediated microRNA silencing in non-human primates Nature 2008 452 7189 896 899 18368051
- Elmén J Lindow M Silahtaroglu A Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver Nucleic Acids Res 2008 36 4 1153 1162 18158304
- Lanford RE Hildebrandt-Eriksen ES Petri A Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection Science 2010 327 5962 198 201 19965718
- Li Y Kowdley KV MicroRNAs in common human diseases Genomics Proteomics Bioinformatics 2012 10 5 246 253 23200134
- Asikainen S Rudgalvyte M Heikkinen L Global microRNA expression profiling of Caenorhabditis elegans Parkinson’s disease models J Mol Neurosci 2010 41 1 210 218 20091141