?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Metal ions play an important role in biological processes and in metal homeostasis. Metal imbalance is the leading cause for many neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. 8-Hydroxyquinoline (8HQ) is a small planar molecule with a lipophilic effect and a metal chelating ability. As a result, 8HQ and its derivatives hold medicinal properties such as antineurodegenerative, anticancer, antioxidant, antimicrobial, anti-inflammatory, and antidiabetic activities. Herein, diverse bioactivities of 8HQ and newly synthesized 8HQ-based compounds are discussed together with their mechanisms of actions and structure–activity relationships.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
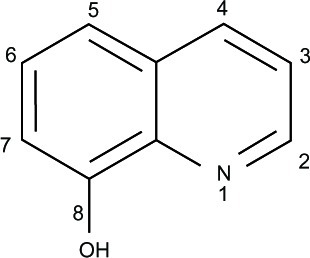
8-Hydroxyquinoline (8HQ) (), a quinoline derivative originating in plants as well as from synthesis, has been used as a fungicide in agriculture and a preservative in the textile, wood, and paper industries.Citation1 8HQ possesses potent coordinating ability and good metal recognition properties, which means it is widely used for analytical and separation purposes as well as for metal chelation.Citation2
Metal ions play a very important role in biological processes, and metal homeostasis is required for the maintenance of metal balance.Citation3,Citation4 Many diseases arise from the loss of homeostasis including metal overload and deficiency, which are caused by abnormal metal metabolism or metal absorption. Of all the hydroxyquinoline derivatives, 8HQ is the most interesting one to be explored, owing to its multifunctional properties, such as diverse bioactivities and therapeutic potentials.Citation5
8HQ is the only one, among seven isomeric monohydroxyquinolines, capable of forming complexes with divalent metal ions through chelation.Citation6 Most bioactivities of 8HQ and its derivatives originate from their chelating ability. As previously mentioned, metal imbalance is the leading cause for many diseases, therefore, 8HQ is a potent chelator that may restore metal balance and be useful for the treatment of metal-related diseases. In this review, the bioactivities, mechanisms of actions of newly synthesized 8HQ-based compounds, and their structure–activity relationships (SAR) will be discussed.
Antineurodegenerative activity
Transition metals such as Fe, Zn and Cu are found in the brain at relatively high concentration and are required for the brain’s cellular processes including synaptic neuronal activity and metalloenzyme function; for instance, Cu/Zn superoxide dismutase (SOD), cytochrome C oxidase, etc.Citation3
Metal homeostasis dysregulation is generally accepted as a key predisposing factor in many neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis and others.Citation4,Citation7 Increasing levels of redox-reactive metal ions, such as Cu and Fe, in specific brain regions can generate reactive oxygen species (ROS) that cause lipid peroxidation and toxic reactive aldehyde products. These finally lead to damage of cellular components.Citation7 The proteasome is a cellular system that degrades unwanted or abnormal proteins.Citation4 It is worth noting that metal ions can interact with proteins in the brain and induce their conformational change, leading to protein misfolding and rendering them resistant to proteasomes. Moreover, metal–protein interaction facilitates aggregation and accumulation of misfolded proteins in regions of the brain,Citation8 leading to neurotoxicity, neuronal dysfunction, and neuronal cell death.Citation7,Citation9 It has been suggested that dysregulation of metal homeostasis and metal ion–protein interactions are involved in the pathogenesis of neurodegenerative diseases.Citation10–Citation12 Therefore, metal chelation therapy has been proposed to be a promising approach in restoring metal balance and reducing neurotoxicity caused by metal–protein interaction.Citation13
An ideal metal chelator for neurodegenerative treatment had been suggested to be a low molecular weight (MW) and lipophilic (uncharged) compound capable of crossing the blood–brain barrier to reach target sites in the brain.Citation3 In addition, the selectivity of compounds in chelating certain metal ions but not affecting metalloenzymes would also be required for cellular functions.Citation3 It is necessary that the compound would be able to chelate metal ions in accumulated proteinsCitation3 in order to reverse proteasome resistance, thereby allowing misfolded proteins to be degraded.Citation14 Moreover, the drug itself is required to have minimal toxicity and side effects.Citation3 However, merely controlling misfolded protein levels may not be sufficient to reverse the neurodegenerative progression in the brain.Citation14 Therefore, a new therapeutic strategy for preventing metal–protein interaction has been recently proposed.Citation14
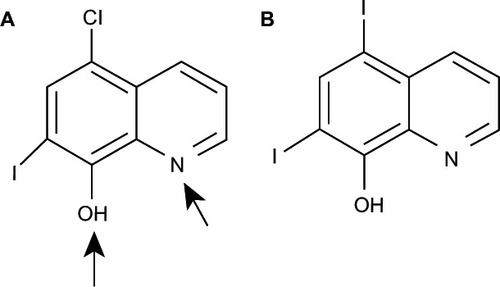
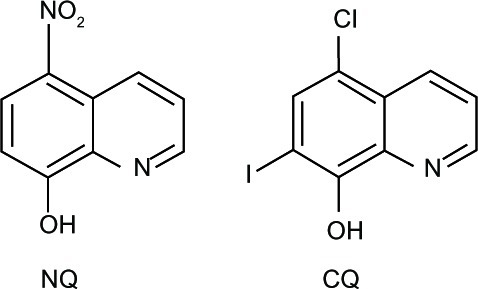
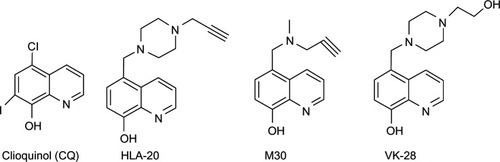
A series of 8HQ derivatives, such as 5-chloro-7-iod o-8-hydroxyquinoline, or clioquinol (CQ), 5-((4-(prop-2-ynyl)piperazin-1-yl)methyl)quinolin-8-ol (HLA-20), 5-((methyl(prop-2-ynyl)amino)methyl)quinolin-8-ol (M30), and 5-((4-(2-hydroxyethyl)piperazin-1-yl)methyl)quinolin-8-ol (VK-28) (), have been reported to exert potent antineurodegenerative effects.Citation15 Among these, CQ has reached pilot Phase II of clinical trials in AD patients.Citation16–Citation20
Figure 2 8-Hydroxyquinoline derivatives with potent antineurodegenerative activity.
CQ was originally used as an antimicrobial for amoebic dysentery (traveler’s diarrhea); however, after its neurotoxicity was reported among the Japanese in the late 1960s, this drug was withdrawn from oral usage.Citation21 The proposed mechanism of toxicity is that CQ decreases vitamin B12 bioavailability, which results in neurological symptoms.Citation22 However, the neurotoxicity can be reversed by vitamin supplementation and dosage control.Citation20
CQ is a potent chelator containing two electron donor sites located at the quinoline ring nitrogen atom and phenolate oxygen atom, which give rise to its chelating ability (). Moreover, halogen groups are known to increase its lipophilicity to allow its absorption to target sites in the brain.Citation9 CQ selectively chelates Cu and Zn ions,Citation9,Citation23 which play a vital role in misfolded protein production, aggregation, and accumulation that ultimately leads to neurotoxicity in AD.Citation9,Citation24 However, the selectivity of the compound can minimize the chance of developing a depletion in systemic metal ions.Citation9
Recently, a dual mechanism of CQ action based on metal–protein interaction has been proposed.Citation14 Firstly, as Cu and Zn chelators, CQ can inhibit misfolded protein production and aggregation. Moreover, the chelation of accumulated Zn in misfolded proteins can reverse proteasome resistance and promote misfolded protein degradation.Citation14 However, the affinity of the compound to chelate Zn is not enough to alter Zn metalloenzymes.Citation14 Secondly, CQ can function as a metal chaperone in transporting metal ions into cells and promoting redistribution of ions that consequently activate cell signaling involved in neuroprotective cascades.Citation25 Thus, the activity of CQ can be attributed to two aspects: the prevention of neurotoxicity initiated by metal–protein interaction and the redistribution of metal ions into cells to promote protective functions.Citation14 It has been reported that CQ is a potent antineurodegenerative agent that can improve cognitive functions in AD patients;Citation3 however, this compound was not further developed owing to manufacturing difficulties. This was due to the presence of a small amount of the carcinogenic contaminant 5,7-diiodo-8-hydroxyquinoline () that forms during large-scale chemical synthesis.Citation14 Thus, PBT2, a second generation CQ, was developed to solve the problem of CQ and to improve its solubility and its ability to cross the blood–brain barrier.Citation14 It was observed that PBT2 could selectively chelate Cu and Zn and form neutral soluble complexes capable of passing through cellular membranes. Due to its moderate affinity to metal ions, after entering cells it can release metal ions from the complex. This leads to bioavailable delivery of Cu and Zn into cells.Citation26–Citation28 So far, PBT2 has shown improvement of cognitive function as noted for CQ in Phase IIa of clinical trials in AD patients.Citation29,Citation30 Results supported by in vitro and in vivo studies suggested that antineurodegenerative efficacies of both CQ and PBT2 are based on their chelating ability and metal ion delivery into cells.Citation26–Citation28 Moreover, metal binding affinity of both compounds is high enough to inhibit misfolded protein production and aggregation but not high enough to alter the actions of metalloenzymes.Citation27,Citation31
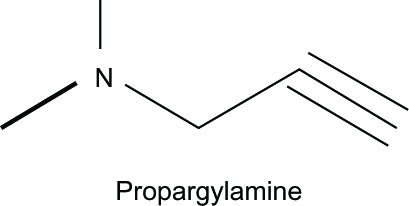
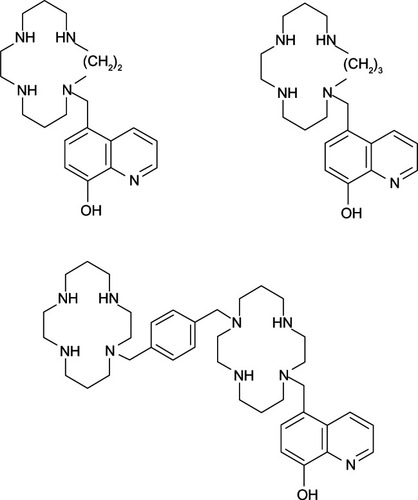
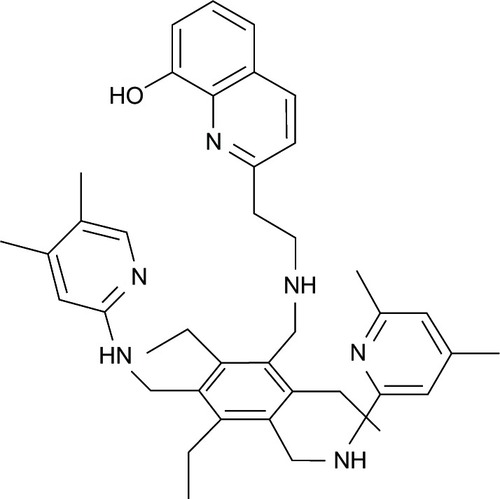
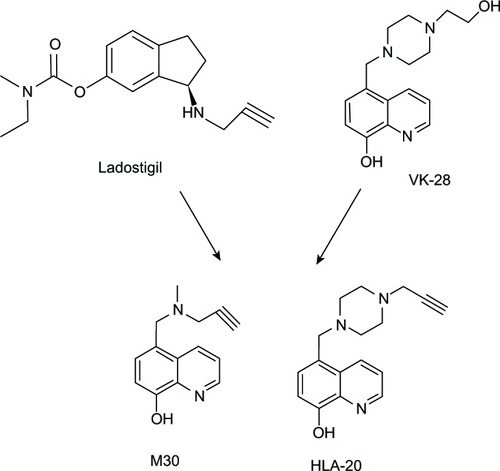
The Fe ion is considered to be another redox-reactive metal ion that causes oxidative stress via the Fenton reaction. It is found to be elevated in many neurodegenerative diseases, such as AD,Citation3 Parkinson’s disease,Citation32 and amyotrophic lateral sclerosis.Citation33 In view of its multifunctional roles, 8HQ-based compounds have been utilized in the treatment and improvement of neurodegenerative patients. For example, M30 and HLA-20 () are novel multifunctional 8HQ-based drugs synthesized by combining an Fe chelating compound possessing an antioxidant activity (VK-28) with the Parkinson’s drug (Ladostigil) containing the N-propagylamine moiety (), which affords the neuroprotective propertyCitation34
Figure 4 M30 and HLA-20 are hybrids of Ladostigil and VK-28.
As outlined in , VK-28 can chelate excessive Fe ions in the brain, thereby preventing the Fenton reaction that produces reactive hydroxyl radicals (OH). It is capable of directly scavenging OH, which gives rise to antioxidant effects.Citation35,Citation36 Ladostigil contains the propargylamine moiety that accounts for its inhibition of the monoamine oxidase enzyme.Citation34 This enzyme is involved in dopamine oxidation, which generates hydrogen peroxide (H2O2) that initiates the Fenton reaction in the presence of Fe2+, leading to oxidative stress in neurons.Citation32 Compounds M30 and HLA-20 have moderate chelating affinity toward Fe, Cu, and, Zn, with the order Fe3+ > Cu2+ > Zn2+, and they strongly inhibit mitochondrial membrane peroxidation in vitro with a half maximal inhibitory concentration (IC50) in the micromolar range.Citation15,Citation37 In vitro studies indicated that M30 upregulates expression of
regulated hypoxia inducible factor, which is a hypoxia mimetic regulator, resulting in neuronal prosurvival and cytoprotective effects.Citation38–Citation40 In addition, M30 has been reported to exhibit neurorescue and neuroprotective activities in animal models.Citation41
Figure 6 Metabolism of dopamine and actions of 8-hydroxyquinoline derivatives.
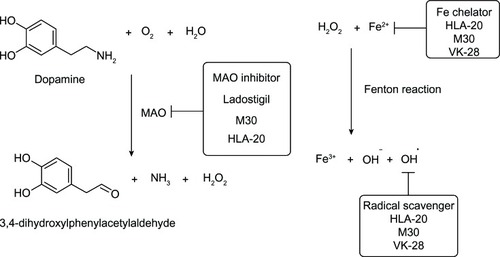
Therefore, both HLA-20 and M30 are novel multifunctional drugs that exhibit promising antioxidant and neuroprotective effects as well as antidepressant activity. These bioactivities arise from the ability of the compounds to elevate levels of dopamine, serotonin, and norepinephrine in the brain through the inhibition of the monoamine oxidase enzyme.Citation42
Anticancer activity
It has been well recognized that redox-active metal ions do not only play important roles in normal cells but are also essential in cancer cells. Some transition metal ions, such as Fe and Cu are considered as cancer risk factors.Citation43–Citation50
In normal cells, Fe serves as a prosthetic group in many enzymes that are required for physiological processes, such as oxidase, catalase, and ribonucleotide reductase. In contrast, it generates ROS, leading to lipid peroxidation and damage to cellular components, such as lipids, proteins, and DNA.Citation51,Citation52 Thus, Fe plays essential roles in cancer via the generation of ROS as well as serving as a nutrient for the growth of cancer cells.Citation43
Most Fe that exists in the human body is in the protein-bound form that cannot promote lipid peroxidation or ROS formation.Citation51 In addition, free Fe per se is a poor catalyst for reactive oxygen metabolites, but Fe toxicity arises when it binds to a low-MW chelator. Therefore, the formed Fe-chelator complex causes the dissociation of H2O2 into OḢ.Citation53 The chelating ability of 8HQ has been proposed to account for its observed cytotoxic activity as afforded by the Fe-8HQ complex.Citation54
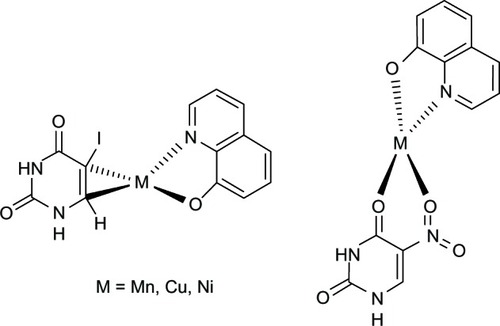
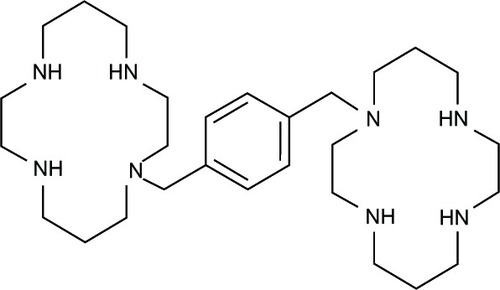
The formed Fe-8HQ lipophilic complex is capable of entering and being distributed within cells,Citation55 causing massive breakage of DNA strands. In order to repair damaged DNA, large quantities of adenosine triphosphate are required, which consequently leads to cellular adenosine triphosphate depletion and finally cell death.Citation56 As such, possible mechanisms of DNA damage were proposed. The Fe-8HQ complex may be formed at specific sites that break the phosphodiester backbone of DNA, acting as chemical nucleases, causing oxidative degradation at the deoxyribose moiety.Citation57 In other words, the Fe-8HQ complex acts as a cytostatic drug.Citation58 Another possible mechanism is that the Fe-chelator complex induces membrane damage, that leads to loss of calcium homeostasis, which triggers endonuclease to cleave DNA in an apoptotic-like manner.Citation54 Results from SAR studies demonstrated that 8HQ is a crucial scaffold for anticancer activity.Citation59 This relationship is derived from the ability of the compound to form chelate complexes with metal ions, incorporated with essential enzymes for DNA synthesis,Citation60 possibly, ribonucleotide reductase.Citation61 Moreover, bis-type structure of 8HQ is required for potent anticancer activity.Citation62 In fact, S1 [bis-N-(8HQ-5-ylmethyl)benzylamine] has been reported to form Fe complexes with higher affinity to exert higher antiproliferative effects as compared to o-trensox (ie, the reference drug). However, o-trensox is a very high affinity Fe chelator in hepatocyte cultures.Citation60 The results indicated that S1 is a promising starting point for anticancer drug development.Citation60 In addition, metal complexes of mixed ligands of 8HQ-uracils () have been reported to provide significant cytotoxicity against human cancer cells (ie, HepG2, A549, HuCCA-1, and MOLT-3).Citation63
Recently, great interest in metal complex compounds has extensively increased due to their wide range of applications.Citation64 The interaction of metal complexes with DNA has been studied for biotechnology and medical applications including their use as anticancer drugs.Citation65 The metal complex binds reversibly to DNA via noncovalent interactions, such as electrostatic binding, groove binding, and intercalative binding.Citation66,Citation67 Intercalation between metal complexes and DNA bases is considered to be the most important binding mode giving rise to antitumor activity.Citation68 This causes DNA conformational changes, which finally leads to DNA strand stress and breakage.Citation69
The intercalating ability of metal complex compounds are dependent on the planarity of the ligands, the coordination geometry, types of ligand donor atoms, and metal ions.Citation70 Sulfonamide-substituted 8HQ metal complexes have been reported to exhibit higher DNA binding affinity than that of free ligands.Citation69 The highest binding efficiency among metal complexes that are formed using the same ligands was found to be that of Cu complexes.Citation69 It was suggested that pharmacological activities of metal complexes are dependent on the nature of both the ligands and the metal ions.Citation71 This notion was demonstrated for metal complexes synthesized from different types of metal ions using the same ligand; such metal complexes were found to exert different bioactivities.Citation72,Citation73
Cu ions are a risk factor predisposing to cancer, and they also serve as an essential cofactor for tumor angiogenesis, that is crucial for tumor growth and metastasis.Citation44–Citation47 High levels of Cu in tissue or serum has been found in many cancer patients including those with breast, prostate, colon, lung, and brain cancer.Citation74–Citation78 It was suggested that Cu could be used as one of the selective targets for cancer treatments.Citation79
The anticancer effects of 8HQ derivatives, such as CQ, are related to Cu and Zn ions. As a Cu chelator, CQ exerts selective antiangiogenesis activityCitation80 toward breast cancerCitation81, prostate cancer,Citation79 leukemia, and myeloma,Citation82 with less effect on normal cells. In addition, the antitumor activity of CQ has been proposed to be tightly associated with proteasome inhibitory ability,Citation79 which is elicited through ionophore actions.
Ionophore is a subset of metal-binding drugs that are capable of transferring multiple metal ions across biological membranes, either in or out of cells.Citation83–Citation85 Two properties are required for metal-binding compounds to act as ionophore, which are described as follows. First, compounds should have low to moderate metal affinity, allowing them to bind metal ions in higher concentration areas and release them in lower concentration areas.Citation80 Second, a suitable logarithmic measure of acid dissociation constant value (pKa) is necessary for compounds to be protonated upon entering cell compartments, which induces the release of metal ions from the complex.Citation80 If the extracellular pH is higher than the pKa of the ionophore, the compound will form a complex with the metal ion. Once the metal complex passes into the cell, where the pH is lower than the pKa, the metal ions will be released.Citation80 Both properties have been noted for CQ thereby allowing it to act as an ionophore with the capability to transport Cu and Zn ions into cells.Citation25,Citation26,Citation86
As a Cu ionophore, CQ has been reported to be able to deliver metal ions into cells, where it exerts its activity.Citation79 CQ has been found to interact with Cu ions in tumor cells to form active Cu complexes which target the proteasome.Citation87 Either Cu1+ or Cu2+ interact with electron donors, such as thiol and amino groups that are located outside the active site of proteasomeCitation88–Citation90 thereby causing its conformational changes. These effects finally lead to proteasome inhibitionCitation79,Citation87 and apoptosis of tumor cells.Citation87 Some organic Cu complexes including CQ-Cu complexes have been reported to exhibit potent proteasome inhibitory effects on tumor cells but not on normal cells.Citation81,Citation91 Moreover, in vivo study of the effect on prostate cancer cells and xenografts by CQ was reported.Citation79 The results showed that CQ alone can form an active metal complex with Cu of tumor cells, leading to androgen receptor repression, angiogenesis reduction, and apoptosis induction. Particularly, androgen receptor overexpression was found in all stages of prostate cancer,Citation92,Citation93 indicating that CQ may serve as an excellent antiandrogen receptor agent for prostate cancer treatment and prevention.Citation79
Besides acting as the Cu ionophore, CQ also provides anticancer activity as the Zn ionophore.Citation84 The CQ-Zn complex is a known proteasome inhibitor; however, its growth inhibitory effect is weaker than that exerted by the CQ-Cu complex.Citation84 In addition, CQ relays Zn ions to lysosomesCitation85 and inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activityCitation84,Citation94 thereby leading to lysosomal disruption and cell apoptosis.Citation84,Citation95
The ionophore property of CQ was confirmed by adding metal ions, such as Cu and Zn, which can potentiate its cytotoxic activity instead of reversing the effects, as can be expected in the case of metal chelators.Citation80 This indicated that CQ can transport metal ions into cells and exhibits cytotoxic activity.Citation80 Furthermore, the anticancer activity of 8HQ derivatives on human cancer cells indicated that the effect is enhanced by Cu ions, a redox-reactive metal ion, as it leads to an elevation of ROS. However, such effects were not observed for Zn ions, which are non-redox reactive metal ions.Citation96
It has been further demonstrated that nitro containing 8HQ derivatives such as nitroxoline (8-hydroxy-5-nitroquinoline; NQ) () exerted more potent anticancer activity, with a IC50 of 5–10-fold less than that of CQ (halogenated 8HQ derivative), and may be less neurotoxic.Citation96 Unlike CQ, the antitumor effect of NQ is mainly exhibited via an increasing level of ROS in cells. The nitro moiety of NQ is a nitrogen radical source that initiates redox reactions, that consequently alters intracellular signaling thereby leading to antiangiogenesis and inhibition of tumor cell growth. These effects are enhanced by Cu but not Zn ions. This hypothesis was supported by studies that demonstrated that NQ acted as an antiangiogenic agent both in vitro and in vivo.Citation84
It is notable that Cu ions enhanced the cytotoxic activity found in NQ.Citation96 While Zn ions are known to enhance cytotoxic activity, the activity is found only in association with compounds containing an iodine moiety on the C-7 position of quinoline rings, such as CQ.Citation96 However, the mechanism by which Zn enhances the cytotoxic activity has not been fully elucidated.Citation96 The neurotoxicity of CQ has been reported to be involved with the Zn transporting activity.Citation86,Citation97 CQ that contains iodine at the C-7 position is capable of acting as a Zn ionophore, while NQ does not.Citation96 Such an observation explains why NQ is less neurotoxic than CQ. Moreover, neurological diseases have not been reported in patients treated with NQ,Citation98 suggesting that NQ is a novel compound with less neurotoxicity and should be further developed as an anticancer drug.
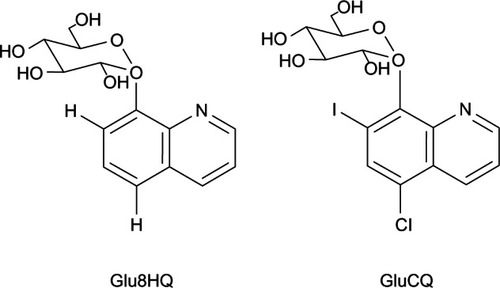
Recently, glucoconjugates of 8HQ derivatives () were developed as anticancer prodrugs in order to improve the selectivity and to avoid chelation of systemic metal ions.Citation99 It was reported that glucose avidity, increased glycolysis rate, and overexpression of glucose transporters were found in cancer cells.Citation100 The study indicated that glucoconjugates could enhance drug delivery owing to the presence of the glucose moiety in drug molecules. The molecular structures of conjugated glucose can mask the chelating properties of compounds until they reach their target sites. Moreover, the presence of glucose in the drug structure promotes a more selective action by exploiting glucose transporters, which were found to be overexpressed in cancer cells.Citation99 Therefore, glucoconjugated drugs are more selective to cancer cells and can cause less systemic side effects.Citation99 After glucoconjugates are trapped in target cells, glucose moieties are hydrolyzed by specific β-glucosidases, which allows the compounds to display chelation and exert antiproliferative effects.Citation101 It has been demonstrated that 8HQ-glucoconjugates are novel compounds with potential for further development as selective anticancer treatment.Citation99
Antimicrobial activity
Antimicrobial effects of 8HQ and its derivatives encompassing antibacterial,Citation102–Citation104 antimalarial,Citation105–Citation107 antiviral,Citation108 antitubercular,Citation109 and antidental plaque activitiesCitation110,Citation111 have been previously reported.
Antibacterial activity
Antitubercular activity
Nonreplicating Mycobacterium tuberculosis (TB) or latent TB is more tolerant to most antituberculosis drugs than the replicating type of TB and requires a more prolonged treatment.Citation109 In fact, more than 200 8HQ-like compounds were identified to have an inhibitory effect against replicating TB.Citation112 Results showed that 8HQ itself exerted the most potent activity among other compounds in its class.Citation113 8HQ can kill both replicating and nonreplicating TB in vitro, with a more potent effect noted for the nonreplicating type.Citation109 Moreover, toxicity toward mammalian cells was not observed within the tested range of concentrations (0.1–10 μM), suggesting its safety in humans.Citation109 Insight into its mechanism of action was not fully elucidated; however, the bidentate chelating property of 8HQ was probably not the primary mechanism. This was supported by the finding that the minimum inhibitory concentration (MIC) value was not changed by the addition of metal ions such as Fe, Cu, Mn, Zn, or Ni.Citation109 Therefore, it was suggested that 8HQ interacts with several molecular targets to evade TB resistance.Citation109
Most of the clinically used antituberculosis drugs are known to be more active against replicating TB and are able to kill both types of TB. The more potent actions were exhibited against the nonreplicating type as was observed in 8HQ, which has not been reported in the literature.Citation109 Thus, 8HQ could be a promising compound for the improvement of TB treatment.Citation109
The antitubercular activity of CQ-metal complexes were also reported.Citation113 In particular, a series of mixed ligand metal complexes using CQ and 1,10-phenanthroline as ligands to coordinate with transition metal ions were synthesized and tested for antitubercular and antifungal activities.Citation113 It was found that the Mn(II) complex was active against TB (MTCC200) with comparable MIC to the standard drug rifampicin with MIC values of 45 μg/mL and 40 μg/mL, respectively,Citation113 whereas the Co(II) complex showed more potent activity with MIC 6.4-fold less than that of rifampicin.Citation113 This study showed that free ligands and metal complexes exerted higher antitubercular activity than that of metal salts.Citation113
Inhibitory effect on Escherichia coli
A previous study reported the antimicrobial effects of 8HQ and its derivatives including 2-hydroxyquinoline (2HQ), 4-hydroxyquinoline (4HQ), and 6-hydroxyquinoline (6HQ) as well as 2-methyl-8HQ against human intestinal bacteria (Bifidobacterium longum, Clostridium difficile, Clostridium perfringens, E. coli, Lactobacillus acidophilus, and Lactobacillus casei).Citation103 The results from the paper disc agar diffusion method demonstrated that only 8HQ could exhibit anti-intestinal bacterial activity. Strong inhibition was also observed for E. coli and C. difficile at a concentration of 0.5 mg/disc and for C. perfringens at a concentration of 0.1 mg/disc. The SAR study indicated that different positions of the hydroxyl group but not the methyl group on the quinoline ring gave rise to growth inhibitory activity against intestinal bacteria. Particularly, compounds with an OH group at the C-8 position displayed effective E. coli, C. difficile, and C. perfringens inhibitions.Citation103 On the other hand, 2HQ, 4HQ, and 6HQ showed no growth inhibition against all of the tested intestinal bacteria.
Inhibitory effect on Staphylococcus aureus
Aqueous formulation of 8HQ (0.5% 8HQ), or commercially available as AQ+, was reported to strongly inhibit the growth of S. aureus including methicillin-resistant S. aureus (MRSA), methicillin-susceptible S. aureus, and vancomycin-intermediate S. aureus and displayed median MIC of 0.25%, which is equal to an active ingredient concentration of 12.5 μg/mL at optimum pH of 9.2.Citation1 Lowering of the pH value caused a reduction in its efficacy: pH 7.5 yielded 4-fold reduction, and pH 5.5 resulted in 8-fold reduction.Citation1 Interestingly, MRSA and vancomycin-intermediate S. aureus were equally susceptible to AQ+ as was observed for methicillin-susceptible S. aureus. It was suggested that the susceptibility to AQ+ was not influenced by antibiotic resistance determinants of the microbe.Citation1 Data from electron microscopy indicated that AQ+ actively disrupts bacterial cell walls thereby leading to cell lysis.Citation1 A time-killing study showed that AQ+ killed 99.9% of all bacterial cells from tested isolates within 6 hours. The time-killing curve of AQ+ was similar to that of gentamicin. Moreover, at higher concentration of AQ+, a more rapid killing effect was observed.Citation1 MRSA is carried in the anterior nares, and it should be noted that mupirocin-containing nasal ointment is currently being used to prevent transmission; however, antibiotic resistance had been reported to increase.Citation113 Owing to the lipophilicity of 8HQ and its potency against various S. aureus strains as well as its rapid killing nature, it has been suggested that this compound could be used as topical hand cleansing agent to prevent MRSA transmission.Citation1
The efficacy of 8HQ in inhibiting S. aureus is dependent on its chelating ability and is enhanced in the presence of Cu. In addition, 8HQ derivatives were found to inhibit S. aureus strains.Citation102 Quantitative structure-activity relationships (QSAR) study performed on 24 substituted 8HQ derivatives showed that ten three-dimensional descriptors such as molecular refraction (MR), partition coefficient (logP), total energy (E), standard Gibbs free energy (G), lowest unoccupied molecular orbital (LUMO), highest occupied molecular orbital (HOMO), total molecular energy (TotE), Wien index (WInd), Balaban index (BInd), and octanol-water ClogP were significant for the development of highly predictive (R 2 = 0.988) model.Citation102 Moreover, potent activity was found in ester derivatives rather than styryl derivatives.Citation102
Antidental plaque activity
Antidental plaque activity of 8HQ derivatives has been reported.Citation110,Citation111 Dental plaque is a combination of oral microorganisms colonized on oral surfaces in which a microbial consortium or oral biofilm is formed.Citation115 Mutans streptococci and Porphyromonas gingivalis are the most important among such oral microorganisms since they are pathogens of dental caries and periodontal diseases, respectively.Citation115 At equilibrium, oral biofilms are beneficial for the prevention of exogenous and potentially pathogenic species colonization. However, unfavorable disruption of dynamic balance between host and microbial community at local sites eventually leads to an overgrowth of virulent or pathogenic species causing diseases.Citation116 Mutans streptococci comprises two species, Streptococcus mutans and S. sobrinus, in which S. mutans is highly prevalent in dental plaque and is considered as an etiological pathogen for dental caries.Citation117 An in vitro study of antidental plaque activity of three 8HQ derivatives, namely 8HQ sulfate, 5-chloro-7-iodo-8HQ (or CQ), and 5,7-dichloro-8HQ, against S. mutans, Streptococcus sanguis, Actinomyces viscosus, and Actinomyces naeslundii was reported. The result showed that all 8HQ derivatives differentially inhibited each of the tested organisms.Citation111 These compounds were prepared in percentage concentrations in polyethylene glycol owing to their sparingly water-soluble nature.Citation111 CQ and 5,7-dichloro-8HQ exerted a bactericidal effect at 0.05% concentration on S. mutans (cariogenic) and A. viscosus (periodontogenic), whereas 8HQ sulfate showed a bacteriostatic effect against both pathogens at a higher (0.3%) concentration.Citation111 It was demonstrated that halogenated 8HQs were more potent dental plaque inhibitors.Citation110,Citation111 The low water solubility is a limitation in using these bioactive compounds via an aqueous vehicle such as mouth rinse.Citation111 However, such compounds could be used via a polyethylene glycol vehicle as an ointment or additive ingredient in dentifrice to control dental diseases.Citation111
It is widely known that the antibacterial activity of 8HQ is closely related with its chelating ability, therefore, Fe or Cu chelation is required for the activity.Citation110 Previous SAR studies indicated that substitution near the nitrogen atom or the phenolic group could alter the chelating ability, which led to a reduction of the antimicrobial activity.Citation118,Citation119 This study demonstrated that the hydrophobic logP parameter alone is not adequate for accurate computational prediction. Thus, electronic parameters such as pKa and steric parameters including MW and molecular refractivity are required for antidental plaque prediction.Citation110 The QSAR study showed that two main factors are involved in antidental plaque activity of 8HQ against S. mutans.Citation110 The results revealed that compounds with increased lipophilicity and electron-withdrawing substituents at the 5-position led to improvements in the activity, whereas bulky substituent groups afforded a decrease in the activity.Citation110 According to these findings, the most potent S. mutans inhibitor should contain small C-5 substituents with lipophilicity and electron-withdrawing properties.Citation110 Notably, CQ contains a 5-chloro group with lipophilicity and an electron-withdrawing nature, making this compound a good S. mutans inhibitor.Citation110
Antimicrobial activity of metal complexes and novel compounds
Metal-8HQ complexes
The antimicrobial activity of divalent metal-8HQ complexes and their mechanisms of action have been proposed.Citation120 It was assumed that 8HQ uses its high lipophilicity to penetrate bacterial cell membranes in order to reach its target site of action, which could possibly be a metal-binding site of bacterial enzymes. The metal-8HQ complex will dissociate into a 1:1 ratio of 8HQ-metal charged complex and 8HQ free ligand.Citation120 The charged 8HQ metal complex can bind and block the metal-binding sites on bacterial enzymes, which gives rise to the antimicrobial effect.Citation119 Therefore, the lipophilicity, as indicated by the logP, is considered to be an important factor for antimicrobial activity of the investigated compounds.Citation120 In addition, the dissociated free ligand of 8HQ possesses high chelating ability that could bind metallic prosthetic groups of microbial enzymes thereby leading to the inhibition of enzymatic activity.Citation5,Citation120
Recently, 8HQ-uracil metal complexes bearing antimicrobial activity () have been reported.Citation121 The complexes exhibited growth inhibition against many strains of Gram-positive and Gram-negative bacteria including resistant pathogens, such as S. aureus, Enterococcus faecalis, and Candida albicans.Citation121
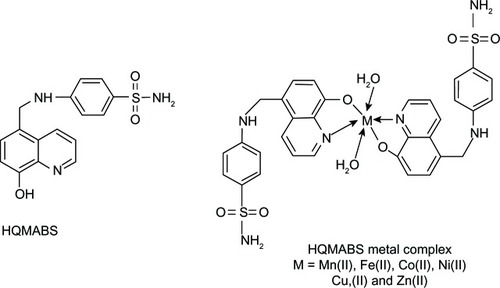
Previously, 4-benzenesulfonamide (HQMABS), shown in , is a hybrid of 8HQ and sulfanilamide and was reported to be a ligand for metal complexes.Citation5 This study showed that HQMABS exhibited more potent antimicrobial activity with higher sensitivity against Gram-positive bacteria as compared to their individual parent compounds (ie, 8HQ and sulfanilamide).Citation5 This demonstrates that there is a synergistic effect of 8HQ and sulfanilamide that facilitates the penetration of HQMABS into the site of action in bacterial cells.Citation5 Therefore, HQMABS exhibited antimicrobial effects through a similar mechanism to that of 8HQ, as a membrane active agent via metal ion chelation.Citation122 On the other hand, all metal complexes of HQMABS displayed weak to moderate activity as compared to their respective free ligand, HQMABS. Moreover, the antimicrobial activity of these compounds is dependent on the nature of the ligands, concentration and lipophilicity of the compound, nature of metal ions, geometry of the complex, and coordinate sites.Citation5
8HQ-based quaternary cationic surfactant
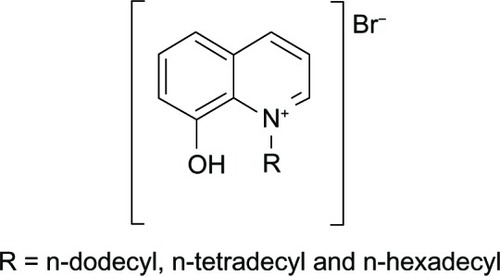
Quaternary cationic surfactants () were synthesized from the reaction of 8HQ and long chain alkyl halides.Citation104 The study showed that cationic amphiphilic structures of quaternary salts allowed the compounds to interact with the bacterial lipid bilayer membrane.Citation122 The effect may alter the membrane itself or cause toxicity to the membrane thereby leading to bacterial cell death.Citation123 The activity of these 8-hydroxyquinolium derivatives is dependent on both the polar heads (ie, size and electronic charge distribution) and the hydrocarbon chain length.Citation104 It was found that the activity increased from C-12 to C-14 carbon atoms and decreased in the case of C-16.Citation104 This suggested that cationic and long chain hydrocarbons of an appropriate length facilitate bacterial killing via membrane attack.
Antiviral activity
It is well recognized that nucleic acid binding ability is important for RNA-dependent-DNA polymerase inhibition, which is essential for antiviral activity.Citation124 Among the groups of tested metal-binding compounds, 8HQ exhibited high antiviral activity with approximately 50-fold higher activity.Citation124 Moreover, the binding activity of the Cu complexes of 8HQ and its derivatives were significantly higher than their respective free ligand forms.Citation124 Interestingly, this activity was enhanced markedly when an equimolar concentration of Cu was added.Citation124 According to Albert et al,Citation119 the ratio of metal complex and their free ligand was shown to affect their antibacterial and antifungal activities; particularly only the 1:1 ratio provided the activity. It was found that increasing the amount of ligands resulted in the formation of more inactive complexes, thereby resulting in decreased activity – which is known as concentration quenching. This phenomenon was observed when high concentrations of drugs gave rise to lower inhibition of DNA synthesis in comparison to using a low drug concentration.Citation124 Despite binding to viral nucleic acid, another possible mechanism of antiviral activity is its binding to Zn in enzymes, thereby leading to the inactivation of viral enzymes.Citation125,Citation126 However, the stability of the Cu complex is much greater than that of the Zn complex.Citation127 This was supported by the study demonstrating that the 8HQ-Cu complex inhibited RNA-dependent-DNA polymerase as well as inactivating Rous sarcoma virus and herpes simplex virus with comparable activity as to that of 8HQ free ligand.Citation112 Therefore, antiviral activity as exerted by ligand binding to Zn metalloenzymes may not be possible.Citation112
Macrocyclic polyamines such as AMD3100 () has reached Phase II of clinical trials and is considered to be a prototype for an antihuman immunodeficiency virus compound.Citation108 AMD3100 is known to block host cell entry via blocking cell surface G-protein-coupled receptors, such as CCR5 and CXCR4, which are chemokine receptors.Citation128–Citation130 Hydroxyquinoline-polyamine conjugates () were synthesized using hydroxyquinoline conjugation with polyamine backbones or polyazamacrocycles in order to mimic chemokine receptor antagonists.Citation108 The results showed that the conjugated compounds elicited antihuman immunodeficiency virus activity against two viral strains, Human immunodeficiency virus (HIV), 1 LAV and HIV-1 BaL, whereas CQ and polyazamacrocycle were shown to be inactive.Citation108 Interestingly, AMD3100 (the reference compound) is only active against HIV-1 LAV thereby suggesting that the quinoline moiety is necessary for conjugated polyamine compounds as anti-HIV agents against both viral strains.Citation108
Antiparasitic activity
Antimalarial activity
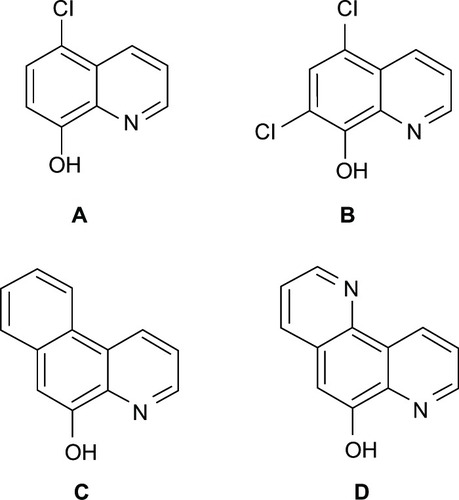
Malaria is considered to be a life-threatening infectious disease worldwide.Citation131 Quinoline-containing compounds have been used for malarial treatment, such as quinine.Citation132 Unfortunately, drug-resistance has been continuously reportedCitation107, thus, the search for novel quinolone-based compounds is a demanding issue.Citation133 Studies have shown that high sensitivity of human malaria to such compounds is mainly due to high lipid-water logP and metal-binding constants.Citation134–Citation139 It was noted that the inhibition of Plasmodium falciparum multiplication and the chelating ability of the compounds were directly correlated.Citation106 Chelators are known to interact with parasitic enzymes in different ways, such as by interacting with sulfhydryl groups, with amino groups, and with certain metal ions of enzymes.Citation137 8HQ as a potent chelator with high lipophilicity, and is known to possess an antimalarial effect against the intracellular stage of malaria in red blood cells by inhibiting a variety of metalloprotein oxidase enzymes, thereby resulting in the inhibition of glycolysis and parasitic growth.Citation135 In vivo toxicity of 8HQ derivatives as diabetogenic agents () has previously been reported. However, a small group of substituents at the C-5 and C-7 positions on the quinoline ring can markedly lower the toxicity in higher animals.Citation106
Substitution with a chlorine group at the C-5 or at both the C-5 and C-7 positions of 8HQ (, respectively) was shown to increase the lipid solubility and chelating ability of the compounds. These increases are expected to be an effect of the phenolic group that leads to improvement in metal chelation.Citation106 Surprisingly, in this case, an improvement of the antimalarial activity was not observed.Citation106 Unlikely to be found in bacterial systems, the addition of an extra aromatic ring at the C-5 and C-6 positions (, respectively) was shown to not only increase its lipophilicity but also led to a decrease in its antimalarial activity. This could be attributed to the bulky structures of the aromatic ring, that may cause steric effects and thereby hinder the compound from interacting with macromolecules on plasmodial enzymes or receptor sites.Citation106 It was suggested that the substitution of C-5 or C-7 positions with electron-withdrawing groups or aromatic rings could improve the lipophilicity of the compounds and is likewise beneficial for drug delivery to target an intracellular site of action. Thus, improved chelating ability and reduced in vivo toxicity were observed, but antimalarial activity showed no improvement. This indicated that the antimalarial effect as afforded by these compounds may be contributed to other factors.Citation106
Linkages of antiparasitic and anticancer activities
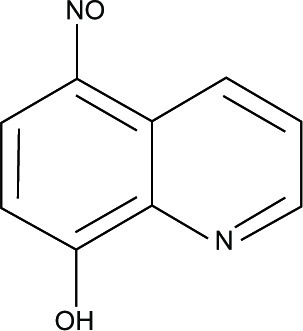
Metabolic pathways in intracellular parasites (ie, T. gondii and P. falciparum) and in cancer cells are more sensitive to oxidative stress than normal cells and are dependent upon glycolysis in order to produce energy. In addition, some anticancer drugs were found to have antimalarial activity.Citation138–Citation140 Results from computational screening of quinoline-based antitumor compounds showed that nitrogen substitution at the C-5 position of the quinoline ring is required for antiprotozoal activity. Apparently, 5-nitroso-8HQ (NSC3852) () exerted the most potent predicted activity and is therefore relevant to the experimentally observed activity against T. gondii, with a half maximal effective concentration of 78.6 nM. But, its activity against P. falciparum was found to be eight times less active than that for T. gondii.Citation105 So far, NSC3852 was reported to exert anticancer activity as elicited through the production of superoxide anion and nitric oxide (NO) against breast cancer cells.Citation141 In contrast, antiprotozoal activity of NSC3852 was attributed to different mechanisms.Citation105 Particularly, it was hypothesized that NSC3852 increased the intracellular oxidative stress via indirect mechanisms. This involves arylation of protein sulfhydryls and the depletion of intracellular glutathione,Citation142 which resulted in the metabolism of NSC3852 to non-redox cycling naphthoquinone and NO release from the nitroso group of the compound.Citation105
Interestingly, antiparasitic and anticancer activities of 8HQ derivatives are potentiated upon complexation with metal ions such as Cu and Zn.Citation84 A series of antimony-8HQ complexes were synthesized and found to exhibit more potent antitrypanosomal and cytotoxic activities when compared to Sb salt (SbCl3) and their free ligands, which are 8HQ, 5-chloro-8HQ, and 5-chloro-7-iodo-8HQ (or CQ).Citation143
Antioxidant activity
Oxidative damage is frequently found in many diseases such as aging, atherosclerosis, cancer, diabetes,Citation144 and neurodegenerative diseases.Citation7 Free radicals are continuously produced in cells through a wide range of biological processes.Citation144 For example, the changing oxidation stage of Cu, which is a cofactor of SOD, results in the generation of ROS.Citation145 Therefore, antioxidant defenses, such as those afforded by tocopherol, ascorbic acid, SOD enzyme, and catalases, are necessary in the maintenance of homeostasis.Citation146 Many phenolic compounds, derived from either natural sources or synthetic methods, have been reported as potent antioxidants.Citation147–Citation149 An effective antioxidant activity of phenolic compounds is dependent on the stability of the phenoxyl radical formed in the reactionCitation150 as well as the position of a substituent which affects the phenoxyl radical.Citation151
8HQ derivatives have been reported as potent antioxidants,Citation32,Citation37,Citation152,Citation153 which arises from their chelating ability. It is widely known that mixed ligand metal complexes can commonly occur in biological fluids from various bioactive ligands with metal ions.Citation154 Interest in the area of metal complexation has steadily increased. SOD is one of the most useful antioxidant enzymes, known to convert superoxide into H2O2 and oxygen.Citation155 However, this enzyme has certain limitations such as short shelf-life, low lipid solubility, low penetration into the cell,Citation155 and high MW.Citation156 The SOD structure has a central metal atom surrounded by the protein structure, thus, accordingly, great focus has been reported on the synthesis of small-sized lipophilic metal complexes in efforts to mimic the SOD activity.Citation63,Citation157,Citation158
A series of mixed ligand metal complexes using 8HQ, 5-iodouracil, and 5-nitrouracil as ligands were synthesized and studied for their antioxidant activity using SOD assay.Citation63 The results showed that amongst the different tested metal complexes, the 5-iodouracil-Mn-8HQ complex was shown to exert the highest activity, with a IC50 of about 3-fold less than that of the free ligand 8HQ.Citation63 This indicated that the coordination of metal ion into the free ligand can lead to enhancement of SOD activity.Citation63 However, complexation of different types of metal ions with the same combination of ligands resulted in different bioactivities.Citation65
Anti-inflammatory activity
Nitric oxide is a short-lived free radical product generated by the conversion of L-arginine to L-citrulline, which is facilitated by nitric oxide synthase (NOS).Citation159 There are three isoforms of NOS comprised of endothelial NOS, inducible NOS (iNOS), and neural NOS.Citation160 Small amounts of NO produced by endothelial NOS and neural NOS were shown to play important roles in the maintenance of homeostasis. In contrast, large amounts of NO from iNOS is generated in pathological conditions and inflammation.Citation161 NO production is regulated by the expression of the iNOS gene,Citation162 which is mainly modulated at the transcriptional level via the binding of transcription factors to sites such as NF-κB sites, activator protein-1, interferon regulatory factor 1, and CCAAT/enhancer-binding protein β (C/EBPβ).Citation163 The NF-κB site is essential for lipopolysaccharide-mediated NO production in response to inflammation.Citation164 Macrophage-derived NO acts as a host-defense mechanism against microbes and tumors, as well as a regulator of proinflammatory genes in vivo.Citation159 It has been found that 8HQ inhibits lipopolysaccharide-induced NO production. Particularly, it suppresses iNOS mRNA expression and iNOS promoter activity by inhibition of NF-κB activation and C/EBPβ DNA-binding activity.Citation160 The study demonstrated that 8HQ possesses anti-inflammatory activity and could be further developed for the treatment of inflammatory diseases.Citation160
Pb transportation across erythrocyte membranes
Long-term exposure to Pb from the environment causes lead poisoning. Pb accumulation is found in bone tissueCitation165 and is also distributed to other organs.Citation166 Moreover, Pb accumulation in bone tissue is correlated with an increased risk of cardiovascular mortality,Citation167 cognitive changes, and neurodegenerative diseases.Citation168,Citation169 The majority of Pb in whole blood is not found in the plasma but is sequestered in erythrocytes, which take up Pb via anion exchangers.Citation83 The accumulation of Pb in erythrocytes affects erythrocyte enzymes, especially Δ-ALA dehydrataseCitation170 and 5-nucleotidase.Citation171 Such accumulation also accelerates the vascular clearance of red blood cellsCitation172 and thrombin generation,Citation173 which may lead to an increased risk of developing cardiovascular disease.Citation174 Most of the chelators that are employed for chelation therapy are not capable of passing across the erythrocyte membrane. Thus, the effective reduction of the total body Pb level is not achieved and gives only a short period of therapeutic benefit.Citation83,Citation175 Therefore, a combination of Pb ionophore and Pb chelating agent was recommended as a new strategy for lowering total body Pb accumulation.Citation176
Δ-ALA dehydratase is well recognized as a Zn-containing erythrocyte enzyme.Citation83 Pb is believed to displace Zn from the enzyme, thereby leading to enzyme inactivation. However, the addition of Zn has been reported to restore Δ-ALA dehydratase activity in vivo.Citation177,Citation178 Since Pb ions enter erythrocytes through anion channels, if they were not trapped in extracellular areas they could reenter cells.Citation83 Thus, the increase of intracellular Zn may reverse Pb binding and facilitate the release of Pb from the enzyme. This potentiates intracellular Pb ions to exit from erythrocytes into the extracellular space, where they are effectively eliminated by Pb chelators.Citation83
CQ is a hydrophobic halogenated 8HQ derivative known to act as a Zn ionophore. It is capable of transporting Zn across the cellular membrane.Citation80 An in vitro study demonstrated that CQ acting as a Zn ionophore could facilitate Pb escape from erythrocytes into the extracellular space. An increased intracellular Zn level thereby allows more effective chelation.Citation83 This suggested that 8HQ derivatives and other classes of Zn ionophore could be further developed as a combined agent, acting as Pb chelators by lowering the total body Pb accumulation.Citation83
Building block for artificial carbohydrate receptors
Artificial carbohydrate receptors exploiting carbohydrate-based molecular recognition processes via noncovalent interactions of sugar bindingCitation179–Citation181 have been developed () for diagnostic and therapeutic purposes.Citation182 Binding preference in carbohydrate recognition is an important factor for effective systemic outcome.Citation183 An in vitro study demonstrated that 8HQ-based receptors elicited higher affinity to β-galactoside as compared to that of quinoline-based receptors.Citation183 Moreover, the addition of more 8HQ moieties into the receptor can potentiate higher affinity.Citation183 It was suggested that the quinoline hydroxyl group plays an important role in complex formation and molecular recognition of carbohydrates. Therefore, 8HQ-based compounds could potentially be used as a building block for artificial carbohydrate receptors.Citation183
Potential antidiabetic activity
Insulin/insulin-like growth factor-1 signaling pathway (IIS) is an evolutionarily conserved pathway, which regulates lifespan and longevity in various species from nonvertebrates to humans.Citation184 In nonhuman organisms, decreased IIS has been found to be associated with extended lifespan and protection against oxidative stress damage.Citation185,Citation186 In addition, experiments on mouse models also demonstrated a significant role of down-regulated IIS in the maintenance of metabolic homeostasis and oxidative defense.Citation184 In humans, individuals with decreased IIS as found in those with Laron syndrome were shown to exhibit a lower rate in the development of diseases of civilization including acne, cancer, and diabetes mellitus (DM).Citation184
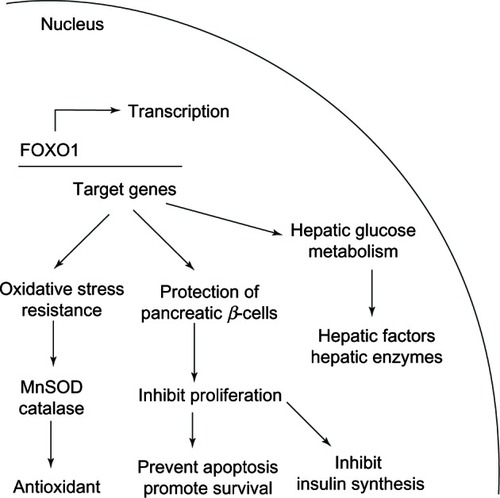
Forkhead box proteins (FOXO) are crucial regulators against oxidative stress conditions. Activation of FOXO in the nucleus results in an expression of many downstream effectors including antioxidant enzyme genes such as superoxide dismutase (MnSOD) and catalase. At the promoter level, FOXO1 induces the expression of the Hmox1 gene (heme oxygenase-1) thereby leading to the reduction of mitochondrial respiration and ROS formation.Citation185 Moreover, in pancreatic β-cell, FOXO1 functions to inhibit β-cell proliferation and prevent β-cell apoptosisCitation187 as shown in . Therefore, the level of nuclear FOXO is important for oxidative stress resistance and cytoprotection of cells.Citation187,Citation188
Figure 17 FOXO1 functions are related to glucose homeostasis and providing protection against oxidative stress.
Overstimulated IIS is closely associated with the pathogenesis of DM. Overconsumption of hyperglycemic diet can cause high blood glucose levels, which induces glucose/FOXO1-mediated β-cell proliferation in order to produce insulin for controlling blood glucose.Citation188 This long-term phenomenon generates insulin resistance and hyperinsulinemia, which are the hallmarks of DM type 2.Citation188 IIS is found to regulate nuclear distribution of FOXO proteins.Citation185 The induction of IIS, caused by hyperglycemic diet, induces Zn-dependent phosphorylation of nuclear FOXO1,Citation189 that subsequently triggers their exclusion from the nucleus.Citation185
Reduction of nuclear FOXO1 levels as a result of phosphorylation leads to three events. First, an impairment of oxidative stress resistance of β-cells as caused by down-regulation of MnSOD and catalase gene expressions.Citation184 Second, an increased proliferation and apoptosis of β-cells. Third, the inhibition of FOXO1 serves as key regulators for the inhibition of hepatic gluconeogenesis. Therefore, FOXO1 inhibition suppresses hepatic enzymes necessary for glucose production, such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, at the transcriptional level.Citation189 Obviously the first two events promote β-cell apoptosis thereby leading to impaired insulin synthesis and altered blood glucose level, while the last event facilitates the lowering of blood glucose as shown in .
Figure 18 The role of CQ as Zn ionophore in controlling blood glucose level via inhibition of FOXO1.
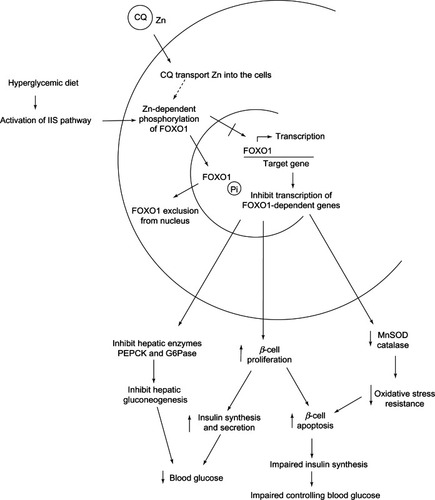
8HQ has been reported as a diabetogenic agent owing to its ability to harm β-cells.Citation190 As a Zn ionophore, 8HQ carries Zn into cells in the form of lipophilic, uncharged complexes and releases Zn inside the cells to promote Zn-dependent FOXO1 phosphorylation thereby leading to oxidative damage and apoptosis of β-cell.Citation190 In addition, acidic proton (H+) released from the –OH group of 8HQ causes damage to β-cells.Citation190
The SAR study of 8HQ and its derivatives indicated that their binding affinity to Zn2+, charge of complex, and acidity are determinant factors for diabetogenicity of the investigated compounds.Citation190 As mentioned, FOXO1 phosphorylation is Zn-dependent, therefore, compounds that possess affinity to bind and form uncharged complexes with Zn are also capable of penetrating into cells thereby leading to the induction of FOXO1 phosphorylation and the triggering of its exclusion from the nucleus.Citation190 Reduced nuclear FOXO1 levels ultimately lead to β-cell destruction and apoptosis.Citation190
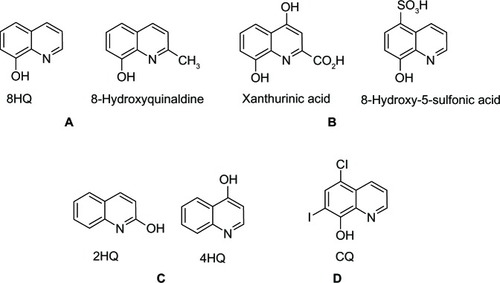
A series of 8HQ and derivatives containing OH groups at different positions on the quinoline ring (ie, 2HQ and 4HQ) as well as ionizable functional groups (ie, CO2H and SO3H) and polar amino groups were investigated in vitro.Citation190 The results showed that 8HQ and 8-hydroxyquinaldine () displayed diabetogenic effects and strongly induced FOXO1 phosphorylation in the presence of Zn2+. However, all 8HQ derivatives with substituents at C-2 (CO2H) and C-5 (SO3H) () positions showed no diabetogenicity, and no induction of FOXO1 phosphorylation. This could be attributed to anionic charge groups (CO2 − and SO3 −) that inhibited their penetration into the cell and specific compartments. Furthermore, 2HQ and 4HQ () displayed no diabetogenic effects and no induction of FOXO1 phosphorylation. This suggested that the OH group in the C-8 position on the quinoline ring was crucial for the observed diabetogenic effect. Interestingly, CQ (), a derivative of 8HQ-bearing substitutions with hydrophobic groups at C-5 (chlorine) and C-7 (iodine) positions induced FOXO1 phosphorylation without diabetogenic effects. The phosphorylation of FOXO1 arises from its CQ property as the Zn ionophore. It was suggested that increased hydrophobicity alone is unlikely to account for the nondiabetogenicity of CQ; however, other factors such as electronic and steric effects may be involved in the lack of diabetogenic effects.
Figure 19 8HQ derivatives with different substitutions.
According to IIS regulatory roles on glucose homeostasis, an ideal antidiabetic agent should be capable of inducing FOXO1 phosphorylation as well as minimizing oxidative damage to β-cells. Recent studies suggested that the scope of drug design may focus on small Zn-binding FOXO1 regulators targeting lipophilic compounds that do not release H+ after Zn-binding.Citation191 Therefore, CQ is a potential candidate for DM treatment.Citation190
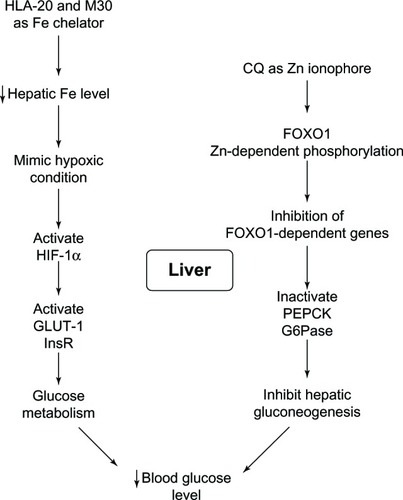
Antidiabetic activity of other 8HQ derivatives, such as M30 and HLA-20, have been reported to function through different mechanisms.Citation34,Citation152 The liver is the center of Fe homeostasis, and hepatic enzymes are found to be key regulators of hepatic gluconeogenesis.Citation34 Glucose metabolism is directly controlled by hepatic factors that in turn are regulated by Fe levels, as is the case for the glucose transporter and the insulin receptor.Citation34 As Fe chelators, M30 and HLA-20 generate low levels of Fe, thereby mimicking hypoxic conditions.Citation34 Such conditions increase the expression of
regulated hypoxia inducible factor. The activation of hypoxia inducible factor leads to the expression of downstream effector genes including the glucose transporter and the insulin receptor, which consequently causes lower blood glucose levelsCitation34 as shown in .
Figure 20 Liver functions on controlling glucose metabolism and antidiabetic actions of 8-hydroxyquinoline derivatives.
In addition, both compounds exhibit protective effects against oxidative damage to β-cells.Citation152 A study on pancreatic β-cell lines indicated that cytoprotective effects of HLA-20 and M30 arise from combined actionsCitation152 in which they act as Fe chelators. M30 and HLA-20 directly decrease ROS formation by inhibiting the Fenton reaction as well as indirectly increasing catalase activity,Citation152 possibly by maintaining nuclear FOXO1 levels.Citation184 Furthermore, both compounds promote antiapoptotic effects against H2O2 stress-induced mitochondrial dysfunction.Citation152 Mitochondria are known to play important roles on cell cycle regulation,Citation152 and their dysfunction is a critical event leading to cell apoptosis.Citation152 M30 and HLA-20 exert antiapoptotic effects by maintenance of a mitochondrial factor called ΔψM, which is necessary for mitochondria survival.Citation152
Due to IIS and the FOXO1 phosphorylation inductive ability and significant cytoprotective effects, 8HQ and its derivatives could be a promising class of compound that merits further development for the treatment of DM.Citation152,Citation190
Conclusion
Metal imbalance plays a crucial role in the etiology of many diseases that affect quality of life. The objectives of treatment are not only aimed at restoring metal balance but also at minimizing cellular damage. 8HQ and its derivatives possess diverse pharmacological and biological activities, which are a result of their chelating ability (). Interestingly, such bioactivities originate from multiple mechanisms (). These mechanisms of actions function in restoring metal homeostasis as well as promoting protective effects. Therefore, 8HQ and its derivatives are considered as promising candidates that should be further developed as therapeutics for many diseases.
Table 1 Brief review of 8-hydroxyquinoline (8HQ) and its derivatives
Table 2 Bioactivities and specific mechanisms of 8-hydroxyquinoline (8HQ) and its derivatives
Acknowledgments
This project was supported by the Office of the Higher Education Commission, Mahidol University, under the National Research Universities Initiative and Annual Governmental Grant of Mahidol University (2556–2558 BE). VP thanks Chanin Nantasenamat for the proofreading of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Short BR Vargas MA Thomas JC O’Hanlon S Enright MC In vitro activity of a novel compound, the metal ion chelating agent AQ+, against clinical isolates of Staphylococcus aureus J Antimicrob Chemother 2006 57 1 104 109 16319182
- Albrecht M Fiege M Osetska O 8-Hydroxyquinolines in metallosupramolecular chemistry Coord Chem Rev 2008 252 8–9 812 824
- Budimir A Metal ions, Alzheimer’s disease and chelation therapy Acta Pharm 2011 61 1 1 14 21406339
- Crichton RR Dexter DT Ward RJ Metal based neurodegenerative diseases-From molecular mechanisms to therapeutic strategies Coord Chem Rev 2008 252 10–11 1189 1199
- Vanparia SF Patel TS Sojitra NA Synthesis, characterization and antimicrobial study of novel 4-{[(8-Hydroxyquinolin-5-yl) methyl]amino}benzenesulfonamide and its oxinates Acta Chim Slov 2010 57 3 600 667
- Rubbo SD Albert A Gibson MI The influence of chemical constitution on antibacterial activity. V. The antibacterial action of 8-hydroxyquinoline (oxine) Br J Exp Pathol 1950 31 3 425 441 14772392
- Crichton RR Dexter DT Ward RJ Brain iron metabolism and its perturbation in neurological diseases J Neural Transm 2011 118 3 301 314 20809066
- Tõugu V Palumaa P Coordination of zinc ions to the key proteins of neurodegenerative diseases: Aβ, APP, α-synuclein and PrP Coord Chem Rev 2012 256 19–20 2219 2224
- Bush AI The metallobiology of Alzheimer’s disease Trends Neurosci 2003 26 4 207 214 12689772
- Bush AI Tanzi RE Therapeutics for Alzheimer’s disease based on the metal hypothesis Neurotherapeutics 2008 5 3 421 432 18625454
- Gaeta A Hider RC The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy Br J Pharmacol 2005 146 8 1041 1059 16205720
- Zatta P Drago D Bolognin S Sensi SL Alzheimer’s disease, metal ions and metal homeostatic therapy Trends Pharmacol Sci 2009 30 7 346 355 19540003
- Perez LR Franz KJ Minding metals: tailoring multifunctional chelating agents for neurodegenerative disease Dalton Trans 2010 39 9 2177 2187 20162187
- Crouch PJ Barnham KJ Therapeutic redistribution of metal ions to treat Alzheimer’s disease Acc Chem Res 2012 45 9 1604 1611 22747493
- Zheng H Gal S Weiner LM Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition J Neurochem 2005 95 1 68 78 16181413
- Kaur D Yantiri F Rajagopalan S Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease Neuron 2003 37 6 899 909 12670420
- Zheng H Youdim MB Weiner LM Fridkin M Synthesis and evaluation of peptidic metal chelators for neuroprotection in neurodegenerative diseases J Pept Res 2005 66 4 190 203 16138857
- Deraeve C Pitié M Mazarguil H Meunier B Bis-8-hydroxyquinoline ligands as potential anti-Alzheimer agents New J Chem 2007 31 2 193 195
- Ritchie CW Bush AI Mackinnon A Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial Arch Neurol 2003 60 12 1685 1691 14676042
- Cherny RA Atwood CS Xilinas ME Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice Neuron 2001 30 3 665 676 11430801
- Tateishi J Subacute myelo-optico-neuropathy: clioquinol intoxication in humans and animals Neuropathology 2000 20 Suppl S20 S24 11037182
- Yassin MS Ekblom J Xilinas M Gottfries CG Oreland L Changes in uptake of vitamin B(12) and trace metals in brains of mice treated with clioquinol J Neurol Sci 2000 173 1 40 44 10675578
- Budimir A Humbert N Elhabiri M Osinska I Biruš M Albrecht-Gary AM Hydroxyquinoline based binders: promising ligands for chelatotherapy? J Inorg Biochem 2011 105 3 490 496 20926137
- LeVine H Ding Q Walker JA Voss RS Augelli-Szafran CE Clioquinol and other hydroxyquinoline derivatives inhibit Abeta (1-42) oligomer assembly Neurosci Lett 2009 465 1 99 103 19664688
- White AR Du T Laughton KM Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity J Biol Chem 2006 281 26 17670 17680 16648635
- Adlard PA Cherny RA Finkelstein DI Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta Neuron 2008 59 1 43 55 18614028
- Crouch PJ Savva MS Hung LW The Alzheimer’s therapeutic PBT2 promotes amyloid-β degradation and GSK3 phosphorylation via a metal chaperone activity J Neurochem 2011 119 1 220 230 21797865
- Adlard PA Bica L White AR Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease PLoS One 2011 6 3 e17669 21412423
- Lannfelt L Blennow K Zetterberg H PBT2-201-EURO study group Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial Lancet Neurol 2008 7 9 779 786 18672400
- Faux NG Ritchie CW Gunn A PBT2 rapidly Improves cognition in Alzheimer’s disease: additional Phase II analyses J Alzheimers Dis 2010 20 2 509 516 20164561
- Crouch PJ Tew DJ Du T Restored degradation of the Alzheimer’s amyloid-beta peptide by targeting amyloid formation J Neurochem 2009 108 5 1198 1207 19141082
- Gal S Fridkin M Amit T Zheng H Youdim MBH M30, a novel multifunctional neuroprotective drug with potent iron chelating and brain selective monoamine oxidase-ab inhibitory activity for Parkinson’s disease J Neural Transm Suppl 2006 70 447 456 17017567
- Kasarskis EJ Tandon L Lovell MA Ehmann WD Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study J Neurol Sci 1995 130 2 203 208 8586987
- Pollak Y Mechlovich D Amit T Effects of novel neuroprotective and neurorestorative multifunctional drugs on iron chelation and glucose metabolism J Neural Transm 2013 120 1 37 48 22446839
- Riederer P Sofic E Rausch WD Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains J Neurochem 1989 52 2 515 520 2911028
- Paris I Martinez-Alvarado P Cárdenas S Dopamine-dependent iron toxicity in cells derived from rat hypothalamus Chem Res Toxicol 2005 18 3 415 419 15777081
- Zheng H Weiner LM Bar-Am O Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer’s, Parkinson’s, and other neurodegenerative diseases Bioorg Med Chem 2005 13 3 773 783 15653345
- Avramovich-Tirosh Y Amit T Bar-Am O Zheng H Fridkin M Youdim MB Therapeutic targets and potential of the novel brain-permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer’s disease J Neurochem 2007 100 2 490 502 17144902
- Avramovich-Tirosh Y Bar-Am O Amit T Youdim MB Weinreb O Up-regulation of hypoxia-inducible factor (HIF)-1α and HIF-target genes in cortical neurons by the novel multifunctional iron chelator anti-Alzheimer drug, M30 Curr Alzheimer Res 2010 7 4 300 306 20043814
- Kupershmidt L Weinreb O Amit T Mandel S Carri MT Youdim MB Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis FASEB J 2009 23 11 3766 3779 19638399
- Weinreb O Mandel S Bar-Am O Amit T Iron-chelating backbone coupled with monoamine oxidase inhibitory moiety as novel pluripotential therapeutic agents for Alzheimer’s disease: a tribute to Moussa Youdim J Neural Transm 2011 118 3 479 492 21360301
- Youdim MB Weinstock M Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation Neurotoxicology 2004 25 1–2 243 250 14697899
- Stevens RG Kalkwarf DR Iron, radiation, and cancer Environ Health Perspect 1990 87 291 300 2269234
- Brem S Angiogenesis and cancer control: from concept to therapeutic trial Cancer Control 1999 6 5 436 458 10758576
- Brewer GJ Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson’s disease Exp Biol Med (Maywood) 2001 226 7 665 673 11444102
- Theophanides T Anastassopoulou J Copper and carcinogenesis Crit Rev Oncol Hematol 2002 42 1 57 64 11923068
- Daniel KG Harbach RH Guida WC Dou QP Copper storage diseases: Menkes, Wilsons, and cancer Front Biosci 2004 9 2652 2662 15358588
- Gupte A Mumper RJ Elevated copper and oxidative stress in cancer cells as a target for cancer treatment Cancer Treat Rev 2009 35 1 32 46 18774652
- Andrews NC Disorders of iron metabolism N Engl J Med 1999 341 26 1986 1995 10607817
- Le NT Richardson DR The role of iron in cell cycle progression and the proliferation of neoplastic cells Biochim Biophys Acta 2002 1603 1 31 46 12242109
- Halliwell B Gutteridge JM Cross CE Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 1992 119 6 598 620 1593209
- Cerutti PA Oxy-radicals and cancer Lancet 1994 344 8926 862 863 7916406
- Leanderson P Tagesson C Rapid and sensitive detection of hydroxyl radicals formed by activated neutrophils in the presence of chelated iron: hydroxylation of deoxyguanosine to 8-hydroxydeoxyguanosine Agents Actions 1992 36 1–2 50 57 1414689
- Leanderson P Tagesson C Iron bound to the lipophilic iron chelator, 8-hydroxyquinoline, causes DNA strand breakage in cultured lung cells Carcinogenesis 1996 17 3 545 550 8631142
- Jonas SK Riley PA The effect of ligands on the uptake of iron by cells in culture Cell Biochem Funct 1991 9 4 245 253 1725507
- Schraufstatter IU Hinshaw DB Hyslop PA Spragg RG Cochrane CG Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide J Clin Invest 1986 77 4 1312 1320 2937805
- Zhou ZH Ando S Furutsuka D Ikebe M Characterization of Ca2+/calmodulin-dependent protein kinase II from smooth muscle Biochem J 1995 310 Pt 2 517 525 7654190
- Hecht SM DNA strand scission by activated bleomycin group antibiotics Fed Proc 1986 45 12 2784 2791 2429877
- Shaw AY Chang CY Hsu MY Synthesis and structure-activity relationship study of 8-hydroxyquinoline-derived Mannich bases as anticancer agents Eur J Med Chem 2010 45 7 2860 2867 20359788
- Lescoat G Léonce S Pierré A Gouffier L Gaboriau F Antiproliferative and iron chelating efficiency of the new bis-8-hydroxyquinoline benzylamine chelator S1 in hepatocyte cultures Chem Biol Interact 2012 195 2 165 172 22197641
- Moor EC In methods in enzymology Colowick SP Kaplan ND Method in Enzymology New York, NY Academic 1967 12 Pt A 155
- Yamato M Hashigaki K Yasumoto Y Synthesis and antitumor activity of tropolone derivatives. 6. Structure-activity relationships of antitumor-active tropolone and 8-hydroxyquinoline derivatives J Med Chem 1987 30 10 1897 1900 3116257
- Prachayasittikul S Worachartcheewan A Pingaew R Metal complexes of uracil derivatives with cytotoxicity and superoxide scavenging activity Lett Drug Des Dis 2012 9 3 282 287
- Boerner LJ Zaleski JM Metal complex-DNA interactions: from transcription inhibition to photoactivated cleavage Curr Opin Chem Biol 2005 9 2 135 144 15811797
- Colak A Terzi Ü Col M DNA binding, antioxidant and antimicrobial activities of homo- and heteronuclear copper(II) and nickel(II) complexes with new oxime-type ligands Eur J Med Chem 2010 45 11 5169 5175 20817331
- Mathur S Tabassum S New homodi-and heterotrinuclear metal complexes of Schiff base compartmental ligand: Interaction studies of copper complexes with calf thymus DNA Cent Eur J Chem 2006 4 3 502 522
- Cowan JA Chemical nucleases Curr Opin Chem Biol 2001 5 6 634 642 11738172
- Tan J Wang B Zhu L DNA binding, cytotoxicity, apoptotic inducing activity, and molecular modeling study of quercetin zinc(II) complex Bioorg Med Chem 2009 17 2 614 620 19097913
- Dixit RB Patel TS Vanparia SF Kunjadiya AP Keharia HR Dixit BC DNA-binding interaction studies of microwave assisted synthesized sulfonamide substituted 8-hydroxyquinoline derivatives Sci Pharm 2011 79 2 293 308 21773067
- Xu H Zheng KC Chen Y Effects of ligand planarity on the interaction of polypyridyl Ru(II) complexes with DNA Dalton Trans 2003 11 2260 2268
- Delaney S Pascaly M Bhattacharya PK Han K Barton JK Oxidative damage by ruthenium complexes containing the dipyridophenazine ligand or its derivatives: a focus on intercalation Inorg Chem 2002 41 7 1966 1974 11925195
- Siddiqi ZA Khalid M Kumar S Shahid M Noor S Antimicrobial and SOD activities of novel transition metal complexes of pyridine-2,6-dicarboxylic acid containing 4-picoline as auxiliary ligand Eur J Med Chem 2010 45 1 264 269 19897283
- Serbest K Colak A Güner S Karaböcek S Kormali F Copper(II)– manganese(II) complexes of 3,3′-(1,3-propanediyldiimine)bis-(3-methyl-2-butanone)dioxime with superoxide dismutase-like activity Transit Metal Chem 2001 26 6 625 629
- Saheki T Ueda A Hosoya M Qualitative and quantitative abnormalities of argininosuccinate synthetase in citrullinemia Clin Chim Acta 1981 109 3 325 335 6784969
- Nayak SB Bhat VR Upadhyay D Udupa SL Copper and ceruloplasmin status in serum of prostate and colon cancer patients Indian J Physiol Pharmacol 2003 47 1 108 110 12708132
- Huang YL Sheu JY Lin TH Association between oxidative stress and changes of trace elements in patients with breast cancer Clin Biochem 1999 32 2 131 136 10211630
- Rizk SL Sky-Peck HH Comparison between concentrations of trace elements in normal and neoplastic human breast tissue Cancer Res 1984 44 11 5390 5394 6488192
- Turecký L Kalina P Uhliková E Námerová S Krizko J Serum ceruloplasmin and copper levels in patients with primary brain tumors Klin Wochenschr 1984 62 4 187 189 6323815
- Chen D Cui QC Yang H Clioquinol, a therapeutic agent for Alzheimer’s disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts Cancer Res 2007 67 4 1636 1644 17308104
- Ding WQ Lind SE Metal ionophores – an emerging class of anticancer drugs IUBMB Life 2009 61 11 1013 1018 19859983
- Daniel KG Chen D Orlu S Cui QC Miller FR Dou QP Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells Breast Cancer Res 2005 7 6 R897 R908 16280039
- Mao X Li X Sprangers R Clioquinol inhibits the proteasome and displays preclinical activity in leukemia and myeloma Leukemia 2009 23 3 585 590 18754030
- Lind SE Park JS Drexler JW Pyrithione and 8-hydroxyquinolines transport lead across erythrocyte membranes Transl Res 2009 154 3 153 159 19665691
- Ding WQ Liu B Vaught JL Yamauchi H Lind SE Anticancer activity of the antibiotic clioquinol Cancer Res 2005 65 8 3389 3395 15833873
- Yu H Zhou Y Lind SE Ding WQ Clioquinol targets zinc to lysosomes in human cancer cells Biochem J 2009 417 1 133 139 18764784
- Andersson DA Gentry C Moss S Bevan S Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+ Proc Natl Acad Sci U S A 2009 106 20 8374 8379 19416844
- Ruschak AM Slassi M Kay LE Schimmer AD Novel proteasome inhibitors to overcome bortezomib resistance J Natl Cancer Inst 2011 103 13 1007 1017 21606441
- Geraki K Farquharson MJ Bradley DA Concentrations of Fe, Cu and Zn in breast tissue: a synchrotron XRF study Phys Med Biol 2002 47 13 2327 2339 12164590
- Dìez M Cerdàn FJ Arroyo M Balibrea JL Use of the copper/zinc ratio in the diagnosis of lung cancer Cancer 1989 63 4 726 730 2914279
- Yoshida D Ikeda Y Nakazawa S Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors J Neurooncol 1993 16 2 109 115 8289088
- Daniel KG Gupta P Harbach RH Guida WC Dou QP Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells Biochem Pharmacol 2004 67 6 1139 1151 15006550
- Dorkin TJ Neal DE Basic science aspects of prostate cancer Semin Cancer Biol 1997 8 1 21 27 9299578
- Arnold JT Isaacs JT Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault Endocr Relat Cancer 2002 9 1 61 73 11914183
- Yu H Lou JR Ding WQ Clioquinol independently targets NF-kappaB and lysosome pathways in human cancer cells Anticancer Res 2010 30 6 2087 2092 20651355
- Ding WQ Yu HJ Lind SE Zinc-binding compounds induce cancer cell death via distinct modes of action Cancer Lett 2008 271 2 251 259 18639975
- Jiang H Taggart JE Zhang X Benbrook DM Lind SE Ding WQ Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline) Cancer Lett 2011 312 1 11 17 21899946
- Arbiser JL Kraeft SK van Leeuwen R Clioquinol-zinc chelate: a candidate causative agent of subacute myelo-optic neuropathy Mol Med 1998 4 10 665 670 9848083
- American Academy of Pediatrics Committee on Drugs: Clioquinol (iodochlorhydroxyquin, vioform) and iodoquinol (diiodohydroxyquin): blindness and neuropathy Pediatrics 1990 86 5 797 798 2146587
- Oliveri V Giuffrida ML Vecchio G Aiello C Viale M Gluconjugates of 8-hydroxyquinolines as potential anti-cancer prodrugs Dalton Trans 2012 41 15 4530 4535 22354329
- Kawamura T Kusakabe T Sugino T Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival Cancer 2001 92 3 634 641 11505409
- Arafa HM Possible contribution of beta-glycosidases and caspases in the cytotoxicity of novel glycoconjugates in colon cancer cells Invest New Drugs 2010 28 3 306 317 19415182
- Pavlov A Takuchev N Georgieva N Drug design by regression analyses of newly synthesized derivatives of 8-quinolinol Biotechnol Biotechnol Equip 2011 26 1 164 169
- Jeon JH Lee CH Lee HS Antimicrobial activities of 2-methyl-8-hydroxyquinoline and its derivatives against human intestinal bacteria J Korean Soc Appl Biol Chem 2009 52 2 202 205
- Ahmed SM Ismail DA Synthesis and biological activity of 8-hydroxyquinoline and 2-hydroxypyridine quaternary ammonium salts J Surfact Deterg 2008 11 3 231 235
- Strobl JS Seibert CW Li Y Inhibition of Toxoplasma gondii and Plasmodium falciparum infections in vitro by NSC3852, a redox active antiproliferative and tumor cell differentiation agent J Parasitol 2009 95 1 215 223 18837587
- Scheibel LW Adler A Antimalarial activity of selected aromatic chelators. III. 8-Hydroxyquinolines (oxines) substituted in positions 5 and 7, and oxines annelated in position 5,6 by an aromatic ring Mol Pharmacol 1982 22 1 140 144 6811856
- Madrid PB Sherrill J Liou AP Weisman JL Derisi JL Guy RK Synthesis of ring-substituted 4-aminoquinolines and evaluation of their antimalarial activities Bioorg Med Chem Lett 2005 15 4 1015 1018 15686903
- Moret V Dereudre-Bosquet N Clayette P Synthesis and anti-HIV properties of new hydroxyquinoline-polyamine conjugates on cells infected by HIV-1 LAV and HIV-1 BaL viral strains Bioorg Med Chem Lett 2006 16 23 5988 5992 17000109
- Darby CM Nathan CF Killing of non-replicating Mycobacterium tuberculosis by 8-hydroxyquinoline J Antimicrob Chemother 2010 65 7 1424 1427 20435781
- Warner VD Musto JD Sane JN Kim KH Grunewald GL Quantitative structure-activity relationships for 5-substituted 8-hydroxyquinolines as inhibitors of dental plaque J Med Chem 1977 20 1 92 96 401891
- Tanzer JM Slee AM Kamay B Scheer E Activity of three 8-hydroxyquinoline derivatives against in vitro dental plaque Antimicrob Agents Chemother 1978 13 6 1044 1045 98105
- Ananthan S Faaleolea ER Goldman RC High-throughput screening for inhibitors of Mycobacterium tuberculosis H37Rv Tuberculosis (Edinb) 2009 89 5 334 353 19758845
- Kharadi GJ Patel JR Dholakiya BZ Antituberculosis, antifungal and thermal activity of mixed ligand transition metal complexes Appl Organomet Chem 2010 24 11 821 827
- Irish D Eltringham I Teall A Control of an outbreak of an epidemic methicillin-resistant Staphylococcus aureus also resistant to mupirocin J Hosp Infect 1998 39 1 19 26 9617681
- Takahashi N Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases Proceedings of the International Symposium for Interface Oral Health, Sendai, Japan, 2–3 February 2005 International Congress Series 2005 1284 103 112
- Rathsam C Eaton RE Simpson CL Two-dimensional fluorescence difference gel electrophoretic analysis of Streptococcus mutans biofilms J Proteome Res 2005 4 6 2161 2173 16335963
- Lemos JA Abranches J Burne RA Responses of cariogenic streptococci to environmental stresses Curr Issues Mol Biol 2005 7 1 95 107 15580782
- Albert A Magrath D The choice of a chelating agent for inactivating trace metals: II. Derivatives of oxine (8-hydroxyquinoline) Biochem. J 1947 41 4 534 530
- Albert A Gibson MI Rubbo SD The influence of chemical constitution on antibacterial activity. VI. The bactericidal action of 8-hydroxyquinoline (oxine) Br J Exp Pathol 1953 34 2 119 130 13051519
- Anjaneyulu Y Rao RP Swamy RY Eknath A Rao KN In vitro antimicrobial-activity studies on the mixed ligand complexes of Hg(II) with 8-hydroxyquinoline and salicylic acids Proc Indian Acad Sci (Chem Sci) 1982 91 2 157 163
- Srisung S Suksrichavalit T Prachayasittikul S Ruchirawat S Prachayasittikul V Antimicrobial activity of 8-hydroxyquinoline and transition metal complexes Int J Pharmacol 2013 9 2 170 175
- Shen AY Chen CP Roffler S A chelating agent possessing cytotoxicity and antimicrobial activity: 7-morpholinomethyl-8-hydroxyquinoline Life Sci 1999 64 9 813 825 10075114
- Badawi AM Mohamed MA Mohamed MZ Khowdairy MM Surface and antitumor activity of some novel metal-based cationic surfactants J Cancer Res Ther 2007 3 4 198 206 18270394
- Rohde W Mikelens P Jackson J Blackman J Whitcher J Levinson W Hydroxyquinolines inhibit ribonucleic acid-dependent deoxyribonucleic acid polymerase and inactivate Rous sarcoma virus and herpes simplex virus Antimicrob Agents Chemother 1976 10 2 234 240 185949
- Auld DS Kawaguchi H Livingston DM Vallee BL RNA-dependent DNA polymerase (reverse transcriptase) from avian myeloblastosis virus: a zinc metalloenzyme Proc Natl Acad Sci U S A 1974 71 5 2091 2095 4134617
- Valenzuela P Morris RW Faras A Levinson W Rutter WJ Are all nucleotidyl transferases metalloenzymes? Biochem Biophys Res Commun 1973 53 3 1036 1041 4125981
- Albert A Quantitative studies of the avidity of naturally occurring substances for trace metals. III. Pteridines, riboflavin and purines Biochem J 1953 54 4 646 654 13058968
- De Clercq E Antiviral drug discovery and development: where chemistry meets with biomedicine Antiviral Res 2005 67 2 56 75 16046240
- De Clercq E HIV-chemotherapy and -prophylaxis: new drugs, leads and approaches Int J Biochem Cell Biol 2004 36 9 1800 1822 15183346
- Hatse S Princen K Gerlach LO Mutation of Asp(171) and Asp(262) of the chemokine receptor CXCR4 impairs its coreceptor function for human immunodeficiency virus-1 entry and abrogates the antagonistic activity of AMD3100 Mol Pharmacol 2001 60 1 164 173 11408611
- Gilles HM Management of Severe Malaria: A Practical Handbook 2nd ed Geneva World Health Organization 2000
- Coatney GR Cooper WC Eddy NB Greengerg J Survey of antimalarial agents: chemotherapy of Plasmodium gallinaceum infections; toxicity; correlation of structure and action Public Health Monogr 1953 9 1 322 13037947
- De D Krogstad FM Cogswell FB Krogstad DJ Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro Am J Trop Med Hyg 1996 55 6 579 583 9025680
- Scheibel LW Adler A Trager W Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum Proc Nat Acad Sci U S A 1979 76 10 5303 5307
- Scheibel LW Adler A Antimalarial activity of selected aromatic chelators Mol Pharmacol 1980 18 2 320 325 6775184
- Scheibel LW Adler A Antimalarial activity of selected aromatic chelators. II. Substituted quinolines and quinoline-N-oxides Mol Pharmacol 1981 20 1 218 223 6793844
- Owens RG Studies on the nature of fungicidal action. I. Inhibition of sulfhydryl-, amino-, iron-, and copper-dependent enzymes in vitro by fungicides and related compounds Contr Boyce Thompson Inst 1953 17 3 221 242
- Denton H Roberts CW Alexander J Thong KW Coombs GH Enzymes of energy metabolism in the bradyzoites and tachyzoites of Toxoplasma gondii FEMS Microbiol Lett 1996 137 1 103 108 8935663
- McCarthy SM Davis CD Prooxidant diet provides protection during murine infection with Toxoplasma gondii J Parasitol 2003 89 5 886 894 14627133
- Pino P Foth BJ Kwok LY Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii PLoS Pathog 2007 3 8 e115 17784785
- Martirosyan A Leonard S Shi X Griffith B Gannett P Strobl J Actions of a histone deacetylase inhibitor NSC3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells J Pharmacol Exp Ther 2006 317 2 546 552 16497787
- Henry TR Wallace KB Differential mechanisms of cell killing by redox cycling and arylating quinones Arch Toxicol 1996 70 8 482 489 8783811
- Reis DC Pinto MC Souza-Fagundes EM Investigation on the pharmacological profile of antimony(III) complexes with hydroxyquinoline derivatives: anti-trypanosomal activity and cytotoxicity against human leukemia cell lines Biometals 2011 24 4 595 601 21221718
- Halliwell B Gutteridge JM Oxygen toxicity, oxygen radicals, transition metals and disease Biochem J 1984 219 1 1 14 6326753
- Valko M Morris H Cronin MT Metals, toxicity and oxidative stress Curr Med Chem 2005 12 10 1161 1208 15892631
- Mau JL Lin HC Song SF Antioxidant properties of several specialty mushrooms Food Res Int 2002 35 6 519 526
- Roby MHH Sarhan MA Selim KAH Khalel KI Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts Ind Crops Prod 2013 43 827 831
- Ma L Liu Z Zhou B Yang L Liu Z Inhibition of free radical induced oxidative hemolysis of red blood cells by green tea polyphenols Chin Sci Bull 2000 45 22 2052 2056
- Prachayasittikul V Prachayasittikul S Ruchirawat S High therapeutic potential of Spilanthes acmella: A review EXCLI J 2013 12 291 312
- Burton GW Ingold KU Vitamin E: application of the principles of physical organic chemistry to the exploration of its structure and function Acc Chem Res 1986 19 7 194 201
- Spiteller G Linoleic acid peroxidation – the dominant lipid peroxidation process in low density lipoprotein – and its relationship to chronic diseases Chem Phys Lipids 1998 95 2 105 162 9853364
- Mechlovich D Amit T Mandel SA The novel multifunctional, iron-chelating drugs M30 and HLA20 protect pancreatic beta-cell lines from oxidative stress damage J Pharmacol Exp Ther 2010 333 3 874 882 20237072
- Fernández-Bachiller MI Pérez C González-Munoz GC Novel tacrine – 8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties J Med Chem 2010 53 13 4927 4937 20545360
- Çolak AT Çolak F Yeşilel OZ Büyükgüngör O Synthesis, spectroscopic, thermal, voltammetric studies and biological activity of crystalline complexes of pyridine-2,6-dicarboxylic acid and 8-hydroxyquinoline J Mol Struct 2009 936 1–3 67 74
- Czapski G Goldstein S Requirements for SOD mimics operating in vitro to work also in vivo Free Radic Res Commun 1991 12–13 Pt 1 167 171 22422014
- Wang Y Yang ZY Wang BD Synthesis, characterization and anti-oxidative activity of cobalt(II), nickel(II) and iron(II) Schiff base complexes Transit Metal Chem 2005 30 879 883
- Suksrichavalit T Prachayasittikul S Nantasenamat C Isarankura-Na-Ayudhya C Prachayasittikul V Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities Eur J Med Chem 2009 44 8 3259 3265 19375194
- Suksrichavalit T Prachayasittikul S Piacham T Isarankura-Na-Ayudhya C Nantasenamat C Prachayasittikul V Copper complexes of nicotinic-aromatic carboxylic acids as superoxide dismutase mimetics Molecules 2008 13 12 3040 3056 19078847
- MacMicking J Xie QW Nathan C Nitric oxide and macrophage function Annu Rev Immunol 1997 15 323 350 9143691
- Kim YH Woo KJ Lim JH 8-Hydroxyquinoline inhibits iNOS expression and nitric oxide production by down-regulating LPS-induced activity of NF-kappaB and C/EBPbeta in Raw 264.7 cells Biochem Biophys Res Commun 2005 329 2 591 597 15737626
- Alderton WK Cooper CE Knowles RG Nitric oxide synthases: structure, function and inhibition Biochem J 2001 357 Pt 3 593 615 11463332
- Rao KM Molecular mechanisms regulating iNOS expression in various cell types J Toxicol Environ Health B Crit Rev 2000 3 1 27 58 10711324
- Lee AK Sung SH Kim YC Kim SG Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation Br J Pharmacol 2003 139 1 11 20 12746218
- Xie QW Kashiwabara Y Nathan C Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase J Biol Chem 1994 269 7 4705 4708 7508926
- Hu H Shih R Rothenberg S Schwartz BS The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues Environ Health Perspect 2007 115 3 455 462 17431499
- Kosnett MJ Becker CE Osterloh JD Kelly TJ Pasta DJ Factors influencing bone lead concentration in a suburban community assessed by noninvasive K x-ray fluorescence JAMA 1994 271 3 197 203 8277545
- Menke A Muntner P Batuman V Silbergeld EK Guallar E Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults Circulation 2006 114 13 1388 1394 16982939
- Shih RA Hu H Weisskopf MG Schwartz BS Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead Environ Health Perspect 2007 115 3 483 492 17431502
- Weisskopf MG Proctor SP Wright RO Cumulative lead exposure and cognitive performance among elderly men Epidemiology 2007 18 1 59 66 17130688
- Bergdahl IA Grubb A Schütz A Lead binding to delta-aminolevulinic acid dehydratase (ALAD) in human erythrocytes Pharmacol Toxicol 1997 81 4 153 158 9353844
- Paglia DE Valentine WN Dahlgren JG Effects of low-level lead exposure on pyrimidine 5′-nucleotidase and other erythrocyte enzymes. Possible role of pyrimidine 5′-nucleotidase in the pathogenesis of lead-induced anemia J Clin Invest 1975 56 5 1164 1169 1184742
- Boas FE Forman L Beutler E Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia Proc Nat Acad Sci U S A 1998 95 6 3077 3081
- Chiu D Lubin B Roelofsen B van Deenen LL Sickled erythrocytes accelerate clotting in vitro: an effect of abnormal membrane lipid asymmetry Blood 1981 58 2 398 401 7248527
- Shin JH Lim KM Noh JY Lead-induced procoagulant activation of erythrocytes through phosphatidylserine exposure may lead to thrombotic diseases Chem Res Toxicol 2007 20 1 38 43 17226925
- Rogan WJ Dietrich KN Ware JH Treatment of Lead-Exposed Children Trial Group The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead N Eng J Med 2001 344 19 1421 1426
- Hamidinia SA Erdahl WL Chapman CJ Steinbaugh GE Taylor RW Pfeiffer DR Monensin improves the effectiveness of meso-dimercaptosuccinate when used to treat lead intoxication in rats Environ Health Perspect 2006 114 4 484 493 16581534
- Sakai T Yanagihara S Kunugi Y Ushio K Mechanisms of ALA-D inhibition by lead and of its restoration by zinc and dithiothreitol Br J Ind Med 1983 40 1 61 66 6824601
- Meredith PA Moore MR Goldberg A Effects of aluminium, lead and zinc on delta-aminolevulinic acid dehydratase Enzyme 1977 22 1 22 27 837892
- Jin S Cheng Y Reid S Li M Wang B Carbohydrate recognition by boronolectins, small molecules, and lectins Med Res Rev 2010 30 2 171 257 19291708
- Phillips MD Fyles TM Barwell NP James TD Carbohydrate sensing using a fluorescent molecular tweezer Chem Commun (Camb) 2009 43 6557 6559 19865648
- Ardá A Venturi C Nativi C A chiral pyrrolic tripodal receptor enantioselectively recognizes beta-mannose and beta-mannosides Chemistry 2010 16 2 414 418 19998432
- Davis AP Synthetic lectins Org Biomol Chem 2009 7 18 3629 3638 19707662
- Mazik M Geffert C 8-Hydroxyquinoline as a building block for artificial receptors: binding preferences in the recognition of glycopyranosides Org Biomol Chem 2011 9 7 2319 2326 21321767
- Melnik B John SM Schmitz G Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from Laron syndrome Nutr Metab (Lond) 2011 8 1 41 21699736
- Cheng Z White MF Targeting forkhead box O1 from the concept to metabolic diseases: lessons from mouse models Antioxid Redox Signal 2011 14 4 649 661 20615072
- Salih DA Brunet A FoxO transcription factors in the maintenance of cellular homeostasis during aging Curr Opin Cell Biol 2008 20 2 126 136 18394876
- Buteau J Accili D Regulation of pancreatic beta-cell function by the forkhead protein FoxO1 Diabetes Obes Metab 2007 9 Suppl 2 140 146 17919188
- Rains JL Jain SK Oxidative stress, insulin signaling, and diabetes Free Radic Biol Med 2011 50 5 567 575 21163346
- Cameron AR Anil S Sutherland E Harthill J Rena G Zinc-dependent effects of small molecules on the insulin-sensitive transcription factor FOXO1a and gluconeogenic genes Metallomics 2010 2 3 195 203 21069157
- Cameron AR Wallace K Logie L The anti-neurodegenerative agent clioquinol regulates the transcription factor FOXO1a Biochem J 2012 443 1 57 64 22248233
- Kitamura T Nakae J Kitamura Y The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth J Clin Invest 2002 110 12 1839 1847 12488434