 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ocular drug delivery is one of the most commonly used treatment modalities in the management of glaucoma. We have recently proposed the use of gelatin and poly(N-isopropylacrylamide) (PNIPAAm) graft copolymers as biodegradable in situ forming delivery systems for the intracameral administration of antiglaucoma medications. In this study, we further investigated the influence of carrier characteristics on drug delivery performance. The carboxyl-terminated PNIPAAm samples with different molecular weights were synthesized by varying the molar ratio of mercaptoacetic acid (MAA)/N-isopropylacrylamide (NIPAAm) from 0.05 to 1.25, and were determined by end-group titration. The preparation of gelatin-g-PNIPAAm (GN) copolymers from these thermoresponsive polymers was achieved using carbodiimide chemistry. Our results showed that the carboxylic end-capped PNIPAAm of high molecular weight may lead to the lower thermal phase transition temperature and slower degradation rate of GN vehicles than its low molecular weight counterparts. With a decreasing MAA/NIPAAm molar ratio, the drug encapsulation efficiency of copolymers was increased due to fast temperature-triggered capture of pilocarpine nitrate. The degradation of the gelatin network could greatly affect the drug release profiles. All of the GN copolymeric carriers demonstrated good corneal endothelial cell and tissue compatibility. It is concluded that different types of GN-based delivery systems exhibit noticeably distinct intraocular pressure-lowering effect and miosis action, thereby reflecting the potential value of a MAA/NIPAAm molar ratio in the development of new antiglaucoma formulations.
Introduction
Drug delivery is one of the most commonly used treatment modalities for health-related disorders.Citation1 For successful therapy in patients, the drug should be delivered to the proper sites at adequate levels and released in a controlled manner over a desired time period. The polymeric drug delivery system (DDS) has gained increasing interest because of its potential importance to drug formulation.Citation2 The use of delivery carriers made of naturalCitation3–Citation5 or syntheticCitation6–Citation8 materials is able to increase the action of drug, thereby achieving a greater pharmacological response. It may also improve the treatment efficacy of a drug and reduce its associated side effects.
Stimuli-responsive polymers are a class of materials that exhibit relatively large and abrupt phase changes in response to small variations in environmental factors such as pH and temperature.Citation9 The changes in the polymer properties are due to the balance of competing interactions between electrostatic forces and hydrophobic dehydration,Citation10 which may act as triggers in controlling cell behavior and drug delivery. Poly(N-isopropylacrylamide) (PNIPAAm) is a well-known thermoresponsive polymer that undergoes a phase transition at 32°C (ie, the lower critical solution temperature [LCST]).Citation11 In our laboratory, PNIPAAm has been used for the fabrication of an intelligent cell culture system.Citation12–Citation14 Human corneal endothelial cells can adhere and proliferate on the hydrophobic PNIPAAm-grafted surfaces at body temperature, and spontaneously detach from the hydrophilic substrates when the surrounding temperature is reduced to a level below the LCST. Furthermore, we recently examined the role of particle size and chemical composition in the determination of in vitro biocompatibility of magnetic thermoresponsive nanohydrogel particles of poly(NIPAAm-co-acrylic acid) with Fe3O4 cores.Citation15 These nanohydrogel carriers may exhibit controlled drug release to a target tissue under magnetic fields.
PNIPAAm-based ophthalmic formulations have been reported in the literature. In 2003, investigators have considered the design of in situ thermosensitive drug vehicles for glaucoma therapy.Citation16 The polymeric eye drop containing an antiglaucoma drug was composed of linear poly(NIPAAm-g-2-hydroxyethyl methacrylate) and its gel particles, as well as epinephrine. A study from Liu and SheardownCitation17 has shown that a composite interpenetrating network of poly(dimethyl siloxane) and PNIPAAm with high glucose permeability and great mechanical strength can potentially be developed as a carrier for controlled drug release applications. Cao et alCitation18 have demonstrated that the PNIPAAm-chitosan gel-forming solution of timolol maleate may have a stronger capacity to reduce intraocular pressure (IOP) than a conventional eye drop of the same concentration over a period of 12 hours. Given that the PNIPAAm is essentially not biodegradable and possibly causes side effects in the body, we have recently proposed the concept of using gelatin-g-PNIPAAm (GN) copolymers as biodegradable in situ-forming delivery systems for intracameral administration of antiglaucoma medications.Citation19 By eliminating the need for surgical removal of foreign materials, the bioerodible graft copolymeric carriers can minimize undesirable tissue reactions following drug administration and can achieve sustained release of the drugs for a longer period of time.
It has been reported that the internal structure of PNIPAAm–gelatin gels and the viability of smooth muscle cells encapsulated in the gelling scaffolds strongly depend on the graft architecture, such as the molecular weight of the graft chain.Citation20 These findings reflect the important role of the molecular weight of PNIPAAm in determining the performance of temperature-triggered in situ-forming hydrogels for biomedical applications. Although the biodegradable and thermoresponsive GN materials may have potential for use as an injectable depot formulation for intraocular drug delivery,Citation19 the effect of the molecular weight of the thermoresponsive polymer segment on the gel-forming and controlled-release properties of copolymers remains to be investigated, and this motivates the research in this paper.
For the preparation of the in situ gelling delivery system, mercaptoacetic acid (MAA), a carboxylic acid-containing chain transfer agent, is frequently used in the free radical polymerization of carboxyl-terminated PNIPAAm.Citation21 Thermoresponsive polymers of different molecular weights can be obtained by synthesis in the presence of varying amounts of MAA. Therefore, here we reported the preparation, characterization, and performance evaluation of GN copolymers in an attempt to clarify the influence of the MAA/NIPAAm molar ratio on the development of drug carriers for ophthalmic drug delivery. After determination of their molecular weights, the carboxyl-terminated PNIPAAm samples were reacted with aminated gelatin to form various GN copolymers. Phase transition characterizations were performed to study the LCST of the samples. The extent of the degradation of the GN materials was measured in the presence of matrix metalloproteinase. Drug encapsulation levels and release profiles were evaluated in vitro under physiological conditions. Biocompatibility of copolymeric carriers was assessed through live/dead fluorescence staining with the use of corneal endothelial cells (in vitro) and by intracameral implantation into rabbits (in vivo). Additionally, the performance of biodegradable in situ forming delivery systems was examined by using a rabbit glaucoma model. After injection of GN-containing pilocarpine solutions into the ocular anterior chamber, the therapeutic efficacy was evaluated based on changes in IOP and pupil diameter.
Materials and methods
Materials
Gelatin (type A; 300 Bloom), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC), adipic acid dihydrazide (ADH), matrix metalloproteinase-2 (MMP-2; EC 3.4.24.24), pilocarpine nitrate, and α-chymotrypsin were purchased from Sigma-Aldrich (St Louis, MO, USA). N-isopropylacrylamide (NIPAAm), and N-hydroxysuccinimide were supplied by Acros Organics (Geel, Belgium). In addition, 2,2′-azobisisobutyronitrile (AIBN) was obtained from Otsuka Chemical Co, Ltd (Tokyo, Japan). Before use, NIPAAm and AIBN were purified by recrystallization from n-hexane and methanol, respectively. MAA supplied from Nacalai Tesque, Inc (Kyoto, Japan) was purified by distillation under reduced pressure. Moreover, 1-hydroxybenzotriazole hydrate was purchased from Chem-Impex International, Inc (Wood Dale, IL, USA). Deionized water used was purified with a Milli-Q system (EMD Millipore, Billerica, MA, USA). Then, 2-(N-morpholino) ethanesulfonic acid (MES; JT Baker® Inc, Phillipsburg, NJ, USA) was dissolved in deionized water to form a 0.1 M buffer solution (pH 5.0). Balanced salt solution (BSS; pH 7.4) was obtained from Alcon Laboratories (Fort Worth, TX, USA). Phosphate buffered saline (PBS; pH 7.4) was acquired from Biochrom AG Biotechnologie (Berlin, Germany). Dulbecco’s Modified Eagle’s Medium was purchased from Gibco® (Life Technologies, Carlsbad, CA, USA). Fetal bovine serum and the antibiotic/antimycotic solution (10,000 U/mL of penicillin, 10 mg/mL of streptomycin, and 25 μg/mL of amphotericin B) were obtained from Biological Industries Israel Beit Haemek Ltd (Kibbutz Beit Haemek, Israel). All the other chemicals were of reagent grade and used as received without further purification.
Synthesis of gelatin-g-PNIPAAm
The aminated gelatin was synthesized according to the protocols as described elsewhere.Citation19 In brief, an aqueous solution was obtained by dissolution of 1 g of gelatin and 2.36 g of adipic acid dihydrazide in 100 mL of deionized water. Then, 2.79 g of EDC and 1.83 g of 1-hydroxybenzotriazole hydrate were dissolved in dimethyl sulfoxide/H2O (1:1 v/v; 6.5 mL each) and added to the above reaction mixture. The pH of the solution was adjusted to 5.0 by 1 N HCl. The reaction was allowed to proceed at 25°C. After 24 hours, the preparation was exhaustively dialyzed (MWCO 3500, Spectra/Por® dialysis membrane; Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA) against deionized water for 3 days. Subsequently, NaCl was added to produce a 5% w/v solution and the aminated gelatin was precipitated in ethanol. After dissolution in deionized water, the precipitate was dialyzed for another 3 days to remove the salt. The purified product was lyophilized at −50°C and kept at 4°C.
The carboxylic end-capped PNIPAAm was synthesized according to the protocols, as described elsewhere.Citation19 In brief, 50 g of NIPAAm, 0.36 g of AIBN, and 1.535–38.375 mL of MAA (equivalent to a MAA/NIPAAm molar ratio of 0.05–1.25) were added to a four-necked flask and dissolved in 250 mL of benzene. The solution was bubbled with a nitrogen atmosphere for 10 minutes, and stirred at 60°C for 24 hours under nitrogen. After polymerization, the reaction product was dissolved in acetone and precipitated from diethyl ether. To remove residual monomers and reagents, the carboxylic end-capped PNIPAAm was then exhaustively dialyzed (MWCO 3500, Spectra/Por® dialysis membrane) against deionized water at 4°C for 7 days. The purified product was lyophilized at −50°C and kept in a closed vessel at room temperature. In this study, the carboxylic end-capped PNIPAAm obtained at a MAA/NIPAAm molar ratio of 0.05 was designated as M/N005.
The number–average molecular weight (Mn) of carboxylic end-capped PNIPAAm (PNIPAAm–COOH) was determined by end-group titration.Citation21 After dissolution of 0.1 g of the test sample in 10 mL of deionized water, the solution was titrated with 0.01 N NaOH to determine the concentration of the carboxyl end group. The Mn of carboxylic end-capped PNIPAAm was calculated according to the moles of the carboxyl end groups of PNIPAAm–COOH. Results were the average of four independent measurements.
The GN was synthesized by grafting carboxylic end-capped PNIPAAm onto the aminated gelatin using carbodiimide chemistry. In brief, each type of carboxylic end-capped PNIPAAm was dissolved in 50 mL of MES buffer containing 2.59 g of EDC and 1.55 g of N-hydroxysuccinimide under agitation for 6 hours, followed by the addition of 50 mL of MES buffer containing 1 g of aminated gelatin. The feed molar ratio of the NH2 groups in the aminated gelatin to the COOH groups in the carboxylic end-capped PNIPAAm was controlled at 0.36.Citation19 The reaction was allowed to proceed at 25°C for 24 hours. Then, the reaction product was precipitated at 50°C, followed by centrifugation and resuspension in deionized water. To remove unreacted components, the solution was exhaustively dialyzed (MWCO 50000; Spectra/Por® dialysis membrane) against deionized water at 4°C for 4 days. The GN graft copolymer was lyophilized at −50°C and kept in a closed vessel at room temperature. In this study, the GN prepared from sample M/N005 was designated as G-M/N005.
Phase transition characterizations
The phase transition property of each test sample was investigated using a DSC 2010 differential scanning calorimeter (DSC) (TA Instruments, New Castle, DE, USA). The GN was dissolved in deionized water to a concentration of 10% (w/v). After equilibration at room temperature for 1 hour, the solutions (8 mg) were hermetically sealed in aluminum pans for DSC experiments. Programmed heating was carried out at 3°C/minute in the temperature range of 25°C–45°C under a nitrogen gas flow. The LCST was determined as the onset point of the endothermic peak. Results were averaged on four independent runs.
In vitro degradation tests
To measure the extent of degradation, the GN solutions (10% w/v) were prepared by dissolving the solutes in deionized water at 25°C, and were transferred to a 34°C thermostatically controlled water bath for 10 minutes to allow gelation. Subsequently, the test samples were dried to constant weight (Wi) in vacuo and immersed at 34°C in BSS containing 50 ng/mL of MMP-2. Degradation medium was replaced weekly with fresh buffer solution containing the same concentration of enzyme. At predetermined time intervals, the degraded hydrogels were collected and further dried in vacuo. The dry weight of samples after degradation (Wd) was determined, and the percentage of weight remaining (%) was calculated as follows:Citation22
Results were the average of four independent measurements.
In vitro drug release studies
For the evaluation of drug encapsulation levels, the GN solutions (10% w/v) were prepared by dissolving the solutes in deionized water, mixed with pilocarpine nitrate (2% w/v) at 25°C, and injected into a brown-colored vial containing 1.5 mL of BSS at 34°C. The drug-incorporated hydrogels were formed and then transferred to an empty vial at 25°C. The redissolved polymer solutions were analyzed by high-performance liquid chromatography (HPLC) using a L-2400 ultraviolet detector and L-2130 pump (Hitachi Ltd, Tokyo, Japan) and a Mightysil RP-18 column (4.6 × 250 mm) (Kanto Chemical Co, Inc, Tokyo, Japan). The mobile phase was a mixture of 5% monobasic potassium phosphate in Milli-Q water (pH adjusted to 2.5 with 85% phosphoric acid)/methanol (85:15 v/v) with a flow rate of 0.7 mL/minute. The eluant peak was detected by measuring absorbance at 216 nm. To determine the amount of drug in each sample, photometric reading was referenced to a standard curve of peak area versus pilocarpine nitrate concentration (0.1 to 500 μg/mL). The drug encapsulation efficiency was calculated as the percentage of pilocarpine nitrate entrapped in the polymeric hydrogels, as compared with the amount of initial drug feeding. Results were averaged on four independent runs.
By the temperature-triggered sol–gel phase transition described above, the drug-incorporated GN samples were prepared at 34°C and then transferred to another vial containing 1.5 ml BSS and 75 ng of MMP-2. After incubation at 34°C with reciprocal shaking (60 rpm) in a thermostatically controlled water bath for predetermined time periods, the release buffer was collected and analyzed by HPLC. The concentrations of pilocarpine nitrate released from the polymeric hydrogels were calculated with respect to a calibration curve. Results were the average of four independent measurements.
Biocompatibility studies
The cellular responses to carrier materials were determined according to the protocols as described elsewhere.Citation19 In this study, the bovine corneal endothelial cell line (BCE C/D-1b; ATCC No CRL-2048) was purchased from the American Type Cell Collection (Manassas, VA, USA). The BCE C/D-1b cells were maintained in regular growth medium containing Dulbecco’s Modified Eagle’s Medium, 10% fetal bovine serum, 4 mM L-glutamine, 1.5 mg/mL of sodium bicarbonate, 4.5 mg/mL of glucose, and 1% antibiotic/antimycotic solution. Cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C. The BCE C/D-1b cells with a density of 5 × 104 cells/mL were seeded into 24-well plates by 1 mL/well and incubated overnight to allow cell attachment. Then, the medium was replaced with fresh culture medium. Using cell culture inserts (Falcon 3095; BD, Franklin Lakes, NJ, USA), each well of a 24-well plate was divided into two compartments. Then, 150 μL of sterile GN solution (10% w/v) was added to the inner well of the double-chamber system at 37°C to examine the cultures after 48 hours of exposure to the in situ forming gels. The cells in regular growth medium without test materials served as control groups.
The qualitative and quantitative assays were performed following removal of the inserts and polymer samples. Cell viability was determined using a membrane integrity assay, the LIVE/DEAD® Viability/Cytotoxicity Kit (Life Technologies), which contains calcein acetoxymethyl and ethidium homodimer-1.Citation23,Citation24 The assay depends on the intracellular esterase activity to identify the living cells, which cleaves the calcein acetoxymethyl to produce a green fluorescence. In dead cells, ethidium homodimer-1 can easily pass through the damaged cell membranes to bind to the nucleic acids, yielding a red fluorescence. After washing three times with PBS, the cultures were stained with a working solution consisting of 2 μL of ethidium homodimer-1, 1 mL of PBS, and 0.5 μL of calcein acetoxymethyl. Under fluorescence microscopy (Axiovert 200M; Carl Zeiss Meditec AG, Jena, Germany), three different areas each containing approximately 500 cells were counted at 100× magnification. All experiments were performed in triplicate, and the viability of the BCE C/D-1b cell cultures was expressed as the average ratio of live cells to the total number of cells in these nine different areas.
On the other hand, to determine the tissue–material interaction in the anterior chamber, 24 adult New Zealand White rabbits (National Laboratory Animal Breeding and Research Center, Taipei, Taiwan), weighing from 3.0–3.5 kg and between 16–20 weeks of age, were used for this study. All animal procedures were approved by the Institutional Review Board and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Surgical operation was performed in the single eye of animals, with the normal fellow eye. The rabbits were anesthetized intramuscularly with 2.5 mg/kg body weight of a tiletamine hydrochloride/zolazepam hydrochloride mixture (Zoletil®; Virbac, Carros, France) and 1 mg/kg body weight of xylazine hydrochloride (Rompun; Bayer AG, Leverkusen, Germany), and topically with two drops of 0.5% proparacaine hydrochloride ophthalmic solution (Alcaine; SA Alcon-Couvreur NV, Puurs, Belgium). In the three test groups (M/N005, M/N025, and M/N125) of animals (six rabbits/group), 50 μL of sterile GN solution (10% w/v) was administered by intracameral injection via a 30-gauge needle. The remaining six rabbits injected with BSS only served as a control group. Ophthalmic evaluations were performed before and 2 weeks after surgery. The corneal endothelial cell density (ECD) in rabbit eyes was measured by specular microscopy (Topcon Optical, Tokyo, Japan).Citation25,Citation26 Each data point is an average of three independent observations.
Glaucoma treatment studies
Eighteen adult New Zealand White rabbits (National Laboratory Animal Breeding and Research Center), weighing from 3.0–3.5 kg and between 16–20 weeks of age, were used for this test. After the establishment of the glaucoma animal model, the drugs were applied to the eye. The rabbits were divided into three groups (ie, G-M/N005, G-M/N025, and G-M/N125 groups), consisting of six rabbits each. These glaucomatous eyes received different types of drug-incorporated GN samples.
To produce experimental glaucoma, the rabbits were first anesthetized intramuscularly with 2.5 mg/kg body weight of tiletamine hydrochloride/zolazepam hydrochloride mixture (Zoletil) and 1 mg/kg body weight of xylazine hydrochloride (Rompun). Then, 0.1 mg/mL of α-chymotrypsin was injected into the posterior chamber of the eye by using a 30-gauge needle. The tip of the needle was swept across to homogeneously distribute the enzyme throughout the posterior chamber, and the needle remained in position for an additional 2 minutes before it was withdrawn to avoid any contact of the enzyme with the corneal endothelium. During the first week of follow-up examination, each operated eye received two drops of a tobramycin–dexamethasone ophthalmic solution (Tobradex; SA Alcon-Couvreur NV) and one drop of diclofenac sodium ophthalmic solution (Voltaren®; Novartis International AG, Basel, Switzerland) three times a day to prevent eye inflammation and pain. In this study, the animals were considered to be glaucomatous when the IOP was higher than 20 mmHg in the eye following 4 weeks of α-chymotrypsin injection.
For drug administration to the glaucomatous eye, the anterior chamber was entered by a 30-gauge needle near the limbus, and injected with 50 μL of a mixture of pilocarpine nitrate (2% w/v) and GN solutions (10% w/v). Subsequently, we examined the bilateral eyes of 24 rabbits at predetermined time intervals for 2 weeks. The IOP was measured using a Schiotz tonometer (AMANN Ophthalmic Instruments, Liptingen, Germany), calibrated according to the manufacturer’s instructions.Citation27 For each IOP determination, five readings were taken on each eye, alternating the left and right eyes, and the mean was calculated. The IOP values of the contralateral normal eyes were used as baseline readings. Data were expressed as the difference from baseline values at each time point. The miosis tests were carried out after acclimatization in a room with constant lighting.Citation19 The pupil diameter was measured under standardized conditions using a pupillary diameter gauge (Smith and Nephew Pharmaceuticals, Ltd, Essex, UK). The pupil diameters of the glaucomatous eyes were taken as pretreatment baseline values. The results were presented as the average variation of pupillary diameter at each post-drug-administration time point with respect to basal levels from four independent measurements.
Statistical analysis
Results were expressed as the mean ± standard deviation. Comparative studies of means were performed using one-way analysis of variance. Significance was accepted with P<0.05.
Results and discussion
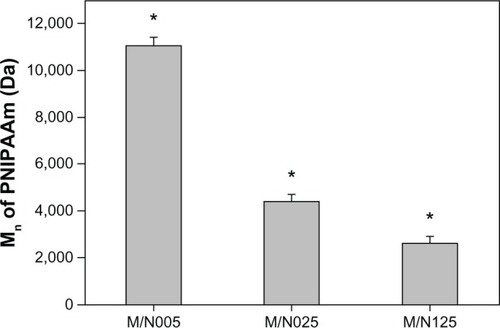
Glaucoma is the second leading cause of blindness after cataracts worldwide.Citation19 It is known that glaucoma patients usually have a documented history of elevated pressure inside the eye (ie, >21 mmHg).Citation28 The pilocarpine eye drop is mainly used to reduce IOP and to treat glaucoma by increasing aqueous outflow via pupil constriction. To improve ocular bioavailability, a GN graft copolymeric carrier has been developed in our laboratory.Citation19 The present study was performed to further delineate the importance of the MAA/NIPAAm molar ratio for the design of new antiglaucoma formulations. First, the carboxylic end-capped PNIPAAm samples with different molecular weights were synthesized by varying the amount of MAA from 1.535 mL to 38.375 mL, and were determined by end-group titration. shows the Mn of various thermoresponsive polymers as a function of the MAA/NIPAAm molar ratio. The number–average molecular weight in the M/N005, M/N025, and M/N125 groups was 11,083 ± 349 Da, 4,436 ± 271 Da, and 2,592 ± 315 Da, respectively. There were significant differences among these groups (P<0.05), indicating that the decrease in Mn is associated with an increased MAA/NIPAAm molar ratio. Takei et alCitation29 have used 3-mercaptopro-pionic acid as a chain transfer agent for the radical oligomerization of NIPAAm and demonstrated that the molecular weight of thermoresponsive polymers can be controlled by the ratio of 3-mercaptopropionic acid to NIPAAm. The present data are compatible with their findings and suggest that the PNIPAAm–COOH with a long chain length is obtained using small amounts of MAA.
Figure 1 Mn of various carboxylic end-capped PNIPAAm samples.
Abbreviations: Mn, number–average molecular weight; PNIPAAm, poly(N-isopropylacrylamide); n, number.
Phase transition characterizations
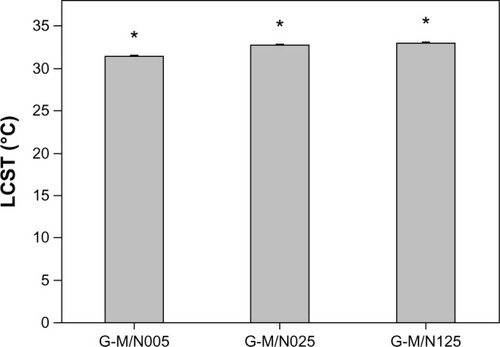
In this work, the GN copolymers are synthesized by the reaction of aminated gelatin with carboxyl-terminated PNIPAAm of varying molecular weights. The temperature-induced changes in hydrophilic–hydrophobic balance of the thermoresponsive drug vehicles were investigated by DSC. shows the LCST of GN prepared from various carboxylic end-capped PNIPAAm samples. The copolymers from the G-M/N125 group had a LCST of 33.0° ± 0.1°C, which was significantly higher than those of the G-M/N005 (31.4° ± 0.2°C) and G-M/N025 (32.7° ± 0.1°C) groups (P<0.05). This finding indicates the potential role of the MAA/NIPAAm molar ratio in governing the molecular weight of thermoresponsive polymer segment for the regulation of phase transition in drug vehicles. Chen and ChengCitation21 have examined the effect of molecular weight on the LCST of the carboxyl-terminated PNIPAAm in deionized water and reported that the increase in molecular weight may decrease the thermal phase transition temperature. Results of the present study further support this view by showing that the LCST of copolymers of gelatin and PNIPAAm–COOH varies as a function of the Mn of the thermoresponsive polymer compound.
Figure 2 LCST of GN prepared from various carboxylic end-capped PNIPAAm samples.
Abbreviations: LCST, lower critical solution temperature; GN, gelatin-g-PNIPAAm; PNIPAAm, poly(N-isopropylacrylamide); n, number.
In vitro degradation tests
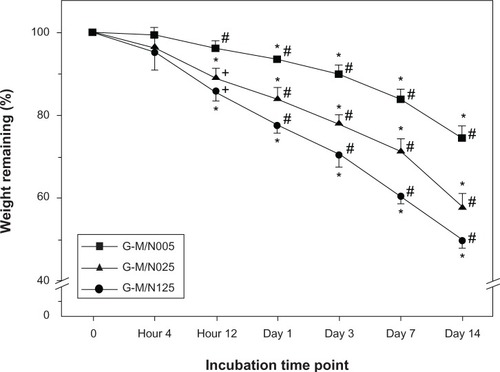
Given that the concentration of MMP-2 in the aqueous humor of glaucoma patients is 50 ng/mL,Citation30 the in vitro degradation tests were performed through the incubation of GN gels in BSS (ie, physiological medium) containing the same concentration of enzyme. shows the weight remaining of various GN samples after degradation at 34°C. During the period ranging from 12 hours to 14 days, the carrier materials from each group gradually degraded and exhibited a significant decrease in weight with time (P<0.05). At the end of the experiment (ie, 14 days), the residual mass percentage in the G-M/N005, G-M/N025, and G-M/N125 groups was 74.7% ± 3.0%, 57.8% ± 3.3%, and 49.6% ± 1.7%, respectively. There were significant differences among these groups (P<0.05), which exhibited distinct extents of degradation of drug vehicles in the presence of MMP-2. The gelatin has received much attention in biomedical fields because of its susceptibility to enzymatic cleavage.Citation31–Citation33 Our previous study has shown that the presence of aggregated PNIPAAm brushes may affect the biodegradability of GN samples under the action of proteinases.Citation19 For the first time, here we demonstrate that the PNIPAAm–COOH with long chain length restricts the access of the enzyme to the active sites of the gelatin chains, thereby leading to slower degradation rates of the graft copolymers.
Figure 3 Time course of the weight remaining of various GN samples after incubation at 34°C in BSS containing MMP-2.
Notes: *Statistically significant differences (P<0.05; n=4) for the mean value of the weight remaining compared to the value at the previous time point. # P<0.05 versus all groups; + P<0.05 versus the G-M/N005 group (compared only within each time point group).
Abbreviations: GN, gelatin-g-poly(N-isopropylacrylamide); BSS, balanced salt solution; MMP-2, matrix metalloproteinase-2; n, number.
In vitro drug release studies
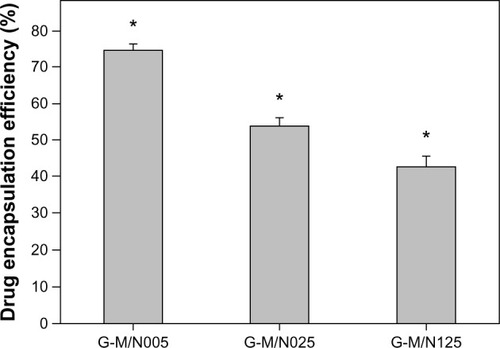
As an ophthalmic in situ gelling delivery system, the synthesized GN material is featured with excellent thermoresponsive characteristics. Therefore, high drug encapsulation efficiency can be achieved through fast temperature-triggered capture of pilocarpine. It is important to determine the drug encapsulation level of GN materials prepared from various carboxylic end-capped PNIPAAm samples. The percentage of pilocarpine nitrate entrapped in the copolymeric hydrogels analyzed by HPLC is presented in . The drug encapsulation efficiency showed significant differences between the G-M/N005 (74.5% ± 1.6%), G-M/N025 (54.1% ± 2.2%), and G-M/N125 groups (42.8% ± 2.9%) (P<0.05). Our results suggest that the variation in the amount of drug entrapped in biodegradable thermosensitive gels is highly correlated with the thermal gelation ability of GN copolymers.
Figure 4 Drug encapsulation efficiency of various GN samples.
Abbreviations: GN, gelatin-g-poly(N-isopropylacrylamide); n, number.
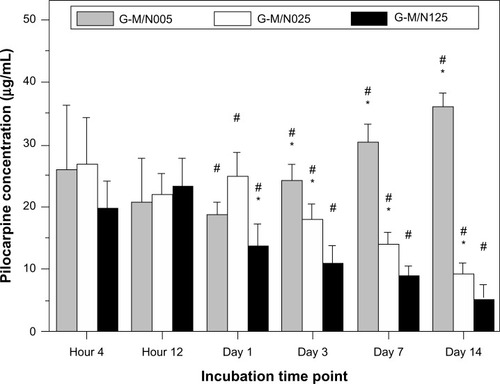
The advantage of using an in situ forming hydrogel as a DDS for therapeutic applications is not only that it is useful for localized drug administration, but also that the carriers can facilitate sustained delivery of drugs over a long period of time.Citation34 In this study, the progressive degradation of the gelatin network may further enhance drug release and bioavailability. The release profiles of pilocarpine nitrate from the biodegradable in situ forming hydrogels were, therefore, investigated. shows the concentration of drug released from various GN samples at 34°C in BSS containing MMP-2. For the G-M/N005 group, the concentration of pilocarpine nitrate at 1 day, 3 days, 7 days, and 14 days of incubation was 18.8 ± 2.0 μg/mL, 24.1 ± 2.6 μg/mL, 30.6 ± 2.8 μg/mL, and 36.0 ± 2.1 μg/mL, respectively, indicating that the amount of released drug was significantly increased with time (P < 0.05). In contrast, for both the G-M/N025 and G-M/N125 groups, the pilocarpine concentration was gradually decreased with the incubation period. At the end of the experiment (ie, at 14 days), the amount of released drug from these GN vehicles reached a relatively low level. In particular, the measured concentration of pilocarpine nitrate in the G-M/N125 group was around 5 μg/mL, which was far below 10–33 μg/mL (ie, the effective concentration range for glaucoma treatment).Citation35 The results of the in vitro release studies of formulations composed of copolymer and pilocarpine nitrate probably reflect the critical role of the degradation of biopolymer backbone in the determination of drug release profiles.
Figure 5 Time course of the concentration of pilocarpine released from various GN samples at 34°C in BSS containing MMP-2.
Notes: *Statistically significant differences (P<0.05; n=4) for the mean value of the pilocarpine concentration compared to the value at the previous time point. # P<0.05 versus all groups (compared only within each time point group).
Abbreviations: GN, gelatin-g-poly(N-isopropylacrylamide); BSS, balanced salt solution; MMP-2, matrix metalloproteinase-2; n, number.
Biocompatibility studies
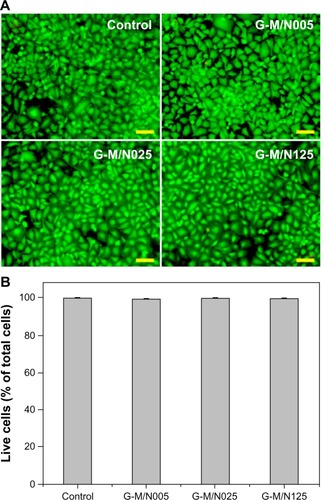
The biomaterials for applications to injectable DDS should be designed to have minimal impact on in vitro and in vivo biocompatibility. In this study, the cellular and tissue responses to the biodegradable in situ gelling system were evaluated by using in vitro cultured corneal endothelial cells and in vivo anterior chamber of the eyes. shows representative fluorescent images of BCE C/D-1b cell cultures, where the live cells fluoresce green and the dead ones fluoresce red. Prominent green fluorescence was found for the control groups, indicating that the cells are almost viable in the absence of the test materials. After a 2-day exposure of the BCE cell cultures to various GN samples, a bright green fluorescence was observed. There were also very few red-stained nuclei present, showing that the cultures were not damaged. shows the results of the quantitative analysis of the mean percentage of live cells. The cell viability did not show a significant difference between the control, G-M/N005, G-M/N025, and G-M/N125 groups (P>0.05) after 2 days in culture. It was noteworthy that these BCE C/D-1b cell lines had a relatively high mean percentage of live cells, suggesting that there was no cytotoxicity of the biodegradable in situ gelling system based on GN.
Figure 6 Cell viability of and mean percentage of live cells in bovine corneal endothelial cell cultures.
Abbreviations: GN, gelatin-g-poly(N-isopropylacrylamide); n, number.
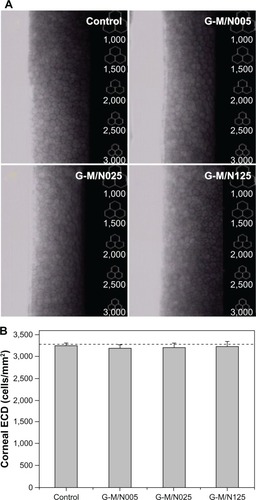
The corneal endothelial cells in rabbit eyes exposed to drug vehicles were further characterized by specular microscopic examinations. shows representative images of rabbit corneal endothelium 2 weeks after intra cameral injection of the GN samples into the ocular anterior chamber. In the control groups, the cells on Descemet’s membrane packed together and exhibited a typical hexagonal shape. Additionally, no change in endothelial cellular hexagonality was found for the G-M/N005, G-M/N025, and G-M/N125 groups. Results of the present study indicate that the rabbits that received BSS or GN injections may have similar corneal endothelial morphological characteristics. shows the results of the quantitative analysis of rabbit corneal endothelium. The mean preoperative ECD was approximately 3,280 cells/mm2; it was not significantly different from that of the control group (3,248 ± 66 cells/mm2) (P>0.05). Furthermore, the corneal ECD in the G-M/N005, G-M/N025, and G-M/N125 groups was 3,186 ± 92 cells/mm2, 3,205 ± 110 cells/mm2, and 3,239 ± 107 cells/mm2, respectively, demonstrating that no significant differences in the endothelial cell count are found between the control and all of the experimental tests (P>0.05). Gelatin is a naturally occurring biopolymer derived from collagen and has already seen medical and pharmaceutical applications.Citation36 The ocular biocompatibility of gelatin has previously been demonstrated in our laboratory.Citation37–Citation40 On the other hand, PNIPAAm-based biomaterials are promising artificial matrices for corneal cell cultivation and regenerative medicine.Citation41 Here, we report the safety of biodegradable and thermoresponsive GN copolymers in the production of ophthalmic drug vehicles.
Figure 7 Typical specular microscope images of rabbit corneal endothelium and specular microscopy measurements of corneal ECD 2 weeks after intracameral injection of various GN samples.
Abbreviations: ECD, endothelial cell density; GN, gelatin-g-poly(N-isopropylacrylamide); BSS, balanced salt solution; n, number.
Glaucoma treatment studies
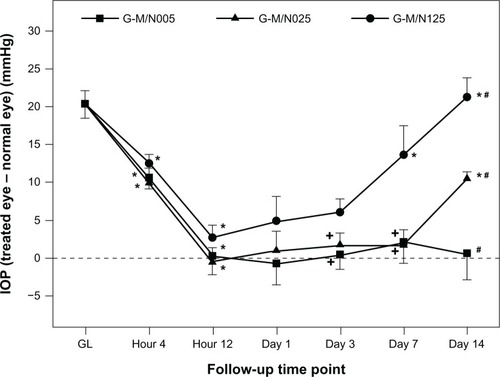
Our group has established an animal model of chronic ocular hypertension by using α-chymotrypsin.Citation19 Given that the α-chymotrypsin injection contributes to the resistance of aqueous humor outflow, the mean IOP values in glaucomatous eyes increased by greater than 20 mmHg (). After intracameral administration of various drug-incorporated GN samples, the IOP was monitored for 2 weeks using a Schiotz indentation tonometer. As shown in , the IOP values for all three experimental groups were significantly decreased within 12 hours (P<0.05) and returned to baseline levels. However, at postoperative day 3, the IOP in the G-M/N125 group was significantly higher than those of the G-M/N005 and G-M/N025 groups (P<0.05). In particular, with increasing follow-up time, a gradual rise in IOP was noted in the G-M/N125 group. A similar phenomenon at later time points (ie, 7–14 days) was also observed in the G-M/N025 group. By contrast, the IOP values remained near normal in the G-M/N005 group until the end of the experiment. Our results indicate that the variation in the IOP-lowering effect may be correlated with the amount of drug released from the biodegradable in situ gelling system. As demonstrated by in vitro drug release studies, the measured concentrations of pilocarpine nitrate at some time points were below 10 μg/mL, which was insufficient to alleviate the IOP elevation. This feature is commonly seen in the cases of either eye drop instillation or free drug injection, which is limited by the relatively short therapeutic action and low drug bioavailability.Citation42
Figure 8 Measurements of IOP after intracameral injection of various GN samples containing pilocarpine in eyes with experimental GL.
P<0.05 versus all groups; +
P<0.05 versus G-M/N125 group (compared only within each time point group).
Abbreviations: IOP, intraocular pressure; GL, glaucoma; GN, gelatin-g-poly(N-isopropylacrylamide); n, number.
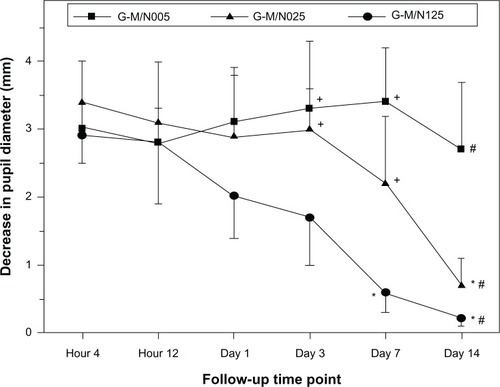
The change in the pupil diameter of rabbits after intracameral injection of various drug-incorporated GN samples was also checked because pilocarpine is a cholinergic agent that has the action of miosis.Citation43 As shown in , the pupil diameter of glaucomatous eyes was significantly decreased 4 hours postoperatively in all groups (P<0.05), indicating that the drug released from the biodegradable in situ gelling system leads to pupillary constriction. For the G-M/N005 group, the intense pharmacological activity was sustained throughout the follow-up period of 2 weeks. However, the duration of miosis in the G-M/N025 group was shorter (7 days). At each time point over the period from 3 days to 14 days, the amplitude of the miotic response in the G-M/N125 group was smaller than that of all other groups (P<0.05). Results of the present study suggest that there is a positive correlation between the decrease in pupil diameter induced by released pilocarpine nitrate and its IOP-lowering effect. The GN graft copolymeric carriers with different drug release profiles exhibited noticeably distinct miosis action, thereby reflecting the importance of the characteristics of biodegradable in situ gelling systems on their treatment performance for glaucoma.
Figure 9 Measurements of pupil diameter after intracameral injection of various GN samples containing pilocarpine in eyes with experimental glaucoma.
Abbreviations: GN, gelatin-g-poly(N-isopropylacrylamide); n, number.
Conclusion
The understanding of the characteristics of biomaterial carriers is particularly important for the design of drug formulations and administration routes relevant to the treatment of glaucoma. In this study, we have evaluated the role of the MAA/NIPAAm molar ratio in the determination of the performance of GN graft copolymers as pilocarpine vehicles. The biodegradable in situ gelling system prepared from carboxyl-terminated PNIPAAm of high molecular weight may have a lower thermal phase transition temperature and slower degradation rate than those from the low molecular weight counterparts. For the G-M/N005 group, high drug encapsulation efficiency can be achieved through fast temperature-triggered capture of pilocarpine nitrate. In addition, the progressive drug release from this carrier enables the measured concentrations of pilocarpine nitrate to rise above the minimum effective concentration for glaucoma treatment throughout the experimental period of 2 weeks. The GN graft copolymeric carriers from all three experimental groups demonstrated good corneal endothelial cell and tissue compatibility. Our findings suggest that different types of GN-based delivery systems exhibit noticeably distinct IOP lowering effects and miosis action, thereby reflecting the potential value of the MAA/NIPAAm molar ratio in the development of new antiglaucoma formulations.
Acknowledgments
This work was supported by grant NSC102-2628-B-182-017-MY3 from the National Science Council of the Republic of China, and grant CMRPD1C0111 from Chang Gung Memorial Hospital (Taoyuan, Taiwan). The author is grateful to Ai-Ching Hsieh (Institute of Biochemical and Biomedical Engineering, Chang Gung University) for providing technical assistance.
Disclosure
The author reports no conflicts of interest in this work.
References
- Roses AD Pharmacogenetics and the practice of medicine Nature 2000 405 6788 857 865 10866212
- Kopeček J Polymer-drug conjugates: origins, progress to date and future directions Adv Drug Deliv Rev 2013 65 1 49 59 23123294
- Anitha A Chennazhi KP Nair SV Jayakumar R 5-flourouracil loaded N,O-carboxymethyl chitosan nanoparticles as an anticancer nanomedicine for breast cancer J Biomed Nanotechnol 2012 8 1 29 42 22515092
- Babu A Jeyasubramanian K Gunasekaran P Murugesan R Gelatin nanocarrier enables efficient delivery and phototoxicity of hypocrellin B against a mice tumour model J Biomed Nanotechnol 2012 8 1 43 56 22515093
- Cover NF Lai-Yuen S Parsons AK Kumar A Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy Int J Nanomedicine 2012 7 2411 2419 22811601
- Sahib MN Abdulameer SA Darwis Y Peh KK Tan YT Solubilization of beclomethasone dipropionate in sterically stabilized phospholipid nanomicelles (SSMs): physicochemical and in vitro evaluations Drug Des Devel Ther 2012 6 29 42
- Deepa G Thulasidasan AK Anto RJ Pillai JJ Kumar GS Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy Int J Nanomedicine 2012 7 4077 4088 22888244
- Li J He Z Yu S Micelles based on methoxy poly(ethylene glycol)-cholesterol conjugate for controlled and targeted drug delivery of a poorly water soluble drug J Biomed Nanotechnol 2012 8 5 809 817 22888752
- Fundueanu G Constantin M Oanea I Harabagiu V Ascenzi P Simionescu BC Prediction of the appropriate size of drug molecules that could be released by a pulsatile mechanism from pH/thermoresponsive microspheres obtained from preformed polymers Acta Biomater 2012 8 3 1281 1289 21945634
- Schmaljohann D Oswald J Jørgensen B Nitschke M Beyerlein D Werner C Thermo-responsive PNiPAAm-g-PEG films for controlled cell detachment Biomacromolecules 2003 4 6 1733 1739 14606903
- Stayton PS Shimoboji T Long C Control of protein-ligand recognition using a stimuli-responsive polymer Nature 1995 378 6556 472 474 7477401
- Lai JY Lu PL Chen KH Tabata Y Hsiue GH Effect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapy Biomacromolecules 2006 7 6 1836 1844 16768405
- Lai JY Chen KH Hsu WM Hsiue GH Lee YH Bioengineered human corneal endothelium for transplantation Arch Ophthalmol 2006 124 10 1441 1448 17030712
- Lai JY Chen KH Hsiue GH Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials Transplantation 2007 84 10 1222 1232 18049106
- Chou FY Lai JY Shih CM Tsai MC Lue SJ In vitro biocompatibility of magnetic thermo-responsive nanohydrogel particles of poly(N-isopropylacrylamide-co-acrylic acid) with Fe3O4 cores: effect of particle size and chemical composition Colloids Surf B Biointerfaces 2013 104 66 74 23298590
- Hsiue GH Chang RW Wang CH Lee SH Development of in situ thermosensitive drug vehicles for glaucoma therapy Biomaterials 2003 24 13 2423 2430 12699680
- Liu L Sheardown H Glucose permeable poly (dimethyl siloxane) poly(N-isopropyl acrylamide) interpenetrating networks as ophthalmic biomaterials Biomaterials 2005 26 3 233 244 15262466
- Cao Y Zhang C Shen W Cheng Z Yu LL Ping Q Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery J Control Release 2007 120 3 186 194 17582643
- Lai JY Hsieh AC A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine Biomaterials 2012 33 7 2372 2387 22182746
- Ohya S Matsuda T Poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as thermoresponsive three-dimensional artificial extracellular matrix: molecular and formulation parameters vs cell proliferation potential J Biomater Sci Polym Ed 2005 16 7 809 827 16128290
- Chen JP Cheng TH Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells Macromol Biosci 2006 6 12 1026 1039 17128421
- Ma DH Lai JY Cheng HY Tsai CC Yeh LK Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells Biomaterials 2010 31 25 6647 6658 20541801
- Lai JY Wang TP Li YT Tu IH Synthesis, characterization and ocular biocompatibility of potential keratoprosthetic hydrogels based on photopolymerized poly(2-hydroxyethyl methacrylate)-co-poly(acrylic acid) J Mater Chem 2012 22 1812 1823
- Lai JY Tu IH Adhesion, phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights Acta Biomater 2012 8 3 1068 1079 22134163
- Lai JY Li YT Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers Biomacromolecules 2010 11 5 1387 1397 20355704
- Lai JY Ma DH Lai MH Li YT Chang RJ Chen LM Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: biopolymer concentration effect PLoS One 2013 8 1 e54058 23382866
- Lai JY Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use J Mater Sci Mater Med 2010 21 6 1899 1911 20238149
- Joffe KM Raymond JE Chrichton A Motion coherence perimetry in glaucoma and suspected glaucoma Vision Res 1997 37 7 955 964 9156192
- Takei YG Aoki T Sanui K Ogata N Okano T Sakurai Y Temperature-responsive bioconjugates. I. Synthesis of temperature-responsive oligomers with reactive end groups and their coupling to biomolecules Bioconjug Chem 1993 4 1 42 46 8431511
- Määttä M Tervahartiala T Harju M Airaksinen J Autio-Harmainen H Sorsa T Matrix metalloproteinases and their tissue inhibitors in aqueous humor of patients with primary open-angle glaucoma, exfoliation syndrome, and exfoliation glaucoma J Glaucoma 2005 14 1 64 69 15650607
- Lai JY Lin PK Hsiue GH Cheng HY Huang SJ Li YT Low bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantation Biomacromolecules 2009 10 2 310 319 19063667
- Lai JY Evaluation of cross-linking time for porous gelatin hydrogels on cell sheet delivery performance J Mech Med Biol 2011 11 967 981
- Lai JY Li YT Cho CH Yu TC Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering Int J Nanomedicine 2012 7 1101 1114 22403490
- Sharma G Italia JL Sonaje K Tikoo K Ravi Kumar MN Biodegradable in situ gelling system for subcutaneous administration of ellagic acid and ellagic acid loaded nanoparticles: evaluation of their antioxidant potential against cyclosporine induced nephrotoxicity in rats J Control Release 2007 118 1 27 37 17258836
- Hsiue GH Guu JA Cheng CC Poly(2-hydroxyethyl methacrylate) film as a drug delivery system for pilocarpine Biomaterials 2001 22 13 1763 1769 11396879
- Young S Wong M Tabata Y Mikos AG Gelatin as a delivery vehicle for the controlled release of bioactive molecules J Control Release 2005 109 1–3 256 274 16266768
- Lu PL Lai JY Tabata Y Hsiue GH A methodology based on the “anterior chamber of rabbit eyes” model for noninvasively determining the biocompatibility of biomaterials in an immune privileged site J Biomed Mater Res A 2008 86 1 108 116 17941023
- Lai JY The role of bloom index of gelatin on the interaction with retinal pigment epithelial cells Int J Mol Sci 2009 10 8 3442 3456 20111679
- Lai JY Li YT Evaluation of cross-linked gelatin membranes as delivery carriers for retinal sheets Mater Sci Eng C Mater Biol Appl 2010 30 5 677 685
- Lai JY Li YT Influence of cross-linker concentration on the functionality of carbodiimide cross-linked gelatin membranes for retinal sheet carriers J Biomater Sci Polym Ed 2011 22 1–3 277 295
- Lai JY Hsiue GH Functional biomedical polymers for corneal regenerative medicine React Funct Polym 2007 67 11 1284 1291
- Guadana R Ananthula HK Parenky A Mitra AK Ocular drug delivery AAPS J 2010 12 3 348 360 20437123
- Bensinger R Shin DH Kass MA Podos SM Becker B Pilocarpine ocular inserts Invest Ophthalmol 1976 15 12 1008 1010 992960