Abstract
Levofloxacin is the synthetic L-isomer of the racemic fluoroquinolone, ofloxacin. It interferes with critical processes in the bacterial cell such as DNA replication, transcription, repair, and recombination by inhibiting bacterial topoisomerases. Levofloxacin has broad spectrum activity against several causative bacterial pathogens of community-acquired pneumonia (CAP). Oral levofloxacin is rapidly absorbed and is bioequivalent to the intravenous formulation such that patients can be conveniently transitioned between these formulations when moving from the inpatient to the outpatient setting. Furthermore, levofloxacin demonstrates excellent safety, and has good tissue penetration maintaining adequate concentrations at the site of infection. The efficacy and tolerability of levofloxacin 500 mg once daily for 10 days in patients with CAP are well established. Furthermore, a high-dose (750 mg) and short-course (5 days) of once-daily levofloxacin has been approved for use in the US in the treatment of CAP, acute bacterial sinusitis, acute pyelonephritis, and complicated urinary tract infections. The high-dose, short-course levofloxacin regimen maximizes its concentration-dependent antibacterial activity, decreases the potential for drug resistance, and has better patient compliance.
Information resources
The medical literature published in any language since 1980 on levofloxacin was searched using PuBMed, MEDLINE, and EMBASE. Additional citations were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also obtained from Ortho-McNeil Janssen Scientific Affairs, LLC (Titusville, NJ).
Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality in adult populations.Citation1–Citation4 The severity and incidence of CAP are significant, especially in the elderly and immunocompromised patients.Citation5–Citation7 CAP affects 6 million people in the US annually.Citation8 Approximately 20% (1.1–1.3 million) of these patients are hospitalizedCitation9 with estimated cost of about US$25,000 per hospitalizationCitation10 resulting in over US$30 billion annual costs for hospitalizations alone; 12% of patients hospitalized for CAP die.Citation9 In patients with severe CAP requiring admission to the intensive care unit (ICU), mortality increases to up to 30%.Citation11–Citation14 The most common cause of CAP is Streptococcus pneumonia.Citation15–Citation18 Other bacterial causes include Haemophilus influenzae, Moraxella catarrhalis, Klebsiella pneumoniae, and the “atypical” CAP pathogens which include Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila.Citation2,Citation17,Citation19–Citation22 Severe CAP, generally requiring admission to the ICU for management, is frequently caused by Staphylococcus aureus and Gram-negative bacilli.Citation13,Citation23–Citation25
Epidemiologic studies reveal that pathogenic organisms are not recovered in >50% of patients exhibiting clinical signs and symptoms of CAP. Thus, microbiological information is frequently unavailable to refine initial empiric antibiotic treatment of CAP in either hospitalized and outpatient settings.Citation9,Citation23,Citation25 The guidelines from the Infectious Diseases Society of America/American Thoracic Society recommend initial empiric therapy with a respiratory fluoroquinolone (eg, levofloxacin 750 mg, moxifloxacin, or gemifloxicin) or a β-lactam plus a macrolide. In adults, fluoroquinolones are recommended for the treatment of CAP caused by penicillin-susceptible S. pneumoniae, penicillin-resistant S. pneumoniae, Legionella pneumophilia, H. influenzae, M. pneumoniae, and C. pneumoniae. Levofloxacin combination therapy with an antipseudomonal β-lactam (or aminoglycoside) should be considered if Pseudomonas aeruginosa infection is a likely cause of pneumonia.Citation24 Antibiotic resistance in S. pneumoniae has been a major problem in the US and worldwide for more than a decade.Citation26 Furthermore, increasing rates of antibiotic resistance (most notably, penicillin, cephalosporin, and macrolide resistance) observed in bacteria that commonly cause CAP have resulted in increased treatment failures and inferior clinical outcomes for many patients with CAP.Citation14,Citation15,Citation27–Citation30 Although there are reports of the emergence of resistance to some fluoroquinolones among S. pneumonia,Citation26 the incidence of levofloxacin-resistant organisms has remained steady with resistance rates of <1% worldwide.Citation31–Citation35
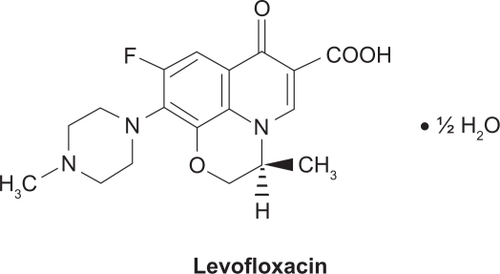
Levofloxacin () is a light yellowish-white crystal or crystalline powder with a molecular weight of 370.38 g/mol. It interferes with critical processes in the bacterial cell, such as DNA replication, transcription, repair, and recombination, by inhibiting bacterial topoisomerases. Human cells lack these topoisomerases, which are essential for bacterial DNA replication, providing specificity against bacterial DNA topoisomerases that are responsible for separating the strands of duplex bacterial DNA, inserting another strand of DNA through the break, and then resealing the originally separated strands.Citation36,Citation37 Levofloxacin is active against a broad range of Gram-positive, Gram-negative, and cell-wall-deficient (atypical) bacteria that may be causative pathogens in community-acquired and nosocomial infections. Levofloxacin is a well-established treatment option for respiratory and urinary tract infections (UTI), particularly since levofloxacin is active against some penicillin – and macrolide-resistant species (eg, S. pneumoniae – the most common causative pathogen for community-acquired bacterial respiratory infections).Citation31–Citation34,Citation38,Citation39 The incidence of penicillin- and macrolide-resistance in many bacterial species is both high and widespread.Citation40 In the US, a high-dose, short-course regimen of levofloxacin (750 mg once daily for 5 days) is approved for the treatment of adults with CAP, acute bacterial sinusitis (ABS), complicated UTI, and acute pyelonephritis (AP). The use of levofloxacin, including some data on the high-dose, short-course treatment regimen, has been reviewed previously.Citation39 This review focuses on the pharmacology of levofloxacin in the treatment of CAP.
Pharmacodynamic properties
Spectrum of activity
Levofloxacin is the L-isomer of the racemic fluoroquinolone ofloxacin.Citation39,Citation41 Topoisomerase IV is the main target for levofloxacin in Gram-positive bacteria and DNA gyrase (topoisomerase II) is the target in Gram-negative bacteria.Citation42 Levofloxacin has a broad spectrum of antibacterial activity that includes several Gram-positive and Gram-negative aerobes and cell-wall-deficient (atypical) bacteria. The minimum inhibitory concentrations (MIC) of levofloxacin required to inhibit the growth of 90% of clinical isolates (MIC90) are used as assessments of the in vitro activity of levofloxacin. The levofloxacin MIC breakpoints for S. pneumoniae defined by the Clinical and Laboratory Standards Institute are: ≤2 mg/L (susceptible), 4 mg/L, (intermediate), and ≥8 mg/L (resistant).Citation41,Citation43 Also, levofloxacin generally demonstrates good in vitro activity against penicillin-resistant S. pneumoniae strains. S. pneumoniae with reduced susceptibility to penicillin commonly cause CAP. The levofloxacin MIC90 for penicillin-susceptible, -intermediate, and -resistant isolates of S. pneumoniae was 1 mg/L in multiple studies, with >97% of isolates testing susceptible to the drug.Citation31–Citation34,Citation38,Citation44
Levofloxacin has variable activity against S. aureus, depending on methicillin susceptibility. Levofloxacin had MIC90 values of 0.25–4.0 mg/L against methicillin-susceptible S. aureus isolates, whereas methicillin-resistant S. aureus isolates exhibited levofloxacin resistance, MIC90 values ranging from >4 to ≥64 mg/L.Citation38,Citation44–Citation46 The in vitro activity of levofloxacin against Enterococcus faecalis was limited (MIC90 of 8 to ≥32 mg/L in vancomycin-susceptible and -resistant strains). Although levofloxacin has limited activity against coagulase-negative staphylococci (>4 mg/L, 54.1%).Citation45 It has demonstrated good in vitro activity against a range of other Gram-positive bacteria, such as Streptococcus pyogenes (1 mg/L, 99.9%)Citation32,Citation33 and other β-hemolytic streptococci (0.5–1 mg/L, 99.1%–100%).Citation47
Generally, levofloxacin has good in vitro activity against Gram-negative bacteria including the common respiratory tract pathogens H. influenzae.Citation31,Citation35,Citation38,Citation44,Citation48–Citation50 Haemophilus parainfluenzae,Citation50 and M. catarrhalisCitation31,Citation35,Citation44,Citation48–Citation50 as well as urinary tract pathogens (K. pneumoniae,Citation38,Citation44,Citation51 Enterobacter cloacae,Citation38,Citation44,Citation51–Citation53 and Proteus mirabilisCitation38,Citation45,Citation48). The values of MIC90 for levofloxacin against isolates of H. influenzae, H. parainfluenzae, and M. catarrhalis were ≤0.06 mg/L with nearly 100% susceptibility rates. Levofloxacin was also highly active against β-lactamase-positive isolates of H. influenzaeCitation31–Citation34,Citation38 and M. catarrhalis,Citation31,Citation44,Citation48–Citation50,Citation54,Citation55 However, the activity of levofloxacin is variable against Escherichia coli and P. aeruginosa. The MIC90 of levofloxacin against E. coli ranged from ≤0.06 mg/L (susceptible) to >8 mg/L (resistant).Citation38,Citation44,Citation45,Citation51,Citation56 Levofloxacin showed lower levels of activity against isolates of P. aeruginosa, MIC90 values ranging from 0.5 mg/L to 64 mg/L and susceptibility rates of 71%–94%.Citation38,Citation44,Citation45,Citation48,Citation51 Levofloxacin also had limited activity against extended-spectrum β-lactamase-producing K. pneumoniae (MIC90 of >8–32 mg/L).Citation45 Levofloxacin has good activity against the cell-wall-deficient (atypical) organisms C. pneumonia.Citation57–Citation60 L. pneumophila,Citation38,Citation44,Citation48,Citation57,Citation61,Citation62, and M. pneumonia,Citation48,Citation57,Citation63–Citation65 MIC90 values being ≤2 mg/L.
Bactericidal activity
The bactericidal activity of levofloxacin is concentration-dependent,Citation66 and the minimum bactericidal concentration (MBC) of levofloxacin was ≤4× the MIC against the majority of isolates for a number of causative pathogens of respiratory tract infections.Citation59,Citation60,Citation64,Citation65,Citation67 The MBC90 of levofloxacin was 1–4× the MIC against the majority of M. pneumoniae isolates (MBC of ≤0.5–1.0 mg/L), as reported by multiple authors.Citation59,Citation60,Citation63–Citation65,Citation67 The MBC of levofloxacin was 1–2× the MIC (≤0.06–4 mg/L) against K. pneumoniae, P. aeruginosa, E. coli, and E. cloacae.Citation51 Levofloxacin has a post-antibiotic effect (PAE) of 2.0–4.5 hours depending on the pathogen.Citation39 The PAE of levofloxacin against S. pneumoniae was up to 4.5 hours at 10× the MIC. Furthermore, levofloxacin has shown PAEs against methicillin-susceptible S. aureus (MSSA), K. pneumoniae, L. pneumophila, and anaerobesCitation39 as well as against erythromycin-resistant and -susceptible strains of L. pneumophila.Citation61
Resistance
Resistance to antibacterial drugs in S. pneumoniae has been a major problem in the US for more than a decade.Citation26 The primary cause of reduced susceptibility of bacteria (particularly S. pneumoniae) to fluoroquinolones is at least one mutation in the parC and parE genes that code for DNA topoisomerase IV or gyrA and gyrB genes that code for DNA gyrase.Citation68,Citation69 Another fluoroquinolone resistance mechanism involves active drug efflux through mutation in the efflux regulatory genes mexR and nfxB.Citation68,Citation70 Although there are reports of the emergence of fluoroquinolone resistance among S. pneumoniae,Citation26 the incidence of levofloxacin-resistant organisms has remained stable to date at ≤1% worldwide.Citation31–Citation35
In the worldwide PROTEKT surveillance program between 1999 and 2000, levofloxacin-resistant isolates of S. pneumoniae were identified; 94% of these isolates had at least one mutation in the genes coding for topoisomerase IV as well as in the genes coding for DNA gyrase.Citation69 The SENTRY surveillance program (1997–2005) identified fluoroquinolone-resistant isolates of β-hemolytic Streptococcus spp. as having significant mutations in the parC or gyrA gene, or both. Only mutations in parC were associated with lower MIC values.Citation47 A report of an in vitro pharmacodynamic model simulating the concentration of levofloxacin in the epithelial lining fluid (ELF) after once daily administration of 500 mg revealed that all five isolates of S. pneumoniae containing the first-step parC mutation had levofloxacin resistance within 48 hours (≥16-fold increase in MIC) and four of the isolates acquired a second-step (gyrA) mutation.Citation71 The acquisition of a second-step mutation appeared to be related with an area under the concentration–time curve (AUC):MIC ratio of ≤256; this indicates that to prevent levofloxacin resistance from being acquired in isolates with a first-step parC mutation, the AUC:MIC ratio target should be >256.Citation71 When the range of free AUCs (fAUCs) of levofloxacin and other fluoroquinolones were simulated, the results demonstrated that fAUC:MIC ratios of ≤82 and ≤86 for levofloxacin were associated with a first-step parC mutation and second-step gyrA mutation in S. pneumoniae. These resistance breakpoints for levofloxacin were significantly higher (P ≤ 0.001) than those for other tested fluoroquinolones (gatifloxacin, gemifloxacin, and moxifloxacin) using post hoc analysis. Furthermore, the higher the fAUC:MIC ratio for each fluoroquinolone, the more delay in the development of first- or second-step mutations was observed.Citation72
In the SENTRY (worldwide, 1997–2004),Citation47 PROTEKT (US and Canada, 1999–2002),Citation32–Citation34 and TRUST (US, 1998–2002)Citation35 surveillance programs, the overall levofloxacin resistance rate in S. pneumoniae isolates was ≤1%; in penicillin-resistant isolates, the overall rate of levofloxacin resistance was 0.9%–2.7%.Citation31,Citation34,Citation35 In the TRUST surveillance program from 2001 to 2005, the rate of S. pneumoniae resistance to levofloxacin changed from 0% to 0.5% and the resistance of these isolates to penicillin resistance increased from 27.4% to 28.9%. Amoxicillin/clavulanic acid resistance increased from 6.5% to 12.9%, and clindamycin resistance increased from 12.1% to 18.6%.Citation73 The levofloxacin 750 mg dose has been directly compared to imipenem–cilastatin in the treatment of nosocomial pneumonia. The average age of the patients was 55 years and 438 patients were randomized. Forty-two percent of patients in the levofloxacin arm were ≥65 years of age. The clinical success rate in the intention-to-treat population was 66.2% in the levofloxacin arm vs 69.4% in the imipenem arm. In the clinically evaluable population, the success rates were 59.3% and 62.5% for levofloxacin and imipenem, respectively.Citation74 Other data from 1998 and 2005 revealed that the levofloxacin-resistant isolates of H. influenzae or M. catarrhalis could not be identified in large worldwide surveillance studies.Citation32–Citation34,Citation49,Citation54,Citation55 However, surveillance studies have demonstrated resistance to levofloxacin in MSSA and methicillin-resistant strains of S. aureus (MRSA) (3.4%–10.1% and 76.6%–79.2%, respectively) and P. aeruginosa (24.7%).Citation45,Citation46,Citation56
Pharmacokinetics and metabolism
Levofloxacin is rapidly absorbed after oral administration and shows linear pharmacokinetics for both single- and multiple-dose (once daily) regimens. The oral solution and tablet formulations are bioequivalent to the intravenous formulation.Citation41 The mean pharmacokinetic parameters obtained in different studies of intravenous and oral levofloxacin in healthy adultsCitation75,Citation76 are comparable to those reported in the manufacturer’s US prescribing information.Citation41 The peak plasma concentration (Cmax) after single 750 mg doses of levofloxacin given to healthy volunteers was 11.3 mg/LCitation75 and 12.1 mg/L for intravenous administration, compared with 7.1 mg/LCitation76 and 9.3 mg/LCitation41 for oral administration. When given in multiple doses levofloxacin had Cmax of 12.1 mg/L and 12.4 mg/L for intravenous administration compared with 8.6 mg/L for oral ones.Citation41,Citation76 Levofloxacin steady-state conditions were reached within 48 hours of initiating once-daily intravenous or oral 750 mg.Citation41 After oral administration, the Tmax of levofloxacin is reached within 1–2 hours with an absolute bioavailability of oral levofloxacin 500 mg and 750 mg of approximately 99%.Citation41,Citation75,Citation76 Systemic exposure to levofloxacin was similar for the intravenous and oral formulations upon administering equal doses of levofloxacin.Citation41 The AUC24 was 103 mg h/LCitation75 and 90.7 mg h/LCitation76 at steady state after intravenous or oral administration of levofloxacin 750 mg once daily, respectively.
The in vitro studies revealed that 24%–38% of levofloxacin was bound to plasma proteins (mainly albumin) and the binding was independent of levofloxacin concentration.Citation41 The volumes of distribution obtained in pharmacokinetic studies ranged from 74–112 L after single or multiple doses of levofloxacin 500 mg or 750 mg.Citation75,Citation76 Levofloxacin is distributed extensively in tissues and fluids throughout the body and accumulates in phagocytic cells.Citation39 Furthermore, the mean concentrations of levofloxacin in tissues, ELF, alveolar macrophages, polymorphonuclear leukocytes, paranasal sinus mucosa, and urine, surpass the concentration of levofloxacin in the plasma.Citation39,Citation77–Citation83 It has been reported that the paranasal sinuses mucosa:plasma concentration ratio was 2.56 at Tmax after a single 500 mg oral dose of levofloxacin. The concentration of levofloxacin in the paranasal sinuses mucosa was generally higher than the MIC90 of the common causative pathogens for upper respiratory tract infections (0.008–2.0 mg/L), including penicillin-susceptible, -intermediate, and -resistant isolates of S. pneumoniae.Citation82 In healthy volunteers, oral levofloxacin (500 or 750 mg) had a mean ELF:plasma concentration ratio at steady state of 1.16 using population pharmacokinetic modeling and 3.18 using Monte Carlo simulation.Citation82 At a lower dosage of levofloxacin (500 mg once daily for 3 days), Cmax and AUC24 values for the drug were significantly (P < 0.01) higher in the polymorphonuclear leukocytes than in plasma.Citation84 Reassuringly, the concentrations of levofloxacin in the ELF and alveolar macrophages were 1.5- to 6-fold higher than that in the plasma at steady state after receiving levofloxacin 500 mg once daily for 5 days in older patients undergoing diagnostic bronchoscopy with a mean age of 62 years.Citation80
Levofloxacin is eliminated mainly through the kidneys, 75%–87% of the dose excreted being unchanged in the urine within 48–72 hours of administering oral levofloxacin 500 or 750 mg; <4% is excreted in the feces.Citation41,Citation75,Citation76 After a single dose of levofloxacin 750 mg, the mean drug concentration in the urine was 475 mg/L at 4 hours and 186 mg/L at 24 hours;Citation77 <5% of the dose is excreted in the urine as inactive metabolites of levofloxacin.Citation41 The mean total body clearance (CL) of levofloxacin in healthy volunteers was reported as 8–9.4 L/hCitation75,Citation76 and 8.6–13.6 L/h.Citation41 Levofloxacin appears to undergo glomerular filtration as well as tubular secretion.Citation41 After single or multiple doses of oral or intravenous levofloxacin 750 mg, the mean terminal plasma elimination half-life (t1/2β) is 7.5–8.8 hours in pharmacokinetic studies.Citation75,Citation76 The t1/2β of levofloxacin is increased and the CL reduced in patients with impaired renal function (creatinine clearance CLCR < 50 mL/min); therefore dosage adjustment is required to avoid drug accumulation as shown in .Citation41 Furthermore, levofloxacin is not cleared effectively by hemodialysis or continuous ambulatory peritoneal dialysis.Citation39,Citation41 The pharmacokinetic properties of levofloxacin are not influenced by age, gender, or race, and they do not show noticeable differences between healthy adults, patients with HIV,Citation39 or patients with severe community-acquired bacterial infections.Citation41 Levofloxacin pharmacokinetics in hepatically-impaired patients have not been investigated; however, because of the limited hepatic metabolism of levofloxacin, hepatic impairment is unlikely to have a prominent effect on the drug pharmacokinetics.Citation41
Table 1 Dosing in patients with diminished renal function
Clinical efficacy
The efficacy of levofloxacin 750 mg once daily (intravenous and oral) for 5 days in adults with CAP,Citation66 ABS,Citation85 and complicated UTICitation86,Citation87 has been assessed in several randomized, double-blind, multicenter, noninferiority trials.Citation66,Citation85–Citation87 The endpoints were the clinical success rate (proportion of patients showing either a clinical cure or improvement with no need for further antimicrobial therapies in both situations) 1–2 weeks after the end of treatment,Citation66 or at 2–3 weeks of the study,Citation85 or the microbiological eradication rate (all pathogens identified in samples at the study entry were eradicated) at 2–3 weeks of the study.Citation86,Citation87 Levofloxacin indications and dosing for patients with normal renal function are summarized in .
Table 2 Levofloxacin indications and dosing for patients with upper respiratory tract infections and with normal renal function
Patients enrolled in the noninferiority trial with CAP were aged ≥18 years and were diagnosed with mild-to-severe CAP. Other inclusion criteria involved one or more signs or symptoms including fever, a white blood cell count of >10,000 cells/mm3, or hypothermia. The exclusion criteria included the following conditions: patients without a confirmed diagnosis of CAP, patients who did not come to the follow-up visit, patients who increased (>120%) or reduced (<80%) the scheduled doses, and patients who had additional antimicrobial therapy during treatment with levofloxacin.Citation66 Patients with mild-to-severe CAP received 750 mg levofloxacin (intravenous or oral) once daily for 5 days or 500 mg once daily for 10 days. Subjects receiving the higher dosage of levofloxacin were given a placebo for the last 5 days of the 10-day treatment regimen.Citation66 Levofloxacin susceptibility testing of the causative pathogens was performed, but initial treatment was empirical. The noninferiority criteria were established as the upper limit of the 2-sided 95% CI for the between-group difference in the clinical success rate <15%, if both treatment groups had a clinical success rate of 80%–90%, or <10%, if both treatment groups had a clinical success rate of ≥90%.Citation66 The results revealed that levofloxacin 750 mg once daily for 5 days was noninferior to 500 mg once daily for 10 days in the treatment of mild-to-severe CAP in the overall patient population,Citation66 as well as for patients with CAP caused by atypical organisms (C. pneumoniae or M. pneumoniae),Citation88 and for elderly patients aged ≥65 years.Citation89
In patients receiving either the levofloxacin 750 mg or 500 mg regimen, baseline characteristics were similar and overall microbiological eradication rates were similar in both groups.Citation66 The eradication rates for both the 750 mg and 500 mg regimens were high for subgroups of micro-biologically evaluable patients infected with aerobic Gram-positive (82.8% vs 85.3%) and Gram-negative (96.2% vs 90.7%) pathogens, as well as other pathogens (93.8% vs 96.2%). Eradication rates for S. pneumoniae, H. influenzae, and H. parainfluenzae in the corresponding post-therapy visit were 86.4% vs 85%, 92.3% vs 85.7% and 100% vs 90%, respectively.Citation66 Retrospective analysis revealed that the clinical success rates in patients with CAP caused by H. influenzae, H. parainfluenzae, or S. pneumoniae were also similar between the levofloxacin 750 mg and 500 mg treatment groups (92.3% vs 92.9%, 100% vs 90%, and 90.9% vs 90%, respectively).Citation66
The efficacy of the high-dose, short-course of levofloxacin in achieving early resolution of symptoms has been studied.Citation90 Resolution of purulent sputum, shortness of breath, chills and cough were 40.6% vs 30.7%, 35.1% vs 27.7%, 54.8% vs 54.2%, and 10% vs 10.1% comparing patients who received the levofloxacin 750 mg or 500 mg regimen, respectively. Furthermore, 99.4% of the 158 pathogens isolated at study entry were susceptible to levofloxacin and there was no significant difference between treatment groups in the time of switching from the intravenous administration of levofloxacin to oral administration of the drug.Citation90 High-dose, short-course of levofloxacin (750 mg once daily for 5 days) also had good efficacy in the subgroup of patients with severe CAP, demonstrating high clinical success rates of >85%. Overall, high microbiological response rates (≥87.5%) were observed in the subgroup of microbiologically evaluable patients receiving levofloxacin regardless of the treatment regimen.Citation91 In the same study, microbiological eradication was observed in 88.2% of typical pathogens identified from respiratory cultures and 90% of atypical pathogens.Citation91
It has been reported that levofloxacin 750 mg once daily for 5 days has good efficacy in patients with CAP caused by atypical organisms.Citation88 The overall clinical success rate of levofloxacin 1–2 weeks after treating CAP caused by a single atypical pathogen, was >95%. Noninferiority of levofloxacin 750 mg once daily for 5 days compared with the 10-day regimen was also established in this study. The overall clinical success rate of the levofloxacin 750 mg regimen was 94.8% for CAP caused by atypical pathogens, compared with 96.5% for the levofloxacin 500 mg regimen.Citation88 Furthermore, the clinical success rates at the 1–2 weeks post-treatment visit for patients with C. pneumoniae, L. pneumophila, and M. pneumoniae were comparable between the groups receiving the levofloxacin 750 mg and 500 mg dosing regimen (90.9% vs 100%, 100% vs 100%, and 95.3% vs 94.4%, respectively).Citation88
Post-marketing surveillance
Post-marketing data demonstrated that levofloxacin simultaneous administered with warfarin may increase the prothrombin time. Therefore, coagulation studies and bleeding should be monitored in patients receiving the two drugs concomitantly.Citation41 Levofloxacin does not currently have a US Food and Drug Administration approved indication in patients aged <18 years. Like other fluoroquinolones, levofloxacin decreases theophylline metabolism and dosage adjustment for theophylline may be required for concurrent administration of both drugs. Concomitant fluoroquinolone administration with cyclosporin resulted in elevated serum concentrations of ciclosporin, but these alterations were not clinically significant.Citation41
Safety and tolerability
Intravenous levofloxacin must be administered slowly as an infusion over a minimum period of 60–90 minutes, depending on the dose. Levofloxacin tablets or oral solution are generally prescribed at dosages of 250, 500, or 750 mg once daily. The tablet formulation of levofloxacin can be taken with or without food; however, the oral solution should be taken 1 hour prior to or 2 hours after meals. In patients receiving levofloxacin, sufficient hydration should be maintained to prevent excessively concentrated urine. Levofloxacin should be administered at least 2 hours apart from some agents such as magnesium- or aluminium-containing antacids, sucralfate, metal cations, zinc-containing multivitamins, or didanosine.
Data from patients aged ≥65 years (phase III clinical trials) demonstrated no difference between elderly and younger patients for safety or effectiveness of levofloxacin. Elderly patients may be more sensitive to levofloxacin, mainly due to the effect of the drug on the QT interval. Thus, caution is required in the simultaneous administration of levofloxacin with drugs that prolong the QT interval such as class IA or class III antiarrhythmics. Although, levofloxacin is a very safe fluoroquinolone, caution and a risk/benefit assessment is required with the use of levofloxacin in the elderly due to the increased risk of severe tendon disorders in this group of patients, particularly if they are receiving corticosteroids.Citation41 However, it should be stated that there is no evidence that tendon rupture is more likely to occur with levofloxacin than with any other fluoroquinolone.Citation92 Blood glucose monitoring is recommended in patients with diabetes mellitus receiving simultaneous hypoglycemic agents and/or insulin, because symptomatic hyperglycemia and hypoglycemia have been reported with levofloxacin administration.Citation41 Concomitant administration of fluoroquinolones (including levofloxacin) with NSAIDs may increase the risk of central nervous system stimulation and convulsive seizures.Citation41
Levofloxacin 750 mg once daily for 5 days is a well-tolerated fluoroquinolone for patients with CAP or UTI.Citation86,Citation87,Citation93 In a pooled analysis of patients with respiratory infections receiving the levofloxacin 750 mg regimen or 500 mg regimen, the results revealed that 4.5% and 4.9% of patients, respectively, had adverse effects during the therapy. The adverse effects in both dosage regimens included nausea, vomiting, diarrhea, dyspepsia, constipation, abdominal pain, headache, insomnia, and dizziness. The incidence of levofloxacin-associated adverse effects was similar between both treatment regimens (8% vs 7.6%).Citation93
The use of fluoroquinolones and exposure to the sun or UV light has been associated with photosensitivity reactions.Citation41 Fluoroquinolones can potentially prolong the QT interval but there are no reported cases of torsade de pointes in any clinical or post-marketing trials.Citation41,Citation93 It has been reported that levofloxacin is associated with Clostridium difficile diarrhea, as are most other antibacterial agents. Severity ranges from mild diarrhea to pseudomembranous colitis.Citation41 The incidence of drug-related adverse effects in patients with CAP or ABS was similar between the levofloxacin 750 mg and 500 mg dosing regimens.Citation93
Regulatory affairs
Levofloxacin is approved for use in the US, Canada, and worldwide in the treatment of CAP, ABS, complicated UTI, and AP.
Conclusion and comments
The respiratory fluoroquinolones are considered to be a substantial component of the anti-infective armamentarium for the treatment of bacterial respiratory infections. Levofloxacin is active against most of the respiratory pathogens and has a good clinical success rate. Its favorable pharmacodynamics, safety, efficacy profile, and tolerability, and also its in vitro activity against the common respiratory pathogens, places levofloxacin among first-line agents for the treatment of community-acquired respiratory tract infections such as CAP.
The Infectious Diseases Society of America/American Thoracic Society guidelines recommend that a respiratory fluoroquinolone (eg, levofloxacin 750 mg) or a β-lactam plus a macrolide be used for the treatment of CAP. The use of fluoroquinolones is a reasonable therapeutic choice for the treatment of respiratory infections caused by penicillin-susceptible S. pneumoniae, penicillin-resistant S. pneumoniae, Legionella pneumophilia, H. influenzae, M. pneumoniae, and C. pneumoniae. Levofloxacin combination therapy with antipseudomonal β-lactam (or aminoglycoside) should be considered if P. aeruginosa is likely to be a causative pathogen of the respiratory infection. S. pneumoniae resistance to antibacterial drugs has been a major problem in the US and worldwide for more than a decade. Although there are reports of the emergence of resistance to some fluoroquinolones among S. pneumoniae, the incidence of levofloxacin-resistant organisms has remained steady at <1% worldwide. In general, levofloxacin shows good in vitro activity against clinically relevant Gram-positive, Gram-negative, and atypical organisms that cause respiratory infections. Levofloxacin is active against penicillin-susceptible and -resistant strains of S. pneumoniae, the Gram-negative species E. cloacae and P. mirabilis, and the atypical organisms C. pneumoniae, L. pneumophila, and M. pneumoniae (MIC90 of ≤2 mg/L). Levofloxacin is highly active against the Gram-negative species H. influenzae, H. parainfluenzae, and M. catarrhalis (MIC90 of ≤0.06 mg/L), including β-lactamase-positive strains of H. influenzae and M. catarrhalis. Because the activity of levofloxacin is concentration-dependent, the most common predictor of microbiological and clinical efficacy is the AUC:MIC ratio. A ratio of >30 was used in some studies to predict in vivo activity, particularly against S. pneumoniae. A higher ratio (>100) is suggested as being predictive of a bactericidal effect, and thus reducing the potential of first-step mutations. Availability of pneumococcal vaccine is decreasing the incidence of pneumococcal infections and decreasing the incidence of infections caused by resistant S. pneumoniae.
In the last 5 years, the rate of resistance of S. pneumoniae to amoxicillin/clavulanic acid, azithromycin, and tetracycline appears to have increased, but the levofloxacin resistance rate of S. pneumoniae remains ≤1% worldwide.Citation94 High-dose, short-term therapy (levofloxacin 750 mg once daily for 5 days) is the standard dosing regimen for levofloxacin in the treatment of CAP worldwide. Increased availability of pneumococcal vaccination programs may decrease the incidence of S. pneumoniae as a cause of CAP in adults over time. Other problematic infections with multidrug-resistant organisms will become the main focus of research in the next 5 years.
Disclosure
The authors declare no conflicts of interest in this work.
References
- AlmirallJBolibarIVidalJEpidemiology of community-acquired pneumonia in adults: a population-based studyEur Respir J200015475776310780770
- GutierrezFMasiaMRodriguezJCEpidemiology of community-acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of SpainClin Microbiol Infect2005111078880016153252
- LohLCKhooSKQuahSYAdult community-acquired pneumonia in Malaysia: prediction of mortality from severity assessment on admissionRespirology20049337938615363012
- O’MearaESWhiteMSiscovickDSLylesMFKullerLHHospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survivalJ Am Geriatr Soc20055371108111616108926
- GutiérrezFMasiáMMireteCThe influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogensJ Infect200653316617416375972
- KaplanVAngusDCCommunity-acquired pneumonia in the elderlyCrit Care Clin200319472974814601717
- ViegiGPistelliRCazzolaMEpidemiological survey on incidence and treatment of community acquired pneumonia in ItalyRespir Med20061001465516046113
- ColiceGLMorleyMAAscheCBirnbaumHGTreatment costs of community-acquired pneumonia in an employed populationChest200412562140214515189934
- NiedermanMSMandellLAAnzuetoAGuidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and preventionAm J Respir Crit Care Med200116371730175411401897
- KollefMHShorrATabakYPGuptaVLiuLZJohannesRSEpidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumoniaChest200512863854386216354854
- BodiMRodriguezASole-ViolanJAntibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survivalClin Infect Dis200541121709171616288392
- TejerinaEFrutos-VivarFRestrepoMIPrognosis factors and outcome of community-acquired pneumonia needing mechanical ventilationJ Crit Care200520323023816253791
- WilsonPAFergusonJSevere community-acquired pneumonia: an Australian perspectiveIntern Med J2005351269970516313544
- WoodheadMWelchCAHarrisonDABellinganGAyresJGCommunity-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme DatabaseCrit Care200610Suppl 2S116934135
- FileTMJrClinical implications and treatment of multiresistant Streptococcus pneumoniae pneumoniaClin Microbiol Infect200612Suppl 3314116669927
- LauderdaleTLChangFYBenRJEtiology of community acquired pneumonia among adult patients requiring hospitalization in TaiwanRespir. Med20059991079108616085210
- LeesikHAniUJuhaniAAltrajaAMicrobial pathogens of adult community-acquired pneumonia in Southern EstoniaMedicina (Kaunas)200642538439416778466
- LunaCMFamigliettiAAbsiRCommunity-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in ArgentinaChest200011851344135411083685
- HuangHHZhangYYXiuQYCommunity-acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patientsEur J Clin Microbiol Infect Dis200625636937416767484
- SaitoAKohnoSMatsushimaTProspective multicenter study of the causative organisms of community-acquired pneumonia in adults in JapanJ Infect Chemother2006122636916648944
- ThibodeauKPVieraAJAtypical pathogens and challenges in community-acquired pneumoniaAm Fam Physician20046971699170615086042
- WoodheadMCommunity-acquired pneumonia in Europe: causative pathogens and resistance patternsEur Respir J Suppl20023620s27s12168744
- FileTMJrGarauJBlasiFGuidelines for empiric antimicrobial prescribing in community-acquired pneumoniaChest200412551888190115136404
- MandellLAWunderinkRGAnzuetoAInfectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adultsClin Infect Dis200744Suppl 2S27S7217278083
- WoodheadMBlasiFEwigSGuidelines for the management of adult lower respiratory tract infectionsEur Respir J20052661138118016319346
- DoernGVRichterSSMillerAAntimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes?Clin Infect Dis200541213914815983908
- BonofiglioLOjedaMIde MierCPhenotypic and genotypic characterization of macrolide resistant Streptococcus pneumoniae recovered from adult patients with community-acquired pneumonia in an Argentinian teaching hospitalInt J Antimicrob Agents200525326026315737523
- FelminghamDComparative antimicrobial susceptibility of respiratory tract pathogensChemotherapy200450Suppl 131015319548
- FullerJDMcGeerALowDEDrug-resistant pneumococcal pneumonia: clinical relevance and approach to managementEur J Clin Microbiol Infect Dis2005241278078816344922
- ReinertRRReinertSvan der LindenMCilMYAl-LahhamAAppelbaumPAntimicrobial susceptibility of Streptococcus pneumoniae in eight European countries from 2001 to 2003Antimicrob Agents Chemother20054972903291315980367
- JonesRNFritscheTRSaderHSStilwellMGActivity of garenoxacin, an investigational des-F(6)-quinolone, tested against pathogens from community-acquired respiratory tract infections, including those with elevated or resistant-level fluoroquinolone MIC valuesDiagn Microbiol Infect Dis200758191717408903
- BrownSDRybakMJAntimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001–2002, as part of the PRO-TEKT US studyJ Antimicrob Chemother200454Suppl 1i7i1515265831
- DoernGVBrownSDAntimicrobial susceptibility among community-acquired respiratory tract pathogens in the USA: data from PROTEKT US 2000–2001J Infect2004481566514667792
- HobanDWaitesKFelminghamDAntimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999–2000: findings of the PROTEKT surveillance studyDiagn Microbiol Infect Dis200345425125912729995
- KarlowskyJAThornsberryCJonesMEFactors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998–2002)Clin Infect Dis200336896397012684907
- WangJCDNA topoisomerasesAnnu Rev Biochem1996656356928811192
- WangJCA journey in the world of DNA rings and beyondAnnu Rev Biochem200978315419489720
- HubandMDCohenMAZurackMIn vitro and in vivo activities of PD 0305970 and PD 0326448, new bacterial gyrase/topoisomerase inhibitors with potent antibacterial activities versus multidrug-resistant gram-positive and fastidious organism groupsAntimicrob Agents Chemother20075141191120117261623
- CroomKFGoaKLLevofloxacin: a review of its use in the treatment of bacterial infections in the United StatesDrugs200363242769280214664657
- KeatingGMScottLJMoxifloxacin: a review of its use in the management of bacterial infectionsDrugs200464202347237715456331
- Levaquin® (levofloxacin tablets, oral solution, injection): US prescribing informationRaritan (NJ)Ortho-McNeil Pharmaceutical, Inc82009
- HurstMLambHMScottLJFiggittDPLevofloxacin: an updated review of its use in the treatment of bacterial infectionsDrugs200262142127216712269858
- Clinical and Laboratory Standards InstituteMethods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard – seventh editionClinical and Laboratory Standards Institute Document M7-A7Wayne, PAClinical and Laboratory Standards Institute12006262
- GordonKASaderHSJonesRNContemporary re-evaluation of the activity and spectrum of grepafloxacin tested against isolates in the United StatesDiagn Microbiol Infect Dis200347137738312967754
- FritscheTRSaderHSJonesRNPotency and spectrum of garenoxacin tested against an international collection of skin and soft tissue infection pathogens: report from the SENTRY antimicrobial surveillance program (1999–2004)Diagn Microbiol Infect Dis2007581192617383139
- GoffDADowzickyMJPrevalence and regional variation in meticillin-resistant Staphylococcus aureus (MRSA) in the USA and comparative in vitro activity of tigecycline, a glycylcycline antimicrobialJ Med Microbiol200756Pt 91189119317761482
- BiedenbachDJTolemanMAWalshTRJonesRNCharacterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997–2004)Diagn Microbiol Infect Dis200655211912716530373
- NiliusAMShenLLHensey-RudloffDIn vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinoloneAntimicrob Agents Chemother200347103260326914506039
- JacobsMRFelminghamDAppelbaumPCGrunebergRNAlexander Project GroupThe Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agentsJ Antimicrob Chemother200352222924612865398
- SorianoFGranizoJJCoronelPAntimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE projectInt J Antimicrob Agents200423329629915164972
- HansenGTBlondeauJMComparison of the minimum inhibitory, mutant prevention and minimum bactericidal concentrations of ciprofloxacin, levofloxacin and garenoxacin against enteric Gram-negative urinary tract infection pathogensJ. Chemother200517548449216323436
- DeshpandeLMDiekemaDJJonesRNComparative activity of clinafloxacin and nine other compounds tested against 2000 contemporary clinical isolates from patients in United States hospitalsDiagn Microbiol Infect Dis1999351818810529885
- RolstonKVFrisbee-HumeSLeBlancBMStreeterHHoDHAntimicrobial activity of a novel des-fluoro (6) quinolone, garenoxacin (BMS-284756): compared to other quinolones, against clinical isolates from cancer patientsDiagn Microbiol Infect Dis200244218719412458127
- ThornsberryCSahmDFKellyLJRegional trends in antimicrobial resistance among clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States: results from the TRUST Surveillance Program, 1999–2000Clin Infect Dis200234Suppl 1S4S1611810606
- KarlowskyJAThornsberryCCritchleyIASusceptibilities to levofloxacin in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children: results from 2000–2001 and 2001–2002 TRUST studies in the United StatesAntimicrob Agents Chemother20034761790179712760850
- ZhanelGGHisanagaTLLaingNMAntibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA)Int J Antimicrob Agents200627646847516713191
- CritchleyIAJonesMEHeinzePDIn vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and EuropeClin Microbiol Infect20028421422112047413
- RoblinPMReznikTKutlinAHammerschlagMRIn vitro activities of rifamycin derivatives ABI-1648 (Rifalazil, KRM-1648): ABI-1657, and ABI-1131 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniaeAntimicrob Agents Chemother20034731135113612604555
- KohlhoffSARoblinPMReznikTHawserSIslamKHammerschlagMRIn vitro activity of a novel diaminopyrimidine compound, iclaprim, against Chlamydia trachomatis and C. pneumoniaeAntimicrob Agents Chemother20044851885188615105151
- HammerschlagMRRoblinPMThe in vitro activity of a new fluoroquinolone, ABT-492, against recent clinical isolates of Chlamydia pneumoniaeJ Antimicrob Chemother200454128128215190026
- DuboisJSt-PierreCComparative in vitro activity and post-antibiotic effect of gemifloxacin against Legionella sppJ Antimicrob Chemother200045Suppl 1414610824031
- StoutJESensKMietznerSObmanAYuVLComparative activity of quinolones, macrolides and ketolides against Legionella species using in vitro broth dilution and intracellular susceptibility testingInt J Antimicrob Agents200525430230715784309
- WaitesKBCrabbDMBingXDuffyLBIn vitro susceptibilities to and bactericidal activities of garenoxacin (BMS-284756) and other antimicrobial agents against human mycoplasmas and ureaplasmasAntimicrob Agents Chemother200347116116512499185
- WaitesKBCrabbDMDuffyLBComparative in vitro activities of the investigational fluoroquinolone DC-159a and other antimicrobial agents against human mycoplasmas and ureaplasmasAntimicrob Agents Chemother200852103776377818663020
- DuffyLBCrabbDMBingXWaitesKBBactericidal activity of levofloxacin against Mycoplasma pneumoniaeJ Antimicrob Chemother200352352752812888583
- DunbarLMWunderinkRGHabibMPHigh-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigmClin Infect Dis200337675276012955634
- WaitesKBCrabbDMDuffyLBComparative in vitro susceptibilities and bactericidal activities of investigational fluoroquinolone ABT-492 and other antimicrobial agents against human mycoplasmas and ureaplasmasAntimicrob Agents Chemother200347123973397514638513
- FelminghamDFeldmanCHryniewiczWSurveillance of resistance in bacteria causing community-acquired respiratory tract infectionsClin Microbiol Infect20028Suppl 2124212427206
- CantonRMorosiniMEnrightMCMorrisseyIWorldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the global PROTEKT surveillance programmeJ Antimicrob Chemother200352694495214585861
- HigginsPGFluitACMilatovicDVerhoefJSchmitzFJMutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosaInt J Antimicrob. Agents200321540941312727072
- DerykeCADuXNicolauDPEvaluation of bacterial kill when modelling the bronchopulmonary pharmacokinetic profile of moxifloxacin and levofloxacin against parC-containing isolates of Streptococcus pneumoniaeJ Antimicrob Chemother200658360160916857688
- LaPlanteKLRybakMJTsujiBLodiseTPKaatzGWFluoroquinolone resistance in Streptococcus pneumoniae: area under the concentration-time curve/MIC ratio and resistance development with gatifloxacin, gemifloxacin, levofloxacin, and moxifloxacinAntimicrob Agents Chemother20075141315132017296740
- SahmDFBenningerMSEvangelistaATYeeYCThornsberryCBrownNPAntimicrobial resistance trends among sinus isolates of Streptococcus pneumoniae in the United States (2001–2005)Otolaryngol Head Neck Surg2007136338538917321864
- WestMBoulangerBRFogartyCLevofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open-label studyClin Ther200325248550612749509
- ChowATFowlerCWilliamsRRMorganNKaminskiSNatarajanJSafety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteersAntimicrob Agents Chemother20014572122212511408234
- ChienSCWongFAFowlerCLDouble-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteersAntimicrob Agents Chemother19984248858889559801
- SteinGESchooleySLNicolauDPUrinary bactericidal activity of single doses (250, 500, 750 and 1000 mg) of levofloxacin against fluoroquinolone-resistant strains of Escherichia coliInt J Antimicrob Agents200832432032518715762
- GotfriedMHDanzigerLHRodvoldKASteady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjectsChest200111941114112211296178
- RodvoldKADanzigerLHGotfriedMHSteady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adultsAntimicrob Agents Chemother20034782450245712878504
- CapitanoBMattoesHMShoreESteady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adultsChest2004125396597315006955
- ConteJEJrGoldenJAMcIverMLittleEZurlindenEIntrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary diseaseInt J Antimicrob Agents200730542242717716873
- DrusanoGLPrestonSLGotfriedMHDanzigerLHRodvoldKALevofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulationAntimicrob Agents Chemother200246258658911796385
- PeaFMarioniGPavanFPenetration of levofloxacin into paranasal sinuses mucosa of patients with chronic rhinosinusitis after a single 500 mg oral dosePharmacol Res20074551384117092740
- GarraffoRLavrutTDurantJIn vivo comparative pharmacokinetics and pharmacodynamics of moxifloxacin and levofloxacin in human neutrophilsClin Drug Investig20052510643650
- PooleMAnonJPagliaMXiangJKhashabMKahnJA trial of high-dose, short-course levofloxacin for the treatment of acute bacterial sinusitisOtolaryngol Head Neck Surg20061341101716399173
- PetersonJKaulSKhashabMFisherACKahnJBA double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritisUrology2008711172218242357
- KlausnerHABrownPPetersonJA trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritisCurr Med Res Opin200723112637264517880755
- DunbarLMKhashabMMKahnJBZadeikisNXiangJXTennenbergAMEfficacy of 750-mg, 5-day levofloxacin in the treatment of community-acquired pneumonia caused by atypical pathogensCurr Med Res Opin200420455556315119993
- ShorrAFZadeikisNXiangJXTennenbergAMWes ElyEA multicenter, randomized, double-blind, retrospective comparison of 5- and 10-day regimens of levofloxacin in a subgroup of patients aged ≥65 years with community-acquired pneumoniaClin Ther20052781251125916199249
- FileTMJrMilkovichGTennenbergAMXiangJXKhashabMMZadeikisNClinical implications of 750 mg, 5-day levofloxacin for the treatment of community-acquired pneumoniaCurr Med Res Opin20042091473148115383197
- ShorrAFKhashabMMXiangJXTennenbergAMKahnJBLevofloxacin 750-mg for 5 days for the treatment of hospitalized Fine Risk Class III/IV community-acquired pneumonia patientsRespir Med2006100122129213616730170
- Levofloxacin revisitedMed Lett Drugs Ther20115313685521738109
- KhashabMMXiangJKahnJBComparison of the adverse event profiles of levofloxacin 500 mg and 750 mg in clinical trials for the treatment of respiratory infectionsCurr Med Res Opin200622101997200617022859
- YeeYCEvangelistaATObot-TuckerMFive-year surveillance (2003–2007) of anti-pneumococcal activity of oral agents recommended for the empirical treatment of community-acquired pneumonia (CAP) in adults [Abstract No C2204]47th Interscience Conference on Antimicrobial Agents and ChemotherapyChicago, ILSep 17–20, 2007