Abstract
Purpose
An extended-release (ER) formulation of the expectorant guaifenesin has recently been launched in India for the treatment of productive cough accompanied by mucus (phlegm). Although the safety profile of ER guaifenesin marketed in the USA is well documented, there were limited safety data available in the Indian population. The aim of this study was to further elucidate the safety profile of ER guaifenesin in patients with acute upper respiratory tract infection (URTI).
Patients and methods
A prospective, post-marketing surveillance study enrolled 552 adults with cough, thickened mucus and chest congestion due to URTI, who took ER guaifenesin 1200 mg (Mucinex®, Reckitt Benckiser; two 600 mg tablets) every 12 hrs for 7 days. Adverse events (AEs) were recorded and questionnaires administered to patients and investigators.
Results
A total of 29 treatment-emergent AEs were recorded in 28/552 patients, including gastrointestinal (n = 11), nervous system (n = 8), psychiatric (n = 3), respiratory, thoracic and mediastinal (n = 2), skin and subcutaneous tissue (n = 2), and general disorders (n = 3). All AEs were mild in severity and no serious AEs or deaths occurred. The majority of both patients and investigators were either satisfied or very satisfied with improvements in treatment outcomes.
Conclusion
This study found that ER guaifenesin was well tolerated and had a favorable safety profile in otherwise healthy patients suffering from symptoms of cough, thickened mucus and chest congestion associated with URTI.
Registered trial NCT03725085 (ClinicalTrials.gov) and CTRI/2014/07/004730 (ctri.nic.in).
Introduction
Mucus production plays an important role in maintaining healthy airways and protecting them from pathogens.Citation1 Mucus is a viscoelastic gel that is produced by goblet cells in the epitheliumCitation1 and submucosal glandsCitation2 of the airways. It forms a thin film on the surface of the airways, protecting the epithelium from damage. Effective clearance of mucus from the airways is dependent on the rheologic properties and volume of secreted mucus, and the ciliary function.Citation1 Under normal, healthy conditions, the secreted mucus traps bacteria, viruses and foreign particles, and removes them from the respiratory tract and lungs, by mucociliary clearance.Citation1,Citation3,Citation4 In contrast, during acute upper respiratory tract infection (URTI), and in chronic airway diseases (such as asthma, chronic obstructive pulmonary disease and cystic fibrosis), mucus production is increased and has greater viscoelasticity, leading to impaired mucociliary clearance and causing chest congestion and coughing.Citation4–Citation7
Treatments for excessive or pathologic mucus include those that decrease mucin production and/or secretion, those that promote mucus clearance, and those that treat airway inflammation or infection.Citation4,Citation6 Expectorants increase hydration and the secretion and expulsion of mucus from the respiratory tract.Citation8 The expectorant guaifenesin is thought to work by stimulating cholinergic muscarinic receptors, which, via the vagus nerve, stimulate submucosal glands.Citation8 This leads to an increased production but decreased viscosity of mucus, thereby relieving chest congestion.Citation9–Citation11
Guaifenesin is considered by the US Food and Drug Administration (FDA) to be an effective expectorant with a good safety profile,Citation12 and is available in several over-the-counter cough and cold products. In patients with URTI, positive but inconsistent changes in sputum thickness and volume,Citation13,Citation14 and symptoms,Citation13–Citation15 have been reported with guaifenesin, although the assessment of any impact on cough or chest symptoms is complicated by differences in study methods and the variety of symptoms experienced by these patients. The FDA has also included in the Monograph a healthcare professional labeling for guaifenesin to “help loosen phlegm and thin bronchial secretions in patients with stable chronic bronchitis”.Citation12
An extended-release (ER) formulation of guaifenesin (MUCINEX, Reckitt Benckiser, Parsippany, NJ, USA)Citation16 has been developed to prolong duration of effect and reduce dosing frequency. This bi-layer tablet formulation combines immediate-release guaifenesin with an ER feature, providing sustained blood levels for 12 hrs.Citation16 The clinical data on the efficacy of ER guaifenesin in patients with URTI are currently limited.Citation17–Citation19 Although the post-marketing safety experience with ER guaifenesin is based on a large number of exposures and is positive, clinical data on the safety and tolerability of ER guaifenesin when used to relieve chest congestion, are limited.Citation17,Citation20 The aim of this study was to evaluate the safety and tolerability of ER guaifenesin (1200 mg twice daily) in patients with symptoms of cough, thickened mucus and chest congestion.
Materials And Methods
Study Design
This was an open-label, non-comparative, single-arm, prospective, post-marketing surveillance (PMS) study conducted across nine study centers in India from January 24, 2015 (first patient enrolled) to October 16, 2015 (last patient completed) and registered on Clinical Trials Registry India (CTRI/2014/07/004730) and ClinicalTrials.gov (NCT03725085). The nine study centers included clinics, and hospital general medical and chest medicine departments, in Gujarat, Maharashtra, Karnataka, and Uttar Pradesh. Ethics committees from all nine study centers approved the study (St. John’s Medical College Hospital, Bangalore; Kempegowda Institute of Medical Sciences, Bangalore; Mandya Institute of Medical Science, Mandya; Mysore Medical College & Research Institute, Mysore; Shree Hospital & Critical Care Centre, Nagpur; King George’s Medical University, Lucknow; Lata Mangeshkar Hospital, Nagpur; Grant Medical Foundation, Pune; AMC Met Medical College & Sheth LG General Hospital, Ahmedabad). The study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization guideline on Good Clinical Practice. This study also complied with the Indian Drugs and Cosmetics Rules 1945, and the Indian Council of Medical Research’s Ethical Guidelines for Biomedical Research on Human Participants. All patients provided written informed consent.
Patients
Adult patients aged ≥18 years with symptoms of cough, thickened mucus and chest congestion, and who were diagnosed with acute bronchitis, a URTI or sinusitis, were enrolled. Patients were non-tobacco users who had not used nicotine or nicotine-containing products in the previous 30 days. Those with asthma, chronic bronchitis, emphysema and other chronic conditions were excluded from the study, as were pregnant or lactating females.
Study Treatment
Eligible patients took ER guaifenesin 1200 mg (two 600 mg bi-layer tablets) every 12 hrs for 7 days (14 doses). This dose is commensurate with the established use of the active ingredient in the USA (200–400 mg every 4 hrs) and with the ER formulation over a 12 hr period.
Study Methods And Procedures
During the first study visit (day 1) patients underwent assessment of signs/symptoms and diagnosis, and a physical/clinical examination. The first dose was administered at the study center and the investigator discussed with the patient the intended timing of dosing and the importance of complying with the 12 hr dosing regimen in order to ensure compliance throughout the course of the study. Patients who missed their scheduled dose by more than 4 hrs were advised to skip the dose and continue with their next regularly scheduled dose. Patients were given a diary at the start of the study, which was used to record details of missed doses, the reason for missed doses, any concomitant medication and any adverse events (AEs). Patients were then assessed at the study center at the end of the 7-day treatment phase. Serious AEs occurring shortly after study completion (within the time period corresponding to five half-lives of the study medication) and judged by the investigator to be drug related were to be followed up and reported.
In order to assess compliance to study medication, patient diaries were reviewed and the number of unused tablets was counted. Any concomitant medication used was reviewed in the context of AEs experienced by the patient.
Study Endpoints
The primary endpoint was the number, type and frequency of AEs as well as the proportion of patients with AEs. The severity, seriousness and the relationship of AEs to treatment were also assessed. Secondary endpoints included the ratings in two questionnaires which were provided to patients and investigators at the end-of-study visit to assess overall satisfaction with the study medication.
Statistical Analysis
No formal sample size estimation was performed for this study. A total of 650 patients were planned to be enrolled to obtain at least 550 evaluable patients, assuming approximately 15% dropouts. All patients who were enrolled were included in the safety analyses. AEs were classified according to the terminology of MedDRA Version 17.1 – Preferred Term and System Organ Class. Statistical analysis was conducted using the SAS® package (SAS® Institute Inc, Cary, NC, USA, and Version 9.2).
Results
Patient Disposition And Demographics
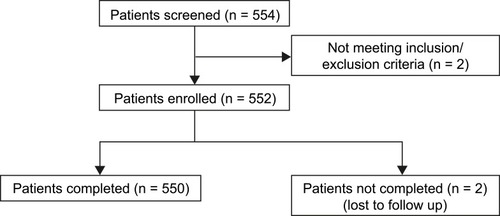
In total, 552 patients were enrolled, of which 550 completed the end-of-study visit (two patients were lost to follow-up) (). Among the 552 enrolled, 349 were males and 203 were females, and all patients were of Asian (Indian) origin ().
Table 1 Baseline Demographics
Primary Endpoint
A total of 29 treatment-emergent adverse events (TEAEs) were recorded in 28 of the 552 patients (5.07%), including gastrointestinal (11 patients), nervous system (eight patients), psychiatric (three patients), respiratory, thoracic and mediastinal (two patients), skin and subcutaneous tissue (two patients), and general disorders (three patients) (). All 29 TEAEs were assessed as mild in severity. Of these, five TEAEs experienced by five patients (0.91%) were assessed as having a probable relationship to the study drug, while 13 TEAEs experienced by 13 patients (2.36%) were assessed as possibly related to the study drug. Three events experienced by two patients (0.36%) were assessed as unlikely to be related to the study drug and eight events experienced by eight patients (1.45%) were assessed as unrelated to the study drug. A total of 26 of the 29 TEAEs, which occurred in 25 patients, did not require a change in the dose of the study drug. Four TEAEs experienced by four patients (upper abdominal pain [n = 1], diarrhea [n = 1], dizziness [n = 1], pain/body ache [n = 1]) required symptomatic treatment during the study period. All 29 TEAEs that occurred in 28 (5.07%) patients resolved during the study period. No serious AEs or deaths occurred and no suspected unexpected serious adverse reactions (SUSARs) were reported during the study.
Table 2 Number And Percentage Of Patients With Treatment-Emergent Adverse Events
Secondary Endpoints
End-Of-Study Patient Questionnaire
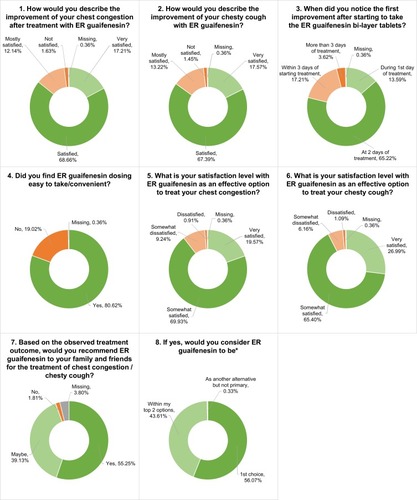
Of the 552 patients enrolled, 550 patients completed the end-of-study questionnaire (). A total of 68.66% of patients were “satisfied” and 17.21% were “very satisfied” with the improvement in their chest congestion after treatment with ER guaifenesin. Similarly, 67.39% of patients were “satisfied” and 17.57% of patients were “very satisfied” with the improvement in their chesty cough. Patients were also asked how soon they noticed an improvement in symptoms; 65.22% of patients noticed an improvement at 2 days of ER guaifenesin intake and 13.59% noticed an improvement during the first day. The majority of patients (80.62%) considered ER guaifenesin easy or convenient to administer. A total of 69.93% and 65.4% of patients were “somewhat satisfied” with ER guaifenesin as an effective option to treat chest congestion and chesty cough, respectively. However, 19.57% and 26.99% of patients were “very satisfied” with ER guaifenesin as an effective option to treat chest congestion and chesty cough, respectively. Overall, 55.25% of patients stated that they would recommend ER guaifenesin to their family and friends. Of these, 56.07% agreed that ER guaifenesin would be the first choice of treatment.
End-Of-Study Investigator Questionnaire
All nine investigators (all physicians) completed the end-of-study investigator questionnaire. The results showed that two investigators were “very satisfied” and five were “satisfied” with the treatment outcome in their patients who were taking ER guaifenesin. Three investigators were “very satisfied” and five investigators were “satisfied” with the patient response to the treatment of chest congestion and chesty cough. Five investigators noticed improvement in symptoms in their patients within 2 days of treatment and four noticed improvement within 3 days, with the majority noticing an improvement in chest congestion rather than other symptoms. A total of eight investigators either “agreed” or “strongly agreed” to prescribe ER guaifenesin for the treatment of chest congestion associated with a URTI. Furthermore, eight investigators agreed that the dosage (1200 mg twice daily) seemed optimal for treating chest congestion compared with other cough preparations.
Discussion
In this study, 5% of patients experienced subjective AEs following administration of ER guaifenesin, all of which were transient and mild in severity. As such, ER guaifenesin (1200 mg twice daily) was shown to have a favorable safety profile and be well tolerated in otherwise healthy patients with symptoms of cough, thickened mucus and chest congestion associated with acute URTI. The AEs observed during this study are consistent with the established safety profile of the drug; in a study evaluating the safety and efficacy of guaifenesin 800 mg/day (200 mg four times daily), 1.7% (4/239) of patients reported AEs.Citation13
Post-marketing periodic safety update reports for guaifenesin are only available from 2014 onwards, however, since its advent in 1949, an estimated 944,499,672 patients have been exposed to the drug (Reckitt Benckiser, data on file). From 01 July 2017 to 30 June 2018, 15,355,869 patients were exposed, and only 930 AEs reported, which is consistent with the excellent safety profile of the drug. Although the PMS safety profile of ER guaifenesin in many millions of consumer exposures is benign, the clinical safety profile is not as well characterized, as only a few studies have reported on its safety and tolerability.Citation17,Citation20 A phase 1 study evaluating the mucociliary clearance of a single dose of ER guaifenesin 1200 mg in 12 healthy adults reported no AEs and no safety concerns.Citation20 Furthermore, in a clinical trial which randomized 378 patients with acute URTI to receive either ER guaifenesin 1200 mg twice daily or matching placebo, AEs were found to be mostly mild in severity and resolved without intervention. None of the AEs were deemed definitely related to the study drug.Citation17 In an open-label trial, 87 physicians reported on 791 completed patients with upper or lower respiratory conditions to assess the efficacy of ER guaifenesin,Citation21 and the observed safety profile was favorable (personal communication). The present study adds to the clinical safety data and seems to confirm the safety profile of ER guaifenesin, showing it to be well tolerated.
This study also assessed the satisfaction of both patients and investigators with ER guaifenesin in the treatment of chest congestion and cough. Caution is needed in interpreting these findings due to the self-limiting (spontaneously resolving) nature of URTICitation22 and the design of the study (not blinded, no control). However, the reported satisfaction rate of approximately 80% of patients and investigators with ER guaifenesin seems to be in line with previously reported treatment effects, both for safety and satisfaction.Citation17,Citation21
Cough is the single most common reason for patients to present to primary care,Citation23 however, the treatment options for productive cough remain limited. Although it is estimated that several billions of dollars are spent on over-the-counter (OTC) cough and cold products annually in the USA,Citation24 there is limited and conflicting evidence on the clinical efficacy of many cough treatments.Citation25 This is mainly due to methodological issues, the limited number of available studies that investigated the efficacy of different cough treatments, and the differences between the studies that make comparison difficult. As such, there is a need for effective and safe OTC therapies for chest congestion and cough.
Some limitations of this study are apparent. As a pure safety study, the design was uncontrolled and unblinded, which limits interpretation of the observations as well as the treatment effects in a spontaneously resolving condition. The study design also increases the risk of bias for ratings of the treatment by patients and investigators. Monitoring of AEs continued for a short time after the study period was complete, with the intent to follow up on any drug-related and potentially serious AEs, had they been reported (there were none); however, any mild AEs occurring after the end of the study would not have been captured. Furthermore, the study was conducted in a single ethnic population, which was intentional. However, based on the solid safety record of guaifenesin, and the PMS experience and data from previous studies with ER guaifenesin, it is not expected that ethnic differences are a significant issue.Citation26
Conclusion
In summary, the study outcomes showed and confirmed that ER guaifenesin bi-layer tablets (1200 mg twice daily) have a favorable safety profile and are well tolerated in otherwise healthy patients with symptoms of cough, thickened mucus and chest congestion associated with URTI. These clinical data are consistent with, and add to, the established safety profile of ER guaifenesin, from PMS and clinical study observations.
Abbreviations
ER, Extended-release; URTI, upper respiratory tract infection; AE, adverse events; FDA, Food and Drug Administration; PMS, post-marketing surveillance; TEAEs, treatment-emergent adverse events; SUSARs, suspected unexpected serious adverse reactions; OTC, over-the-counter.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Statistical support was provided by Sudhakar babu Katakam at Manipal AcuNova Limited, Bangalore, India. The authors would also like to thank Sudhir Patel and Selva Sundaravel (clinical operations), Kumuda Nayak (data management), Dr Shivashankar Parashuram (medical monitor), and Ankur Mehrotra, formerly an employee of Reckitt Benckiser, for his input into the manuscript. Medical writing assistance was provided by Kim Russell at Elements Communications Ltd, Westerham, UK and funded by Reckitt Benckiser.
Data Availability
Data supporting each of the outcomes reported herein are found on ClinicalTrials.gov (NCT03725085). Any additional data that may be available can be obtained by contacting the corresponding author.
Disclosure
The study was funded by Reckitt Benckiser, Gurgaon 122001, Haryana, India. Medical writing assistance was provided by Kim Russell and Saroshi Amirthalingam at Elements Communications Ltd, Westerham, UK and funded by Reckitt Benckiser Healthcare International Ltd, UK. Tim Shea and Gaurav Sharma are employees of Reckitt Benckiser USA and Reckitt Benckiser India, respectively. As investigators of the study, Sanjay Tripathi and Ashish Nikhare received compensation for time and resources in enrolling patients into the study and recording data during the clinical trial. Helmut Albrecht is a paid clinical/medical consultant to Reckitt Benckiser. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52(9):1134–1146.17716382
- Finkbeiner WE. Physiology and pathology of tracheobronchial glands. Respir Physiol. 1999;118(2–3):77–83. doi:10.1016/S0034-5687(99)00080-810647853
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245–278. doi:10.1152/physrev.00008.200616371599
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi:10.1056/NEJMoa101120521121836
- Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J. 1999;13(5):1177–1188. doi:10.1034/j.1399-3003.1999.13e39.x10414423
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury: airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S76–80. doi:10.1164/ajrccm.164.supplement_2.210606711734472
- Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61(2):86–100. doi:10.1016/j.addr.2008.09.01219166889
- Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur Respir Rev. 2010;19(116):127–133. doi:10.1183/09059180.0000351020956181
- Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulm Pharmacol. 1996;9(5–6):299–308. doi:10.1006/pulp.1996.00399232667
- Yuta A, Baraniuk JN. Therapeutic approaches to mucus hypersecretion. Curr Allergy Asthma Rep. 2005;5(3):243–251. doi:10.1007/s11882-005-0044-615842963
- Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res. 2012;13:98. doi:10.1186/1465-9921-13-9823113953
- FDA. Cold, cough, allergy, bronchodilator and antiasthmatic drug products for over-the-counter human use; Final monograph. Fed Regist. 1989;54:8494–8509.
- Robinson RE, Cummings WB, Deffenbaugh ER. Effectiveness of guaifenesin as an expectorant: a cooperative double-blind study. Curr Ther Res. 1977;22(2):284–296.
- Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM Jr. Antitussive effect of guaifenesin in young adults with natural colds. Objective and subjective assessment. Chest. 1982;82(6):713–718. doi:10.1378/chest.82.6.7136754274
- Dicpinigaitis PV, Gayle YE. Effect of guaifenesin on cough reflex sensitivity. Chest. 2003;124(6):2178–2181. doi:10.1378/chest.124.6.217814665498
- Mucinex® (guaifenesin) [package insert]. Parsippany, NJ: Reckitt Benckiser LLC; 2016.
- Albrecht H, Vernon M, Solomon G. Patient-reported outcomes to assess the efficacy of extended-release guaifenesin for the treatment of acute respiratory tract infection symptoms. Respir Res. 2012;13:118. doi:10.1186/1465-9921-13-11823270519
- Hoffer-Schaefer A, Rozycki HJ, Yopp MA, Rubin BK. Guaifenesin has no effect on sputum volume or sputum properties in adolescents and adults with acute respiratory tract infections. Respir Care. 2014;59(5):631–636. doi:10.4187/respcare.0264024003241
- Bennett WD, Kala A, Duckworth H, et al. Effect of a single 1200 mg dose of Mucinex® on mucociliary and cough clearance during an acute respiratory tract infection. Respir Med. 2015;109(11):1476–1483. doi:10.1016/j.rmed.2015.09.01726462765
- Bennet W, Kala A, Zeman K, et al. Effect of oral guaifenesin (Mucinex® 1200 mg) on mucociliary clearance from the lungs of healthy, non-smoking adults. Poster presented at: European Respiratory Society Annual Congress; 9 18–22, 2010; Barcelona, Spain.
- Storms W, Kaliner MA, Javidi M, Efessiou C, Farrar J. An open-label, multicenter, single-group study to evaluate patients’ reactions to single-entity long-acting guaifenesin. Poster presented at: Annual Meeting of the Western Society of Allergy, Asthma, and Immunology (WSAAI); 1, 2008; Kailua-Kona, Hawaii, USA.
- Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190–199. doi:10.1503/cmaj.12144224468694
- Irwin RS. Introduction to the diagnosis and management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):25S–27S. doi:10.1378/chest.129.1_suppl.25S16428688
- Madison JM, Irwin RS. Cough: a worldwide problem. Otolaryngol Clin North Am. 2010;43(1):1–13. doi:10.1016/j.otc.2009.11.00120172252
- Smith SM, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev. 2014;(11):CD001831.25420096
- Dicpinigaitis PV, Allusson VR, Baldanti A, Nalamati JR. Ethnic and gender differences in cough reflex sensitivity. Respiration. 2001;68(5):480–482. doi:10.1159/00005055411694809